Abstract
Suboptimal vitamin B-6 status, as reflected by low plasma pyridoxal 5′-phosphate (PLP) concentration, is associated with increased risk of vascular disease. PLP plays many roles, including in one-carbon metabolism for the acquisition and transfer of carbon units and in the transsulfuration pathway. PLP also serves as a coenzyme in the catabolism of tryptophan. We hypothesize that the pattern of these metabolites can provide information reflecting the functional impact of marginal vitamin B-6 deficiency. We report here the concentration of major constituents of one-carbon metabolic processes and the tryptophan catabolic pathway in plasma from 23 healthy men and women before and after a 28-d controlled dietary vitamin B-6 restriction (<0.35 mg/d). liquid chromatography-tandem mass spectrometry analysis of the compounds relevant to one-carbon metabolism showed that vitamin B-6 restriction yielded increased cystathionine (53% pre- and 76% postprandial; P < 0.0001) and serine (12% preprandial; P < 0.05), and lower creatine (40% pre- and postprandial; P < 0.0001), creatinine (9% postprandial; P < 0.05), and dimethylglycine (16% postprandial; P < 0.05) relative to the vitamin B-6–adequate state. In the tryptophan pathway, vitamin B-6 restriction yielded lower kynurenic acid (22% pre- and 20% postprandial; P < 0.01) and higher 3-hydroxykynurenine (39% pre- and 34% postprandial; P < 0.01). Multivariate ANOVA analysis showed a significant global effect of vitamin B-6 restriction and multilevel partial least squares-discriminant analysis supported this conclusion. Thus, plasma concentrations of creatine, cystathionine, kynurenic acid, and 3-hydroxykynurenine jointly reveal effects of vitamin B-6 restriction on the profiles of one-carbon and tryptophan metabolites and serve as biomarkers of functional effects of marginal vitamin B-6 deficiency.
Introduction
Vitamin B-6, as pyridoxal 5′-phosphate (PLP)15, serves a coenzymatic function in many phases of metabolism. Marginal vitamin B-6 status, defined as plasma PLP of 20–30 nmol/L, is common in the American population (1, 2) and has been associated with increased risk of coronary artery disease and stroke (3–6). The mechanism responsible for these disease associations has not been determined but frequently is not associated with homocysteine elevation.
Vitamin B-6 plays an integral role in one-carbon metabolism, because PLP serves as a coenzyme for glycine decarboxylase of the glycine cleavage system, cytoplasmic and mitochondrial forms of serine hydroxymethyltransferase (SHMT), and cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) in the trans-sulfuration pathway. Inadequate vitamin B-6 status has been associated with cardiovascular disease risk (3–6), possibly due to derangement of one-carbon metabolism. Whether any of these specific metabolic processes are involved in mediating disease risk remains unclear. In vivo kinetic studies and mathematical modeling analysis suggest that most aspects of one-carbon metabolism are sufficiently adaptive to maintain function over the range of mild to moderate deficiency of vitamin B-6 (7–12). However, our recent metabolomic investigation showed extended metabolic changes associated with marginal vitamin B-6 deficiency in controlled nutritional studies, including perturbations of amino acid, lipid, and organic acid profiles (13).
Severe vitamin B-6 deficiency has substantial effects on amino acid metabolism as reflected by changes in the concentration of several amino acids in plasma, tissue, and urine (14–16). Concentrations of glycine and, to a lesser extent, serine have been shown to increase in plasma, urine, and liver after vitamin B-6 depletion (14, 15, 17, 18). A marginal vitamin B-6 deficiency, consistent with plasma PLP concentration of 20-30 nmol/L, (19, 20) has been shown to affect one-carbon metabolism. Previous studies reported significant elevations in plasma glycine and cystathionine after a 28-d dietary vitamin B-6 restriction (8, 9) and an inverse association of cystathionine with plasma B-6 vitamers in a large-scale population study (21). Little is known about the influence of vitamin B-6 deficiency on the pattern of other constituents of one-carbon metabolism and related pathways, especially during marginal deficiency. Mathematical modeling suggested that the PLP-dependent reactions in one-carbon metabolism exhibited little change of flux in vitamin B-6 deficiency (11), but the pattern of the various metabolites was not systematically investigated and is addressed herein.
The involvement of PLP in tryptophan catabolism represents another important role of vitamin B-6 in amino acid metabolism by regulating tryptophan turnover and providing NAD synthesis (22). This pathway is influenced by inflammatory conditions (23–25), cancer (26), pregnancy (27, 28), and oral contraceptive usage (28–32) as well as insufficiency of cellular PLP (33–35). The major pathway of tryptophan oxidation to N-formylkynurenine exists in liver and is catalyzed by tryptophan 2,3-dioxygenase (TDO) (22, 36). TDO is induced by tryptophan (37–39) and hormones such as glucocorticoids (38, 40, 41), glucagon (42), and estrogens (43). Tryptophan oxidation is catalyzed in nonhepatic tissues by indoleamine 2,3-dioxygenase (IDO) (44, 45). In contrast to TDO, IDO is induced primarily by IFNγ (3, 12); thus, IDO is associated with the immune response (24, 44). Various inflammatory conditions frequently are associated with low plasma PLP (23–25), although vitamin B-6 deficiency in otherwise healthy humans does not induce inflammation (8). Despite having similar roles in supplying the tryptophan catabolic pathway, TDO and IDO have distinct functions in regulating tryptophan metabolism (39). Both hepatic and nonhepatic pathways of tryptophan catabolism involve the bifunctional PLP-dependent enzymes kynureninase and kynurenine aminotransferase (46, 47) and are subject to effects of vitamin B-6 deficiency. Kynureninase catalyzes both the conversion of kynurenine to anthranilic acid and 3-hydroxykynurenine to 3-hydroxyanthranilic acid. Kynurenine aminotransferase catalyzes the conversion of kynurenine to kynurenic acid and 3-hydroxykynurenine to xanthurenic acid (48–50). Kynureninase appears to be more sensitive to the effects of vitamin B-6 insufficiency than kynurenine aminotransferase (47), as confirmed by recent mathematical modeling of tryptophan metabolism (51). It has been proposed that induction of the IDO-dependent nonhepatic pathway of tryptophan catabolism and other PLP-dependent enzymes involved in the inflammatory response can account for the association of low plasma PLP and markers of systemic inflammation (23).
Relatively little information is available regarding the response of tryptophan catabolites in blood, tissue, and urine to various levels of vitamin B-6 status. The relationship between circulating B-6 vitamers and plasma tryptophan metabolites has been examined in large-scale studies of individuals with systemic inflammation and showed that elevation of 3-hydroxykynurenine was a biomarker of vitamin B-6 deficiency (52, 53), which complements similar studies of the relationship of plasma B-6 vitamers and trans-sulfuration metabolites (21), although the extent to which inflammation affected the latter relationship is unclear. The sensitivity of tryptophan catabolites to nutritional effects (i.e., controlled vitamin B-6 restriction) is addressed in the present study.
We report here metabolic profile analyses intended to extend our understanding of the functional consequences of marginal vitamin B-6 deficiency in healthy men and women. These investigations involved analysis of the constituents of one-carbon metabolism and related pathways and of tryptophan catabolism of plasma samples from healthy men and women obtained before and after a dietary vitamin B-6 restriction protocol (9, 12). We also interpret findings in relation to simulations using mathematical models of one-carbon metabolism (11) and tryptophan catabolism (51). These studies broaden our understanding of the metabolic effects of vitamin B-6 deficiency and extend recent metabolomic findings (54).
