Abstract
Evidence that depressive symptoms are inversely related to n–3 (ω-3) fatty acids is growing among United States adults. We assessed whether self-reported depressive symptoms were inversely associated with n–3 fatty acid intakes by using a cross-sectional study in 1746 adults (aged 30–65 y) in Baltimore City, MD (2004–2009). The 20-item Center for Epidemiologic Studies–Depression Scale (CES-D) was used, with a CES-D score ≥16 suggestive of elevated depressive symptoms (EDS). By using the mean of two 24-h dietary recalls, n–3 highly unsaturated fatty acids (HUFAs; ≥20 carbons), n–3 polyunsaturated fatty acids (PUFAs; ≥18 carbons), and plausible ratios with n–6 (ω6) fatty acids were estimated. EDS prevalence was 18.1% among men and 25.6% among women. In women, the uppermost tertile (tertile 3) of n–3 PUFAs (compared with tertile 1) was associated with reduced odds of EDS by 49%, with a substantial sex differential. The n–3 PUFA:n–6:PUFA ratio was inversely related to EDS among women (tertile 2 vs. tertile 1, OR: 0.74; 95% CI: 0.41, 1.32; tertile 3 vs. tertile 1, OR: 0.47; 95% CI: 0.27, 0.83). A similar pattern was noted for n–3 HUFA:n–6 HUFA among women. For CES-D subscales, n–3 PUFA (% of energy) was inversely related to somatic complaints, whereas positive affect was directly related to n–3 HUFA (% of energy; total population and among women), n–3 HUFA:n–6 HUFA (women), and n–3 HUFA:n–6 PUFA (total population and among women). In sum, among United States women, higher intakes of n–3 fatty acids [absolute (n–3) and relative to n–6 fatty acids (n–3:n–6)] were associated with lower risk of elevated depressive symptoms, specifically in domains of somatic complaints (mainly n–3 PUFAs) and positive affect (mainly n–3 HUFAs).
Introduction
The role of the ω-3 (n–3) fatty acids as structural membrane lipids in nerve tissue and the retina and as precursors to eicosanoids is well established. Importantly, there is evidence for a relation between n–3 fatty acids and depressive symptoms (1–20). For instance, in ecological studies there is a strong inverse correlation between the prevalence of depression and fish consumption across populations (16). Additionally, both observational and experimental research findings at the individual level, and consistently across study designs, study groups, and contextual settings, suggest that lower intake of n–3 fatty acids (reflected in self-reported intakes and/or in plasma or erythrocyte concentrations) was associated with higher risk of depression or elevated depressive symptoms (EDS)9. For instance, out of 5 cross-sectional studies that were cited in 1 review article (17), 3 found increased depression was reported among “less frequent” compared with “frequent” fish consumers, after adjustment for potential confounders (6, 10, 18). Meta-analyses of randomized controlled trials that controlled for inclusion of participants with severe depressive symptoms and/or n–3 fatty acid composition of the interventions have reported substantial clinical efficacy for reducing depressive symptoms (19, 20).
It is commonly suggested that the prevalence of depression has reached epidemic proportions in recent decades. Whereas external factors may contribute to this increase in prevalence, such as changes in attribution, definition, destigmatization, and help seeking behaviors, Klerman and Weissman (21) ruled out many of those artifactual influences and concluded the presence of a genuine secular increasing trend. For a true increase in any disorder to occur, the risk factors may be genetic or socioenvironmental in nature. Among the latter, the influence of dietary factors and their over-time patterns of change, particularly n–3 fatty acid intake, has gained attention over the past decade.
Generally, n–3 fatty acids are long-chain PUFAs from both plant and marine origins (22). The n–3 fatty acids derived from marine sources consist primarily of EPA (20:5n–3) and DHA (22:6n–3). In contrast, plant-derived n–3 fatty acids (from flaxseed, walnuts, and canola oil) are contributors of α-linolenic acid (ALA; 18:3n–3), the essential n–3 fatty acid that can be converted to EPA and DHA endogenously. The metabolic pathways involved are relatively inefficient among humans (10–15% being converted) (23), and ALA conversion is impaired by excess linoleic acid (LA; 18:2n–6), an n–6 fatty acid (24).
During the last century, per capita consumption of soybean oil, rich in LA, increased 1000-fold (25). This increased the availability of LA from 2.8% to 7.2% of energy, whereas the availability of ALA increased only from 0.39% to 0.72% of energy and was accompanied by declines in n–3 HUFA intakes. The predicted net effects included declines in tissue n–3 HUFA status from 36.8% to 22.9%, primarily due to greater intakes of LA over the century. This is in sharp contrast to the diets in regions of hominid evolution, which were higher in n–3 HUFAs (2.26–17.0 g/d) and lower in LA (range: 2.3–3.6% of energy) (26).