Materials and Methods
Materials
All standards were purchased from Sigma-Aldrich except symmetric dimethylarginine and asymmetric dimethylarginine (ADMA), which were purchased from Axxora (formerly Alexis Biochemicals). Internal standards [[13C2]-glycine; [13C5]-methionine; [15N2]-arginine; [2H3]-serine; [2H3]-sarcosine; [2H6]-dimethylglycine; [2H9]-choline; [2H11]-betaine; [2H3]-creatine; [2H3]-creatinine; [2H2]-threonine; [2H4]-cystathionine; [2H2]-guanidinoacetic acid] were purchased from Cambridge Isotopes. [13C5]Methionine sulfoxide was synthesized from [13C5]methionine by oxidizing with 5% hydrogen peroxide.
Human vitamin B-6 restriction protocols
Participants.
The plasma samples analyzed in this study were obtained from 23 healthy men and women participants in 2 identical dietary vitamin B-6 restriction studies previously reported (9, 12). Nutritional adequacy was indicated by normal concentrations under preprandial conditions of serum folate (>7 nmol/L), serum vitamin B-12 (>200 pmol/L), plasma PLP (>30 nmol/L), and plasma total homocysteine (<12 μmol/L). All participants gave written informed consent. The University of Florida Institutional Review Board and the University of Florida Clinical Research Center Scientific Advisory Committee reviewed and approved this protocol (9, 12).
Dietary protocol.
All meals were prepared by the Clinical Research Center Bionutrition Unit at the University of Florida. Prior to the first blood sample, participants consumed nutritionally adequate meals with standardized composition for 2 d, after which they consumed a low-vitamin B-6 diet (0.37 ± 0.04 mg/d) for 28-d, intended to induce a state of marginal vitamin B-6 deficiency as reflected by plasma PLP between 20 and 30 nmol/L (9, 12). All participants received daily a custom vitamin-mineral supplement containing no vitamin B-6 to maintain adequate status of other micronutrients.
The diet was planned using the following criteria. Actual intake varied depending on participants’ daily intake patterns, with food components adjusted according to food preferences and energy needs. The diets provided: 50–60 g/d protein for females or 60–70 g/d males; 1.0 mg/d vitamin B-6 in the 2-d control diet period and <0.5 mg/d in the 28-d vitamin B-6 restriction period; 400 μg/d dietary folate equivalents; energy adjusted to maintain weight using the Mifflin St.-Jeor equation and individualized activity factors; and macronutrient composition varied depending on individual food preferences. Actual intakes for the 2-d control period were: energy, 10,900 ± 1850 kJ/d; energy from fat, 28.4 ± 3.8%; energy from carbohydrate, 62.0 ± 4.0%; energy from protein, 9.4 ± 1.3; vitamin B-12, 1.7 ± 0.7 μg/d; naturally occurring folate, 150 ± 36 μg/d; folic acid, 240 ± 36 μg/d; and vitamin B-6, 1.02 ± 0.11 μg/d. The actual intakes for the 28-d restriction period were: energy, 11,700 ± 25,10 kJ/d; energy from fat, 29.1 ± 2.6%; energy from carbohydrate, 60.4 ± 2.9%; energy from protein, 10.4 ± 1.0; vitamin B-12, 1.33 ± 0.34 μg/d; naturally occurring folate, 92 ± 29 μg/d; folic acid, 265 ± 28 μg/d, and vitamin B-6, 0.37 ± 0.04 μg/d. Dietary intake data were collected using Nutrition Data System for Research software versions 2005 and 2007, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. Final calculations were completed using NDSR version 2012. The NDSR time-related database updates analytic data while maintaining nutrient profiles true to the version used for data collection.
Postprandial plasma was obtained during steady-state tracer infusion protocols after 8 administrations of hourly portions of a nutritive formula containing protein, carbohydrate, and fat (9, 12). As previously reported (9), hourly administration of this formula beginning 2 h before the infusion maintained a postprandial state. The formula provided a balanced pattern of amino acids at a rate based on requirements of 0.8 g protein · kg−1 · d−1, equivalent to an hourly protein dose of 0.03 g/kg with 5.23 and 5.44 kJ · kg−1 · d−1 for women and men, respectively. The formula also provided an adequate energy intake according to the requirements of 126 and 130 kJ · kg−1 · d−1 for women and men, respectively.
Blood sample collection.
Preprandial and postprandial blood samples were collected in EDTA tubes at the end of the 2-d controlled diet and after the 28-d vitamin B-6–restricted diet. Within 2 h of collection, plasma was separated by centrifuging whole blood at 1650 × g for 15 min at 4°C and then stored at −80°C for later analysis.
Analytical methods
Plasma PLP was determined as the semicarbazone-derivative by reverse-phase HPLC with fluorescence detection (55). Aminothiols were measured as ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate derivatives by reverse-phase HPLC with fluorescence detection (56).
A panel of 16 one-carbon metabolites and related compounds, including betaine, dimethylglycine (DMG), serine, glycine, methionine, methionine sulfoxide, threonine, choline, guanidinoacetate, creatine, creatinine, cystathionine, arginine, leucine, symmetrical dimethylarginine, and symmetrical dimethylarginine, was quantified by LC-tandem MS (MS/MS) at the Biomedical Mass Spectrometry Laboratory, Clinical and Translational Sciences Institute, University of Florida, which was a modification of the methods of Ueland et al. (57). In this analysis, the internal standard mixture (20 μL) was added to 60 μL of plasma and mixed by vortexing. Proteins were precipitated by adding 3 volumes of methanol, mixing, and then storing at 4°C for 30 min, followed by centrifuging at 2000 × g for 10 min at 4°C. The supernatant was removed and analyzed (10 μL sample injection) by LC-MS/MS. Samples were chromatographed on a Luna C18 150- × 4.6-mm column (Phenomenex) and eluted in 0.02% heptafluorobutyric acid using a methanol gradient at 0.6 mL/min. To achieve retention of the early eluting compounds, the column was equilibrated for 3 min with 0.1% heptafluorobutyric acid between injections. Samples were analyzed in ESI mode using a TSQ Quantum triple quadrupole mass spectrometer (Thermo-Finnigan). Analysis was performed in positive ionization mode (capillary temperature, 300°C; vaporizer temperature, 250°C; sheath gas pressure, 50 arbitrary units; auxiliary gas pressure, 20 arbitrary units; collision gas pressure, 1.5 mTorr) using selected reaction monitoring with the m/z transitions shown (Supplemental Table 1). Analyte concentrations were determined by calculating the analyte:internal standard peak area ratios and comparing them with standard concentration curves prepared using authentic standards.
Quantitative analysis of constituents of tryptophan catabolism, including tryptophan, kynurenine, kynurenic acid, anthranilic acid, 3-hydroxykynurenine, xanthurenic acid, 3-hydroxyanthranilic acid, quinolinic acid, N1-methyl-nicotinamide, nicotinamide, and nicotinic acid, was performed by LC-MS/MS (58) at Bevital.
To assess any inflammatory response in this dietary vitamin B-6 restriction protocol, 3 markers of inflammation were evaluated. Neopterin was determined concurrently with the tryptophan metabolites, and the kynurenine:tryptophan ratio also was derived from results of that analysis (58). We also measured C-reactive protein by ELISA in preprandial plasma samples (High Sensitivity C-Reactive Protein kit no. CR120C, Calbiotech).
Statistical analysis
All data are presented as means ± SDs. MANOVA was used to evaluate significance of the overall change across all targeted metabolites from the LC-MS/MS analyses of one-carbon metabolites and tryptophan metabolites for the fasting and fed data separately (59). Differences in concentration after vitamin B-6 restriction were determined after log base 2 transformation to meet the Gaussian requirement. If a significant overall effect was found, stepdown tests with adjustment for multiple testing were performed. For one-carbon metabolites, the resulting adjusted P values were computed with methods that have control of the false discovery rate (60). For the tryptophan metabolites, the adjusted P values were calculated with Sidak adjustment (61). The main effect of the vitamin B-6 restriction protocol on the plasma ratios of cystathionine:creatine, 3-hydroxykynurenine:kynurenic acid (potential biomarkers of vitamin B-6 deficiency), and kynurenine:tryptophan (an indicator of inflammatory response) were evaluated using the 2-way repeated-measures ANOVA. A 2-sample t test was used to assess differences between men and women on the change of plasma PLP from the restriction protocol. The target level of significance was set to 0.05 or lower. All data analyses were performed using SAS 9.3 software.