This imbalance is reflected in the concentration of several plasma and erythrocyte biomarkers (27, 28). Recent research suggests that the imbalance between those 2 classes of fatty acids, specifically deficient intakes of EPA and DHA and high concentrations of the highly unsaturated n–6 fatty acid arachidonic acid (AA; 20:4n–6) in tissues, may contribute to the rising burden of many chronic conditions, including cardiovascular disease and certain types of cancer (29), in addition to cognitive decline (30, 31). Thus, studying this imbalance may be equally important to studying absolute concentrations of dietary n–3 fatty acids when examining depressive symptoms as the outcome, and domains of depressive symptoms should also be targeted separately. Indeed, many of the previous studies had focused on fish consumption and absolute intakes of n–3 fatty acids in relation to total depressive symptom scores without incorporating balance with n–6 fatty acids or domains of depressive symptoms into the analysis [e.g., (2, 4, 7, 9, 11, 12, 32)]
The aim of the present study was to assess whether self-reported depressive symptoms (and related domains), measured by the Center for Epidemiologic Studies–Depression Scale (CES-D) in a sample of 1746 adults residing in Baltimore City, MD, were associated with intakes of n–3 fatty acids (measured with two 24-h recalls), both in absolute terms and relative to n–6 fatty acids.
Participants and Methods
Database and study population
The Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study, initiated in 2004, is an ongoing prospective cohort study that recruited a representative sample of African Americans and whites (30–64 y old) at baseline who were living in Baltimore, Maryland (33). To this end, an area probability sampling design of 13 neighborhoods (defined as groups of contiguous census tracts) was used. Phase 1 of this study consisted of screening, recruitment, and household interviews, whereas phase 2 included examinations in mobile medical research vehicles. All participants provided written informed consent after they were provided with a protocol booklet in layman’s terms and a video explaining all procedures performed in the study, including future recontacts. All materials were approved by the MedStar Institutional Review Board. Our study used cross-sectional data from the baseline HANDLS cohort.
Of 3720 participants in phase 1 (sample 1), 2178 (58.5%) completed their baseline phase 2 examination and had complete dietary data (sample 2). Similarly, 2178 participants had complete data on CES-D scores (sample 3). Our data are restricted to the subset of participants with 2 d of dietary recall and CES-D data (n = 1746; sample 4). The group of participants selected into sample 4 compared with the remaining HANDLS participants in sample 1 (weighted analysis) had a higher percentage of African American (i.e., 67.7% vs. 55.9%), although no substantial differences were found in age, sex, or income level distributions.
Outcome assessment: depressive symptoms
Baseline symptoms of depression were measured by using the CES-D, a 20-item self-report symptom rating scale that assesses affective and depressed mood (34). A score ≥16 on the CES-D indicated EDS, as was done in many studies [e.g., (35)], given that being at this cutoff or higher is highly predictive of clinical depression on the basis of Diagnostic and Statistical Manual of Mental Disorders, 4th edition, criteria (36). Four previously identified subscales of the CES-D shown to have an invariant factor structure between the NHANES I and pilot HANDLS data (37) were studied in separate analyses as continuous variables. These subscales were as follows: 1) somatic complaints, 2) depressive affect, 3) positive affect, and 4) interpersonal problems (37).
Exposure assessment: dietary n–3 fatty acid absolute and relative exposures
With the use of the USDA’s Automated Multiple Pass Method (AMPM), trained interviewers administered two 24-h dietary recalls. AMPM is a standardized 5-step process that was validated for protein, carbohydrate, fat, and energy intakes in both obese and nonobese individuals (38–40). Additional studies provided evidence that the AMPM accurately measures group energy intake in smaller samples (41, 42).
Food consumed was coded by using USDA Food and Nutrient Database for Dietary Studies, version 3 (43), to estimate nutrient intakes. In this study, the mean of the two 24-h recalls was considered after food equivalents and nutrient intakes were summed for each individual per recall day.