Multivariate evaluation of overall effects of the vitamin B-6 restriction protocol on the combined profiles of constituents of one-carbon metabolism and tryptophan catabolism pathways was conducted and the results were visualized using multilevel partial least squares-discriminant analysis, which accounts for the paired structure of the data (62). For this multilevel analysis, we processed the aggregate metabolite profile data according to Westerhuis et al. (62) using Microsoft Excel and then conducted partial least squares-discriminant analysis using SIMCA version 13 (MKS Umetrics). The effect of vitamin B-6 restriction on overall metabolite profiles was visualized with score plots and discriminatory variables (i.e., responding metabolites) were identified using Variable Influence on Projection (VIP) plots in SIMCA version 13 (63). The VIP values in these plots represent weighted sums of squares of the PLS weights of the various metabolites and take into account the proportion of Y-variance in each dimension (64). In the VIP plots, each variable is provided with a VIP value and a 95% CI derived from jack-knifing (64–66). Variables for which the VIP ± 95% CI exceeds 1 are designated as significant biomarkers in this analysis.
Results
Summary of participants and effect of vitamin B-6 restriction protocol.
All participants had serum folate, vitamin B-12, and plasma PLP and total homocysteine in the normal range at the beginning of the study (9, 12) (Table 1). The dietary vitamin B-6 restriction protocol lowered the plasma PLP concentration from 52 ± 14 to 21 ± 5 nmol/L (P < 0.05), consistent with induction of marginal deficiency (9, 12, 54). Following restriction, the PLP values ranged from 12.3 to 29.3 nmol/L. Plasma total homocysteine and cysteine concentrations were not changed by the 28-d vitamin B-6 restriction (9, 12, 54).
TABLE 1.
Baseline characteristics of adults who completed the 28-d dietary vitamin B-6 restriction1
| Men | Women | |
| n | 12 | 11 |
| Age, y | 24 ± 52 | 25 ± 6 |
| BMI, kg/m2 | 24.5 ± 2.7 | 23.6 ± 2.3 |
| Plasma PLP, nmol/L | 59 ± 13 | 45 ± 13* |
| Serum folate, nmol/L | 34 ± 8 | 30 ± 7 |
| Serum vitamin B-12, pmol/L | 349 ± 115 | 388 ± 129 |
| Plasma homocysteine, μmol/L | 7.5 ± 1.0 | 6.4 ± 1.3 |
Data previously reported (9,12,13,80). *Different from men, P < 0.05 (2-sample t test). PLP, pyridoxal 5'-phosphate.
Mean ± SD (all such values).
The 2-sample t test on the change of PLP revealed no difference between male and female participants (P = 0.11). The average decrease for males was 35.8 nmol/L with a SD of 12.8 nmol/L; the average decrease for females was 25.9 nmol/L with a SD of 15.4 nmol/L.
One-carbon metabolism.
Targeted analysis of one-carbon metabolites in both preprandial and postprandial plasma successfully quantified a panel of 16 compounds (Table 2). MANOVA testing showed an effect of vitamin B-6 deficiency on the overall profile in both preprandial and postprandial states (P < 0.05). MANOVA also indicated that the pattern of changes in metabolite concentration in preprandial was different from that of postprandial conditions (P < 0.01), but the differences were not due to changes in concentration of any individual metabolite. After adjusting for multiple comparisons using the false discovery rate method, preprandial plasma concentrations of serine (12.4% increase, adjusted P < 0.05), creatine (40.2% decrease, adjusted P < 0.0001), and cystathionine (53.3% increase, adjusted P < 0.0001) were significantly different after the 28-d vitamin B-6 restriction compared with baseline (Table 2), whereas the changes in preprandial concentrations of creatinine (7.7% decrease, adjusted P = 0.09), glycine (14.8% increase, adjusted P = 0.09), threonine (15.5% increase, adjusted P = 0.09), and DMG (11.3% decrease, adjusted P = 0.09) approached significance. In the postprandial state, DMG (16% decrease, adjusted P < 0.05) and creatinine (8.4% decrease, adjusted P < 0.05), and creatine (40.1% decrease, adjusted P < 0.05) and cystathionine (76.4% increase, adjusted P < 0.001) plasma concentrations significantly differed in the vitamin B-6–restricted state compared with adequate status (Table 2).
TABLE 2.
Concentrations of one-carbon metabolites quantified in plasma of human participants in the preprandial and postprandial states before and after a 28-d dietary vitamin B-6 restriction1
| Preprandial |
Postprandial |
|||
| Baseline | B-6 restricted | Baseline | B-6 restricted | |
| μmol/L | μmol/L | |||
| Betaine | 44.3 ± 15.3 | 39.0 ± 10.6 | 39.2 ± 12.2 | 34.3 ± 9.50 |
| Choline | 9.14 ± 2.48 | 8.57 ± 1.8 | 8.00 ± 1.70 | 7.10 ± 1.30 |
| DMG | 2.65 ± 0.65 | 2.35 ± 0.62 | 2.50 ± 0.60 | †2.10 ± 0.40 |
| Serine | 113 ± 23.5 | †127 ± 38.0 | 110 ± 24.0 | 116 ± 27.1 |
| Glycine | 277 ± 78.8 | 318 ± 99.6 | 283 ± 80.3 | 308 ± 70.5 |
| Threonine | 155 ± 34.2 | 179 ± 51.1 | 155 ± 35.7 | 172 ± 42.7 |
| Guanidinoacetic acid | 3.18 ± 1.23 | 3.00 ± 1.00 | 2.60 ± 0.60 | 2.50 ± 0.60 |
| Creatine | 31.1 ± 13.7 | ‡18.6 ± 6.36 | 32.2 ± 12.1 | ‡19.3 ± 6.70 |
| Creatinine | 80.2 ± 15.0 | 74.0 ± 16.9 | 69.0 ± 10.0 | †63.2 ± 9.20 |
| Cystathionine | 0.15 ± 0.06 | ‡0.23 ± 0.08 | 0.17 ± 0.09 | ‡0.30 ± 0.12 |
| Methionine | 28.9 ± 6.32 | 28.1 ± 5.57 | 30.0 ± 5.40 | 29.9 ± 5.80 |
| Methionine sulfoxide | 0.95 ± 0.31 | 0.98 ± 0.28 | 1.00 ± 0.30 | 1.00 ± 0.30 |
| Leucine | 144 ± 36.8 | 132 ± 27.0 | 128 ± 23.9 | 123 ± 18.7 |
| Arginine | 105 ± 28.5 | 96.3 ± 24.7 | 95.1 ± 17.1 | 88.8 ± 13.3 |
| Symmetric dimethyl arginine | 0.66 ± 0.12 | 0.64 ± 0.12 | 0.59 ± 0.06 | 0.57 ± 0.08 |
| ADMA | 0.75 ± 0.15 | 0.69 ± 0.15 | 0.66 ± 0.11 | 0.62 ± 0.10 |
Values are means ± SDs, n = 23. Significant difference from baseline designated by: †adjusted P < 0.05, ‡adjusted P < 0.001. The adjustment was based on the false discovery rate method. ADMA, asymmetric dimethylarginine; DMG, dimethylglycine.
Tryptophan catabolism.
LC-MS/MS provided a measurement of 11 constituents of the tryptophan catabolic pathway. MANOVA analysis of data from both preprandial and postprandial states showed effects of vitamin B-6 restriction on the tryptophan metabolite profile (P < 0.0002). In this analysis, the magnitude of change of plasma PLP did not have an overall effect (preprandial, P = 0.149; postprandial, P = 0.596) (Table 3). Vitamin B-6 restriction induced the following changes in tryptophan catabolites when assessed under preprandial conditions: kynurenic acid (25.7% decrease, adjusted P = 0.0019), 3-hydroxykynurenine (32.2% increase, adjusted P = 0.0031), and nicotinic acid (15.8% decrease, adjusted P = 0.017). When the effects of vitamin B-6 restriction were evaluated in postprandial plasma, kynurenic acid (22.6% decrease, adjusted P = 0.0013) and 3-hydroxykynurenine (28.9% increase, adjusted P = 0.0088) changed significantly, but the change in nicotinic acid (10.3% decrease, adjusted P = 0.546) was not significant (Table 3).
TABLE 3.
Concentrations of tryptophan metabolites quantified in plasma of human participants in the preprandial and postprandial states before and after a 28-d dietary vitamin B-6 restriction1
| Preprandial |
Postprandial |
|||
| Baseline | B-6 restricted | Baseline | B-6 restricted | |
| Tryptophan, μmol/L | 61.1 ± 14.1 | 69.3 ± 17.8# | 62.3 ± 11.4 | 66.5 ± 14.1 |
| Kynurenine, μmol/L | 1.15 ± 0.244 | 1.18 ± 0.267 | 1.25 ± 0.237 | 1.22 ± 0.196 |
| Kynurenic acid, nmol/L | 30.7 ± 10.1 | 22.8 ± 7.68† | 32.8 ± 8.10 | 25.4 ± 7.48† |
| Anthranilic acid, nmol/L | 14.5 ± 4.32 | 13.5 ± 3.44 | 13.4 ± 3.36 | 13.6 ± 4.87 |
| 3-Hydroxykynurenine, nmol/L | 24.5 ± 9.46 | 32.4 ± 11.0‡ | 27.7 ± 11.8 | 35.7 ± 15.9† |
| Xanthurenic acid, nmol/L | 8.44 ± 4.43 | 7.05 ± 2.43 | 12.9 ± 7.02 | 11.7 ± 6.00 |
| 3-Hydroxyanthranilic acid, nmol/L | 19.9 ± 6.83 | 18.1 ± 6.46 | 24.0 ± 8.47 | 22.3 ± 6.85 |
| Quinolinic acid, nmol/L | 253 ± 59.6 | 274 ± 55.2@ | 264 ± 63.8 | 250 ± 46.4 |
| Nicotinic acid, nmol/L | 77.6 ± 16.4 | 65.3 ± 19.1‡ | 64.8 ± 14.5 | 58.1 ± 8.59 |
| Nicotinamide, nmol/L | 292 ± 184 | 303 ± 238 | 292 ± 152 | 272 ± 147 |
| N1-methylnicotinamide, nmol/L | 157 ± 84.5 | 146 ± 79.2 | 159 ± 99.6 | 124 ± 70.3 |
Values are means ± SDs, n = 23. Significant difference from baseline designated by: †, adjusted P < 0.05; ‡, adjusted P < 0.001. The adjustment was based on the Sidak method. Trend toward significant difference from baseline designated by: #, adjusted P = 0.073; @, adjusted P = 0.062.
Vitamin B-6 effects on biomarkers of inflammation.
Three indicators of inflammatory response were examined in plasma obtained under both preprandial and postprandial conditions before and after the 28-d vitamin B-6 restriction (Table 4). C-reactive protein, which is an acute-phase protein indicative of inflammation, exhibited a small decrease (P = 0.002). Neopterin, which is a pterin indicative of immune activation, exhibited a small increase due to the restriction protocol (P = 0.012). The kynurenine:tryptophan ratio, a marker of cellular immune response, exhibited a small decrease due to the B-6 restriction (P = 0.013) with a small but positive effect of postprandial status (P = 0.008).
TABLE 4.
Indicators of inflammatory status in plasma of human participants in the preprandial and postprandial states before and after a 28-d dietary vitamin B-6 restriction1
| Preprandial |
Postprandial |
P value |
|||||
| Baseline | B-6 restricted | Baseline | B-6 restricted | B-6 restriction | Preprandial status | Interaction | |
| C-reactive protein, mg/L | 2.66 ± 3.56 | 1.00 ± 1.74* | 2.41 ± 3.15 | 0.947 ± 1.36* | 0.002 | 0.236 | 0.618 |
| Neopterin, nmol/L | 6.15 ± 1.15 | 6.95 ± 1.46* | 6.16 ± 1.28 | 6.69 ± 2.08* | 0.012 | 0.357 | 0.278 |
| Kynurenine/tryptophan | 0.0196 ± 0.0057 | 0.0175 ± 0.0040* | 0.0207 ± 0.0057 | 0.0190 ± 0.0044* | 0.013 | 0.008 | 0.640 |
Values are means ± SDs, n = 23. *Different from baseline, P < 0.05. P values are from repeated-measures 2-way ANOVA.
Ratios of one-carbon and tryptophan metabolites as biomarkers of vitamin B-6 deficiency.
We investigated several plasma product:precursor ratios to probe potential effects of vitamin B-6 restriction on cellular processes (Table 5). The ratio of glycine:serine, reflecting relative rates of glycine cleavage and SHMT reactions, increased in the postprandial state but was not affected by vitamin B-6 status. Vitamin B-6 restriction did not affect the betaine:choline or DMG:betaine ratios in either preprandial or postprandial conditions. The ratio of guanidinoacetic acid:creatine, which reflects the choline synthesis process catalyzed by guanidinoacetate N-methyltransferase, increased in vitamin B-6 restriction in both preprandial and postprandial samples and paralleled creatine concentration. The ratio of creatine:cystathionine, which are not directly related metabolically, appears to provide a very sensitive combined biomarker of marginal vitamin B-6 deficiency (Table 5).
TABLE 5.
Ratios of selected one-carbon and tryptophan metabolites quantified in plasma of human participants in the preprandial and postprandial states before and after a 28-d dietary vitamin B-6 restriction1
| Preprandial |
Postprandial |
P value |
|||||
| Baseline | B-6 restricted | Baseline | B-6 restricted | B-6 restriction | Preprandial status | Interaction | |
| Product:precursor ratios | |||||||
| Glycine:serine | 2.49 ± 0.608 | 2.54 ± 0.522 | 2.62 ± 0.571 | 2.73 ± 0.573 | 0.153 | 0.021 | 0.795 |
| Betaine:choline | 5.09 ± 1.99 | 4.59 ± 0.972 | 5.13 ± 1.75 | 4.86 ± 1.26 | 0.513 | 0.252 | 0.575 |
| DMG:betaine | 0.0651 ± 0.0216 | 0.0620 ± 0.0161 | 0.0674 ± 0.0245 | 0.0633 ± 0.0151 | 0.486 | 0.163 | 0.765 |
| Creatine:guanidinoacetic acid | 11.3 ± 6.65 | 6.51 ± 2.33* | 13.9 ± 6.88 | 8.28 ± 3.07* | <0.001 | <0.001 | 0.91 |
| ADMA:arginine | 0.00822 ± 0.00422 | 0.00732 ± 0.00118* | 0.00761 ± 0.00176 | 0.00702 ± 0.00117* | 0.005 | 0.22 | 0.74 |
| Other biomarker ratios | |||||||
| Creatine:cystathionine | 229 ± 94.5 | 85.6 ± 31.1* | 214 ± 91.0 | 74.9 ± 34.3* | <0.001 | 0.056 | 0.26 |
| 3-Hydroxykynurenine: kynurenic acid | 0.903 ± 0.244 | 1.56 ± 0.759* | 0.900 ± 0.530 | 1.47 ± 0.735* | <0.001 | 0.67 | 0.35 |
Values are means ± SDs, n = 23. *Different from baseline, P < 0.05. P values are from repeated-measures 2-way ANOVA. ADMA, asymetric dimethylarginine; DMG, dimethylglycine.