Total n–3 PUFAs [≥18 carbons; i.e., n–3 PUFAs = ALA+DHA+EPA+docosapentaenoic acid (DPA); 22:5n–3] and HUFAs (≥20 carbons; HUFAs = DHA+EPA+DPA) were also expressed as percentages of total energy intake and were considered as 2 main nutrient exposures of interest. Together, these were labeled as “absolute n–3 fatty acid exposure.” In the main analysis, tertiles of main absolute exposures were constructed to assess dose-response relationships to detect threshold values if applicable, consistent with a previous study using HANDLS (44). Moreover, another set of analyses was conducted with main exposures of interest being “relative n–3 fatty acid exposures.” These were namely the ratio of n–3 HUFAs to n–6 HUFAs (i.e., DHA+EPA+DPA:AA), the ratio of n–3 HUFAs to n–6 PUFAs (i.e., DHA+EPA+DPA:LA+AA), and the ratio of n–3 PUFAs to n–6 PUFAs (i.e., ALA+DHA+EPA+DPA:LA+AA). These ratios rather than the typically used ratio of n–6 to n–3 fatty acids were considered as exposures of interest in our analyses for 2 main reasons: 1) to avoid having a zero value for the denominator because n–3 fatty acids in our study population are more likely to be negligibly consumed compared with n–6 fatty acids, particularly among non–fish eaters across the two 24-h recalls; 2) to allow for interpreting results in the hypothesized direction of a protective effect of n–3 fatty acids against depressive symptoms, as well as a protective effect of a good balanced intake of n–3 and n–6 fatty acids.
The adequacy of intakes for each essential fatty acid was assessed by using the Adequate Intake (AI) reference values (45). Proposed recommendations based on benefits associated for DHA+EPA as a percentage of energy have been published: the cutoff points for DHA+EPA are 0.15% of energy for postpartum and bipolar depression prevention and 0.35% of energy for major depression prevention (46, 47).
Covariates
Sociodemographic and lifestyle characteristics.
Covariates related to sociodemographic and lifestyle factors included in our analyses were age, sex, race (white vs. African American), marital status (married vs. unmarried), completed years of education [less than high school (HS), HS, and more than HS], poverty-income ratio (PIR <125%), measured BMI (kg/m2), lifetime use of drugs (opiates, marijuana, or cocaine vs. no drug use), and smoking status (0 = “never or former smoker” and 1 = “current smoker”).
Potential dietary confounders.
Potential confounding by other nutrients (daily intakes) that were formerly linked with depressive symptoms was adjusted for in multiple regression models. These nutrients, expressed as per 1000 kcal of daily energy intake, included B-vitamins [vitamins B-6 and B-12 (mg/1000 kcal) and folate (μg/1000 kcal)] and several antioxidants, namely intakes of total carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein+zeaxanthin, lycopene; μg/1000 kcal), vitamin A (retinol equivalents/1000 kcal), vitamin C (mg/1000 kcal), and α-tocopherol (mg/1000 kcal) (48–63). To emulate a multivariate nutrient density model, total energy intake was also included as a potentially confounding variable (64).
Statistical methods
Stata release 11.0 (StataCorp) survey commands were used, accounting for sampling weights to obtain population estimates of means, proportions, and regression coefficients. Two-sided independent-samples t tests compared means across binary variables, whereas design-based F tests were conducted to examine relationships between categorical variables (65). First, the HANDLS participants’ study sample characteristics were described by sex and CES-D status (high vs. low) by using both independent-samples t tests and design-based F tests. Second, the adequacy of intake for key fatty acids and their combinations was examined and sex differences determined with design-based F tests. Third, Pearson correlation coefficients between pairs of fatty acids and their scatterplot were presented. Fourth, multiple logistic regression models with target outcome being EDS (CES-D ≥16) were conducted to test the association between n–3 fatty acid exposures and depressive symptoms, controlling for potential confounders. Individuals with missing data on covariates such as marital status, education, smoking, and drug use were accounted for in the analysis by adding a dummy variable for “missing.” Effect modification of these associations by sex was tested by adding interaction terms to the multivariate models. Finally, the multiple linear regression models were re-conducted with each CES-D subscale as alternative outcomes to test whether the association of depressive symptoms with n–3 fatty acid exposures was more specific to some symptoms than others. A type I error of 0.05 was used for significance in all analyses, except for interaction terms in multivariate models where a 0.10 concentration was used, given the low power of interaction terms compared with main effects (66).
Results
The weighted prevalences of a CES-D score ≥16 (or EDS) were 18.1% among men and 25.6% among women (P < 0.001 based on a design-based F test) (Table 1). HANDLS participants with a CES-D score ≥16 were generally more likely to have a PIR <125% compared with those with a CES-D score <16 for both sexes. Moreover, among women, those with a CES-D score ≥16 were less likely to be currently married (38% vs. 52%; P = 0.026). The examination of the 5 n–3 fatty acid exposures across depressive symptoms categories revealed an inverse relationship with EDS among women for n–3 PUFAs (% of energy) and n–3 PUFA:n–6 PUFA ratio. Moreover, among women, lower mean intakes of total carotenoids and vitamin B-6 were noted for those with a CES-D score ≥16 compared with those with a score <16 (P < 0.05).
TABLE 1.