Two of the metabolite ratios provided evidence of physiological status. The ADMA:arginine inversely reflects NO synthetase substrate function and ADMA as a competitive inhibitor. In this study, vitamin B-6 restriction yielded an increased ADMA:arginine ratio (P < 0.001) in both pre- and postprandial conditions. The ratio of methionine sulfoxide:methionine, which may be an indicator of oxidative stress, did not change significantly due to vitamin B-6 restriction in either pre- or postprandial samples.
Graphical multivariate analysis of pooled one-carbon and tryptophan catabolite profile data.
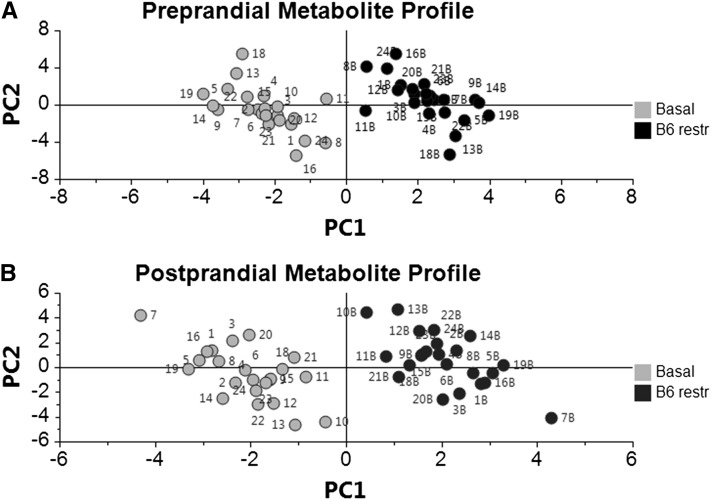
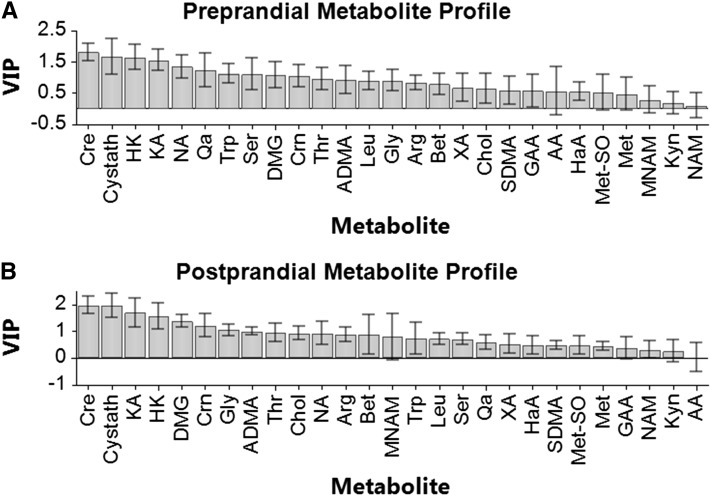
The overall effect of vitamin B-6 restriction on this collection of various constituents of one-carbon metabolism and tryptophan catabolic processes was evaluated further by a multilevel PLS-DA approach (62), which is a graphical method for visualizing overall treatment effects with paired data. The overall metabolite profiles effectively grouped the data according to vitamin B-6 status (i.e., basal vs. restricted) when conducted for preprandial and postprandial samples (Fig. 1A,B). In this analysis of samples in the preprandial state, the first principal component accounted for 85% of the variation modeled in Y and the second component accounted for 5.8%, while 88% of the variation could be predicted by the model. Similarly, for samples in the postprandial state, the first principal component accounted for 85% of the overall variation modeled in Y and the second component accounted for 6.9%, with 88% of the variation predicted by the model. The VIP plot served as an effective means of ranking the variables (metabolites) for biomarker identification (Fig. 2A,B). Using VIP analysis for profiles from preprandial samples, we identified creatine, cystathionine, 3-hydroxykynurenine, and kynurenic acid as the most important terms in the model (i.e., significant discriminating biomarkers of vitamin B-6 restriction). For profiles from postprandial samples, VIP analysis identified creatine, cystathionine, kynurenic acid, 3-hydroxykynurenine, and DMG as significant discriminating biomarkers.
FIGURE 1.
Score plots from multilevel partial least squares-discriminant analysis of the overall one-carbon metabolism and tryptophan catabolism data sets from analysis of plasma sampled before and after 28-d vitamin B-6 restriction. (A) Analysis of metabolite profiles of preprandial plasma. (B) Analysis of metabolite profiles of postprandial plasma. Each data point represents a function of the entire metabolite profile of a human participant (numerical designations provided); e.g., 1 and 1b represent data points for participant 1 in basal and vitamin B-6 restricted states. PC1, principal component 1; PC2, principal component 2; restr, restricted.
FIGURE 2.
VIP plots from multilevel partial least squares-discriminant analysis of the overall one-carbon metabolism and tryptophan catabolism data sets from analysis of plasma sampled before and after 28-d vitamin B-6 restriction. (A) Analysis of metabolite profiles of preprandial plasma. (B) Analysis of metabolite profiles of postprandial plasma. For each metabolite in the VIP analysis, the bar represents a weighted sum of squares with CI. A variable is considered to respond significantly if the VIP value and its 95% CI exceeds 1. AA, anthranilic acid; ADMA, asymmetric dimethylarginine; Arg, arginine; Bet, betaine; Chol, choline; Cre, creatine; Crn, creatinine; Cystath, cystathionine; DMG, dimethylglycine; GAA, Guanidinoacetic acid; Gly, glycine; HaA, 3-hydroxyanthranilic acid; HK, 3-hydroxykynurenine; KA, kynurenic acid; Kyn, kynurenine; Leu, Leucine; Met, methionine; Met-SO, methionine sulfoxide; MNAM, N1-methylnicotinamide; NA, nicotinic acid; NAM, nicotinamide; Qa, quinolinic acid; SDMA, symmetric dimethylarginine; Ser, serine; Thr, threonine; Trp, tryptophan; VIP, variable influence in the projection (63); XA, xanthurenic acid.
Discussion
Marginal vitamin B-6 deficiency has been associated with an increased risk of cardiovascular disease and certain cancers (67–69). The dietary protocol used in these studies serves as an effective tool in inducing short-term, marginal vitamin B-6 deficiency in healthy young adults (7–9, 12, 70). Whether the changes observed in studies of this type successfully predict the metabolic effects of chronic marginal vitamin B-6 deficiency remains to be determined. An important strength of these studies is their focus on the specific nutritional effects of controlled vitamin B-6 deficiency without inflammatory conditions, which often are associated with a low plasma PLP concentration (23, 25). The current work augments recent studies of metabolite profiling of vitamin B-6 status in population-based studies. The results of this study confirm and extend our previous conclusion (8) that short-term vitamin B-6 deficiency in healthy young adults is not, in itself, an inflammatory condition (Table 4). The extent to which dietary vitamin B-6 insufficiency contributes to inflammation in various population subgroups (23, 25, 71, 72) remains unclear.
Because PLP serves as a coenzyme for many aspects of metabolism, there are many possible functional impacts of vitamin B-6 deficiency. We recently published evidence from a metabolomic analysis of the same samples used in the current study suggesting wide-ranging effects of vitamin B-6 restriction, including perturbations of amino acid, lipid, and organic acid profiles (54). However, prediction of the effect of marginal deficiency on specific PLP-dependent enzymes on in vivo fluxes and metabolite concentrations has not been successful. For example, marginal deficiency of vitamin B-6 yields little or no change of in vivo SHMT flux (7, 9) and only subtle changes in serine and glycine concentrations (7, 9, 54), as supported by mathematical modeling (11) and observations of this study (Tables 2 and 4). The trans-sulfuration pathway of homocysteine catabolism, comprised of the PLP-dependent enzymes CBS and CSE, exhibits differential effects of PLP insufficiency. In this case, CBS tends to maintain its activity and in vivo flux (8, 73), whereas CSE is sensitive to loss of activity with an increased proportion of apo-CSE and loss of total CSE activity. However, CSE in vivo flux is maintained by the great increase in cystathionine, which, relative to the high Km of this enzyme, allows net in vivo flux of the trans-sulfuration pathway to be maintained (8, 12, 73). Consequently, as shown here and previously, elevated cystathionine is a sensitive biomarker for marginal vitamin B-6 deficiency, whereas cysteine flux (8, 11) and cysteine concentration are depressed only in more severe deficiency (8, 11, 12, 21, 73, 74).
Several additional findings of interest arose from our metabolite profile analysis. Plasma methionine sulfoxide concentrations and the methionine sulfoxide:methionine ratio (Tables 2 and 5) were not affected by vitamin B-6 restriction, nor were plasma glutathione concentrations (10). Methionine sulfoxide may be formed from oxidation of methionine by reactive oxygen species and thus may serve as an indicator of oxidative stress damage (75). Therefore, the marginal vitamin B-6 deficiency induced in our human participants did not appear to increase oxidative stress. The ADMA:arginine ratio is another unexpected example. Because ADMA arises from the turnover of methylated proteins, the formation of ADMA is not directly related to PLP-dependent processes. However, vitamin B-6 restriction was associated with a reduction in the ADMA:arginine ratio. A reduction in the ADMA:arginine ratio would be expected to improve endothelial function, because ADMA is a competitive inhibitor of endothelial NO synthase-derived NO production (76). The reduction in the ADMA:arginine ratio appears to be in contrast with the association of marginal vitamin B-6 deficiency with a variety of forms of vascular disease (3–6). Perhaps the most unexpected change in the product:precursor ratios was creatine:guinidinoacetate, which reflects the S-adenosylmethionine–dependent guanidinoacetate methyltransferase reaction. Because guanidinoacetate methyltransferase is not PLP dependent and neither guanidinoacetate production nor creatine catabolism involves PLP-dependent steps in metabolic proximity, the mechanism responsible for the association of vitamin B-6 restriction with a lower creatine:guanidinoacetate ratio is unclear. In fasted participants, creatine is the major product of the methylation cycle (77), and the methylation demand of creatine synthesis is second only to PC synthesis in fed participants. Therefore, decreasing creatine synthesis may spare 1C-groups (or more specifically methyl-groups) in the face of vitamin B-6 restriction. Severe vitamin B-6 deficiency (78) in rats yields a reduction in hepatic SAM concentration and decrease in the SAM:SAH ratio. However, such an effect appears unlikely in this study in view of the lack of effect on plasma methionine, homocysteine, or N1-methylnicotinamide (which is formed by methylation of nicotinamide by SAM) concentrations. It is possible that differences in the creatine content of the participants’ prestudy diet and the controlled vitamin B-6 restriction diet could contribute to the observed results. However, we think that observations demonstrate a physiologically real phenomenon in light of our findings of a similar reduction in creatine in HepG2 cells cultured in low vitamin B-6 conditions (V.R. da Silva and J.F. Gregory III, unpublished results).
Our metabolite profiles demonstrate that the tryptophan catabolic pathway is perturbed during marginal vitamin B-6 deficiency. These results support our mathematical modeling (51) and animal studies (79) as well as a recent large-scale study of low vitamin B-6 status in cardiovascular patients with systemic inflammation (52). Our results of this study and the previous findings of Midttun et al. (52), showing increased 3-hydroxykynurenine and decreased kynurenic acid as well as an increased 3-hydroxykynurenine:kynurenic acid ratio, strongly suggest that kynureninase is more susceptible than kynurenine aminotransferase to PLP insufficiency, as previously proposed (47). The mechanisms and metabolic consequences of the association of systemic inflammation, low plasma PLP, and altered tryptophan catabolism, as recently reviewed (23), require further study.
Our multivariate analysis of the pooled metabolites associated with one-carbon metabolism and tryptophan catabolism has provided important leads in the development of better functional diagnostic criteria for marginal vitamin B-6 deficiency. In view of well-documented associations of marginal vitamin B-6 deficiency with disease risk, we propose that biomarkers such as cystathionine, 3-hydroxykynurenine, kynurenic acid, and creatine, especially in combination, may provide useful new assessment tools.
Supplementary Material
Acknowledgments
J.F.G. and P.W.S. designed the research; V.R.d.S., L.R.-A., Y.L., M.A.R., E.P.Q., T.J.G., B.C., M.N.S., S.S.P., Ø.M., and P.M.U. conducted the research; Y.-Y.C., K.E.M., and J.F.G. analyzed the data; and V.R.d.S. and J.F.G. wrote the paper. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ADMA, asymmetric dimethylarginine; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; DMG, dimethylglycine; IDO, indoleamine 2,3-dioxygenase; MS/MS, tandem MS; PLP, pyridoxal 5'-phosphate; SAM, S-adenosylmethionine; SHMT, serine hydroxymethyltransferase; TDO, tryptophan 2,3- dioxygenase; VIP, Variable Influence on Projection.
Literature Cited
- 1.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5'-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87:1446–54. [DOI] [PubMed] [Google Scholar]
- 2.CDC. Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population. Atlanta: Department of Health and Human Services; 2012.
- 3.Cheng CH, Lin PT, Liaw YP, Ho CC, Tsai TP, Chou MC, Huang YC. Plasma pyridoxal 5 '-phosphate and high-sensitivity C-reactive protein are independently associated with an increased risk of coronary artery disease. Nutrition. 2008;24:239–44. [DOI] [PubMed] [Google Scholar]
- 4.Page JH, Ma J, Chiuve SE, Stampfer MJ, Selhub J, Manson JE, Rimm EB. Plasma vitamin B-6 and risk of myocardial infarction in women. Circulation. 2009;120:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, Hennekens C, Stampfer MJ. Folate and vitamin B-6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA. 1998;279:359–64. [DOI] [PubMed] [Google Scholar]
- 6.Robinson K, Arheart K, Refsum H, Brattstrom L, Boers G, Ueland PM, Rubba P, Palma-Reis R, Meleady R, Daly L, et al. Low circulating folate and vitamin B-6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. Circulation. 1998;97:437–43. [DOI] [PubMed] [Google Scholar]
- 7.Davis SR, Scheer JB, Quinlivan EP, Coats BS, Stacpoole PW, Gregory JF III. Dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am J Clin Nutr. 2005;81:648–55. [DOI] [PubMed] [Google Scholar]
- 8.Davis SR, Quinlivan EP, Stacpoole PW, Gregory JF III. Plasma glutathione and cystathionine concentrations are elevated but cysteine flux is unchanged by dietary vitamin B-6 restriction in young men and women. J Nutr. 2006;136:373–8. [DOI] [PubMed] [Google Scholar]
- 9.Lamers Y, Williamson J, Ralat M, Quinlivan EP, Gilbert LR, Keeling C, Stevens RD, Newgard CB, Ueland PM, Meyer K, et al. Moderate dietary vitamin B-6 restriction raises plasma glycine and cystathionine concentrations while minimally affecting the rates of glycine turnover and glycine cleavage in healthy men and women. J Nutr. 2009;139:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamers Y, O'Rourke B, Gilbert LR, Keeling C, Matthews DE, Stacpoole PW, Gregory JF III. Vitamin B-6 restriction tends to reduce the red blood cell glutathione synthesis rate without affecting red blood cell or plasma glutathione concentrations in healthy men and women. Am J Clin Nutr. 2009;90:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nijhout HF, Gregory J, Fitzpatrick C, Cho E, Lamers K, Ulrich C, Reed M. A mathematical model gives insights into the effects of vitamin B-6 deficiency on 1-carbon and glutathione metabolism. J Nutr. 2009;139:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamers Y, Coats B, Ralat M, Quinlivan EP, Stacpoole PW, Gregory JF III. Moderate vitamin B-6 restriction does not alter postprandial methionine cycle rates of remethylation, transmethylation, and total transsulfuration but increases the fractional synthesis rate of cystathionine in healthy young men and women. J Nutr. 2011;141:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregory JF III, Park Y, Lamers Y, Bandyopadhyay N, Chi YY, Lee K, Kim S, da Silva V, Hove N, Ranka S, et al. Metabolomic analysis reveals extended metabolic consequences of marginal vitamin B-6 deficiency in healthy human subjects. PLoS ONE. 2013;8:e63544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada M, Suzuki K. Amino acid metabolism in rats fed a high protein diet without pyridoxine. J Nutr. 1974;104:287–93. [DOI] [PubMed] [Google Scholar]
- 15.Park YK. Linkswil H. Effect of vitamin-B6 depletion in adult man on plasma concentration and urinary excretion of free amino acids. J Nutr. 1971;101:185–91. [DOI] [PubMed] [Google Scholar]
- 16.Swendseid ME, Villalobos J, Friedrich B. Free amino acids in plasma and tissues of rats fed a vitamin B6-deficient diet. J Nutr. 1964;82:206–8. [DOI] [PubMed] [Google Scholar]
- 17.Scheer JB, Mackey AD, Gregory JF. Activities of hepatic cytosolic and mitochondrial forms of serine hydroxymethyltransferase and hepatic glycine concentration are affected by vitamin B-6 intake in rats. J Nutr. 2005;135:233–8. [DOI] [PubMed] [Google Scholar]
- 18.Runyan TJ, Gershoff SN. Glycine metabolism in vitamin B6-deficient and deoxypyridoxine-treated rats. J Nutr. 1969;98:113–8. [DOI] [PubMed] [Google Scholar]
- 19.Leklem JE. Vitamin B-6: a status report. J Nutr. 1990;120 Suppl 11:1503–7. [DOI] [PubMed] [Google Scholar]
- 20.Gregory J. Vitamin B6 deficiency. In: Carmel R, Jacobsen D, editors. Homocysteine in health and disease. Cambridge (UK): Cambridge University Press; 2001. p. 307–20.
- 21.Midttun Ø, Hustad S, Schneede J, Vollset SE, Ueland PM. Plasma vitamin B-6 forms and their relation to transsulfuration metabolites in a large, population-based study. Am J Clin Nutr. 2007;86:131–8. [DOI] [PubMed] [Google Scholar]
- 22.Wolf H. Studies on tryptophan metabolism in man: the effects of hormones and vitamin B6 on urinary excretion of metabolites of the kynurenine pathway. Scand J Clin Lab Invest Suppl. 1974;33:11–87. [PubMed] [Google Scholar]
- 23.Paul L, Ueland PM, Selhub J. Mechanistic perspective on the relationship between pyridoxal 5′-phosphate and inflammation. Nutr Rev. 2013;71:239–44. [DOI] [PubMed] [Google Scholar]
- 24.Oxenkrug GF. Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: implications for aging and aging-associated psychiatric and medical disorders. J Neural Transm. 2011;118:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakakeeny L, Roubenoff R, Obin M, Fontes JD, Benjamin EJ, Bujanover Y, Jacques PF, Selhub J. Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. adults. J Nutr. 2012;142:1280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, Nakamura Y, Inui N, Nakamura H, Chida K. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer. 2010;67:361–5. [DOI] [PubMed] [Google Scholar]
- 27.Sprince H, Lowy RS, Folsome CE, Behrman JS. Studies on the urinary excretion of "xanthurenic acid" during normal and abnormal pregnancy: a survey of the excretion of "xanthurenic acid" in normal nonpregnant, normal pregnant, pre-eclamptic, and eclamptic women. Am J Obstet Gynecol. 1951;62:84–92. [DOI] [PubMed] [Google Scholar]
- 28.Rose DP, Braidman IP. Excretion of tryptophan metabolites as affected by pregnancy, contraceptive steroids, and steroid hormones. Am J Clin Nutr. 1971;24:673–83. [DOI] [PubMed] [Google Scholar]
- 29.Rose DP. Excretion of xanthurenic acid in the urine of women taking progestogen-oestrogen preparations. Nature. 1966;210:196–7. [DOI] [PubMed] [Google Scholar]
- 30.Rose DP, Adams PW. Oral contraceptives and tryptophan metabolism: effects of oestrogen in low dose combined with a progestagen and of a low-dose progestagen (megestrol acetate) given alone. J Clin Pathol. 1972;25:252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bender DA, Tagoe CE, Vale JA. Effects of oestrogen administration on vitamin B6 and tryptophan metabolism in the rat. Br J Nutr. 1982;47:609–14. [DOI] [PubMed] [Google Scholar]
- 32.Price SA, Rose DP, Toseland PA. Effects of dietary vitamin B 6 deficiency and oral contraceptives on the spontaneous urinary excretion of 3-hydroxyanthranilic acid. Am J Clin Nutr. 1972;25:494–8. [DOI] [PubMed] [Google Scholar]
- 33.Yess N, Price JM, Brown RR, Swan PB, Linkswiler H. Vitamin B6 depletion in man: urinary excretion of tryptophan metabolites. J Nutr. 1964;84:229–36. [DOI] [PubMed] [Google Scholar]
- 34.Brown RR, Yess N, Price JM, Linkswiler H, Swan P, Hankes LV. Vitamin B6 depletion in man: urinary excretion of quinolinic acid and niacin metabolites. J Nutr. 1965;87:419–23. [DOI] [PubMed] [Google Scholar]
- 35.Yeh JK, Brown RR. Effects of vitamin B-6 deficiency and tryptophan loading on urinary excretion of tryptophan metabolites in mammals. J Nutr. 1977;107:261–71. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T, Knox WE. The nature and mechanism of the tryptophan pyrrolase (peroxidase-oxidase) reaction of pseudomonas and of rat liver. J Biol Chem. 1959;234:1162–70. [PubMed] [Google Scholar]
- 37.Knox WE, Mehler AH. The adaptive increase of the tryptophan peroxidase-oxidase system of liver. Science. 1951;113:237–8. [DOI] [PubMed] [Google Scholar]
- 38.Schutz G, Killewich L, Chen G, Feigelson P. Control of the mRNA for hepatic tryptophan oxygenase during hormonal and substrate induction. Proc Natl Acad Sci USA. 1975;72:1017–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altman K, Greengard O. Correlation of kynurenine excretion with liver tryptophan pyrrolase levels in disease and after hydrocortisone induction. J Clin Invest. 1966;45:1527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knox WE, Auerbach VH. The hormonal control of tryptophan peroxidase in the rat. J Biol Chem. 1955;214:307–13. [PubMed] [Google Scholar]
- 41.Wolf H, Brown RR. Studies on tryptophan metabolism in male subjects treated with hydrocortisone. J Clin Endocrinol Metab. 1971;33:838–43. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura T, Shinno H, Ichihara A. Insulin and glucagon as a new regulator system for tryptophan oxygenase activity demonstrated in primary cultured rat hepatocytes. J Biol Chem. 1980;255:7533–5. [PubMed] [Google Scholar]
- 43.Braidman IP, Rose DP. Effects of sex hormones on three glucocorticoid-inducible enzymes concerned with amino acid metabolism in rat liver. Endocrinology. 1971;89:1250–5. [DOI] [PubMed] [Google Scholar]
- 44.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–22. [PubMed] [Google Scholar]
- 45.Shimizu T, Nomiyama S, Hirata F, Hayaishi O. Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem. 1978;253:4700–6. [PubMed] [Google Scholar]
- 46.Dalgliesh CE, Knox WE, Neuberger A. Intermediary metabolism of tryptophan. Nature. 1951;168:20–2. [DOI] [PubMed] [Google Scholar]
- 47.Ogasawara N, Hagino Y, Kotake Y. Kynurenine-transaminase, kynureninase and the increase of xanthurenic acid excretion. J Biochem. 1962;52:162–6. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi F, Shibata Y. Kynurenine metabolism in vitamin-B-6-deficient rat liver after tryptophan injection. Biochem J. 1984;220:693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiss O, Fuchs H. [Splitting of kynurenine, oxykynurenine an related substances by the rat liver enzyme]. Experientia. 1950;6:472–3. [DOI] [PubMed] [Google Scholar]
- 50.Nakatani M, Morimoto M, Noguchi T, Kido R. Subcellular distribution and properties of kynurenine transaminase in rat liver. Biochem J. 1974;143:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rios-Avila L, Nijhout HF, Reed MC, Sitren HS, Gregory JF. A mathematical model of tryptophan metabolism via the kynurenine pathway provides insights into the effects of vitamin B6 deficiency, tryptophan loading and induction of tryptophan 2,3-dioxygenase on tryptophan metabolites. J Nutr. 2013:Jul 31 (Epub ahead of print; DOI:10.3945/jn.113.174599). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Midttun O, Ulvik A, Ringdal Pedersen E, Ebbing M, Bleie O, Schartum-Hansen H, Nilsen RM, Nygard O, Ueland PM. Low plasma vitamin B-6 status affects metabolism through the kynurenine pathway in cardiovascular patients with systemic inflammation. J Nutr. 2011;141:611–7. [DOI] [PubMed] [Google Scholar]
- 53.Theofylaktopoulou D, Midttun O, Ulvik A, Ueland PM, Tell GS, Vollset SE, Nygard O, Eussen SJ. A community based study on determinants of circulating markers of cellular immune activation and kynurenines: the Hordaland Health Study. Clin Exp Immunol. 2013;173:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregory JF III, Park Y, Lamers Y, Bandyopadhyay N, Chi Y-Y, Lee K, Kim S, da Silva V, Hove N, et al. Metabolomic analysis reveals extended metabolic consequences of marginal vitamin B-6 deficiency in healthy human subjects. PLoS ONE. 2013;8:e63544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ubbink JB, Serfontein WJ, Devilliers LS. Stability of pyridoxal-5-phosphate semicarbazone: applications in plasma vitamin-B6 analysis and population surveys of vitamin-B6 nutritional-status. J Chromatogr. 1985;342:277–84. [DOI] [PubMed] [Google Scholar]
- 56.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45:290–2. [PubMed] [Google Scholar]
- 57.Ueland PM, Midttun O, Windelberg A, Svardal A, Skalevik R, Hustad S. Quantitative profiling of folate and one-carbon metabolism in large-scale epidemiological studies by mass spectrometry. Clin Chem Lab Med. 2007;45:1737–45. [DOI] [PubMed] [Google Scholar]
- 58.Midttun Ø, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid communications in mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:1371–9. [DOI] [PubMed] [Google Scholar]
- 59.Chi YY, Gribbin M, Lamers Y, Gregory JF, Muller KE. Global hypothesis testing for high-dimensional repeated measures outcomes. Stat Med. 2012;31:724–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Storey JD. A direct approach to false discovery rates. J R Stat Soc: Series B Stat Methodol. 2012;64:479–98. [Google Scholar]
- 61.Arani RB, Chen JJ. A power study of a sequential method of P-value adjustment for correlated continuous endpoints. J Biopharm Stat. 1998;8:585–98. [DOI] [PubMed] [Google Scholar]
- 62.Westerhuis JA, van Velzen EJ, Hoefsloot HC, Smilde AK. Multivariate paired data analysis: multilevel PLSDA versus OPLSDA. Metabolomics. 2010;6:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wold S, Sjostrom M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab. 2001;58:109–30.
- 64.Umetrics M. User guide to SIMCA. Malmö (Sweden): MKS Umetrics AB; 2013.
- 65.Efron B, Gong G. A leisurely look at the bootstrap, the jackknife, and cross-validation. Am Stat. 1983;37:36–48. [Google Scholar]
- 66.Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, Edlund U, Shockcor JP, Gottfries J, Moritz T, Trygg J. Visualization of GC/TOF-MS-based metabolomics data for identification of. Anal Chem. 2008;80:115–22. [DOI] [PubMed] [Google Scholar]
- 67.Larsson SC, Orsini N, Wolk A. Vitamin B-6 and risk of colorectal cancer a meta-analysis of prospective studies. JAMA. 2010;303:1077–83. [DOI] [PubMed] [Google Scholar]
- 68.Lotto V, Choi SW, Friso S. Vitamin B6: a challenging link between nutrition and inflammation in CVD. Br J Nutr. 2011;106:183–95. [DOI] [PubMed] [Google Scholar]
- 69.Eussen SJ, Vollset SE, Hustad S, Midttun O, Meyer K, Fredriksen A, Ueland PM, Jenab M, Slimani N, Boffetta P, et al. Plasma vitamins B2, B6, and B12, and related genetic variants as predictors of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2010;19:2549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cuskelly GJ, Stacpoole PW, Williamson J, Baumgartner TG, Gregory JF III. Deficiencies of folate and vitamin B(6) exert distinct effects on homocysteine, serine, and methionine kinetics. Am J Physiol Endocrinol Metab. 2001;281:E1182–90. [DOI] [PubMed] [Google Scholar]
- 71.Morris MS, Sakakeeny L, Jacques PF, Picciano MF, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr. 2010;140:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ulvik A, Midttun O, Pedersen ER, Nygard O, Ueland PM. Association of plasma B-6 vitamers with systemic markers of inflammation before and after pyridoxine treatment in patients with stable angina pectoris. Am J Clin Nutr. 2012;95:1072–8. [DOI] [PubMed] [Google Scholar]
- 73.Lima CP, Davis SR, Mackey AD, Scheer JB, Williamson J, Gregory JF III. Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J Nutr. 2006;136:2141–7. [DOI] [PubMed] [Google Scholar]
- 74.Ubbink JB, van der Merwe A, Delport R, Allen RH, Stabler SP, Riezler R, Vermaak WJ. The effect of a subnormal vitamin B-6 status on homocysteine metabolism. J Clin Invest. 1996;98:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moskovitz J, Berlett BS, Poston JM, Stadtman ER. Methionine sulfoxide reductase in antioxidant defense. Methods Enzymol. 1999;300:239–44. [DOI] [PubMed] [Google Scholar]
- 76.Böger RH, Bode-Böger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction. Circulation 1998;98:1842–7. [DOI] [PubMed] [Google Scholar]
- 77.Brosnan JT, da Silva RP, Brosnan ME. The metabolic burden of creatine synthesis. Amino Acids. 2011;40:1325–31. [DOI] [PubMed] [Google Scholar]
- 78.Martinez M, Cuskelly GJ, Williamson J, Toth JP, Gregory JF. Vitamin B-6 deficiency in rats reduces hepatic serine hydroxymethyltransferase and cystathionine beta-synthase activities and rates of in vivo protein turnover, homocysteine remethylation and transsulfuration. J Nutr. 2000;130:1115–23. [DOI] [PubMed] [Google Scholar]
- 79.van de Kamp JL, Smolen A. Response of kynurenine pathway enzymes to pregnancy and dietary level of vitamin B-6. Pharmacol Biochem Behav. 1995;51:753–8. [DOI] [PubMed] [Google Scholar]
- 80.Zhao M, Lamers Y, Ralat MA, Coats BS, Chi YY, Muller KE, Bain JR, Shankar MN, Newgard CB, Stacpoole P, et al. Marginal vitamin B-6 deficiency decreases plasma (n-3) and (n-6) PUFA concentrations in healthy men and women. J Nutr. 2012;142:1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.