Characteristics of HANDLS study participants by sex and CES-D score1
| Men |
Women |
P value2 |
|||||||
| CES-D <16 (n = 594) | CES-D ≥16 (n = 161) | All men (n = 755) | CES-D <16 (n = 678) | CES-D ≥16 (n = 313) | All women (n = 991) | Men vs. women | Low vs. high CES-D score among men | Low vs. high CES-D score among women | |
| Percentage | 81.8 | 18.1 | 45.5 | 74.4 | 25.6 | 54.5 | |||
| Depressive symptoms | <0.0013 | <0.0014 | <0.0014 | ||||||
| CES-D | 7.46 ± 0.28 | 20.77 ± 0.56 | 9.87 ± 0.38 | 7.19 ± 0.27 | 22.79 ± 0.73 | 11.19 ± 0.47 | |||
| Sociodemographic and lifestyle factors | |||||||||
| Age, y | 46.7 ± 0.6 | 46.2 ± 1.4 | 46.6 ± 0.5 | 46.2 ± 0.6 | 44.8 ± 0.9 | 45.9 ± 0.5 | 0.71 | 0.82 | 0.37 |
| African American, % | 67.2 | 71.8 | 68.0 | 68.5 | 64.4 | 67.5 | 0.88 | 0.46 | 0.54 |
| Marital status, % | 0.31 | 0.48 | 0.0264 | ||||||
| Married | 52.0 | 60.4 | 53.5 | 52.2 | 38.1 | 48.6 | |||
| Missing | 13.5 | 11.9 | 13.2 | 12.9 | 11.6 | 12.6 | |||
| Education, % | 0.49 | 0.12 | 0.08 | ||||||
| <HS | 4.6 | 5.8 | 4.8 | 3.1 | 7.3 | 4.2 | |||
| HS | 50.1 | 62.2 | 52.2 | 50.7 | 57.0 | 52.4 | |||
| >HS | 40.9 | 31.6 | 39.2 | 43.8 | 34.7 | 41.5 | |||
| Missing | 4.4 | 0.4 | 3.7 | 2.3 | 0.9 | 1.9 | |||
| PIR ≥125%, % | 84.5 | 71.9 | 82.2 | 79.7 | 66.9 | 76.5 | 0.0133 | 0.0044 | 0.0034 |
| Current smoking status, % | |||||||||
| Currently smoking | 46.9 | 58.9 | 49.1 | 31.7 | 44.5 | 34.5 | 0.0023 | 0.21 | 0.09 |
| Missing | 8.1 | 8.4 | 8.2 | 9.8 | 12.0 | 10.4 | |||
| Ever use of illicit drugs, % | |||||||||
| Used any type | 72.6 | 71.3 | 72.4 | 55.1 | 57.1 | 55.6 | <0.0013 | 0.92 | 0.90 |
| Missing | 6.0 | 5.6 | 5.9 | 8.7 | 7.3 | 8.3 | |||
| BMI, kg/m2 | 27.8 ± 0.4 | 27.2 ± 0.9 | 27.7 ± 0.3 | 30.5 ± 0.5 | 31.3 ± 1.3 | 30.7 ± 0.5 | <0.0013 | 0.98 | 0.67 |
| Fatty acid exposures5, % | |||||||||
| n–3 HUFA intake, % energy | 0.77 | 0.69 | 0.05 | ||||||
| T1: 0.0093 ± 0.0005 | 32.0 | 30.1 | 31.7 | 25.9 | 39.4 | 29.4 | |||
| T2: 0.0399 ± 0.0009 | 32.4 | 38.4 | 33.5 | 35.6 | 27.0 | 33.3 | |||
| T3: 0.2506 ± 0.0183 | 35.6 | 31.4 | 34.9 | 38.5 | 33.6 | 37.1 | |||
| n–3 PUFA intake, % energy | 0.43 | 0.68 | 0.0304 | ||||||
| T1: 0.4181 ± 0.0065 | 36.2 | 35.2 | 36.0 | 28.4 | 42.2 | 32.0 | |||
| T2: 0.6586 ± 0.0046 | 31.1 | 26.2 | 30.2 | 35.1 | 33.8 | 34.8 | |||
| T3: 1.1477 ± 0.0303 | 32.7 | 38.7 | 33.8 | 36.4 | 24.0 | 33.3 | |||
| n–3 HUFA:n–6 HUFA ratio | 0.06 | 0.86 | 0.07 | ||||||
| T1: 0.3217 ± 0.0130 | 35.0 | 34.6 | 34.9 | 23.3 | 33.9 | 26.0 | |||
| T2: 1.0377 ± 0.0174 | 34.3 | 31.0 | 33.7 | 37.8 | 39.3 | 38.2 | |||
| T3: 5.9810 ± 0.2558 | 30.7 | 34.3 | 31.4 | 38.7 | 26.9 | 35.7 | |||
| n–3 HUFA:n–6 PUFA ratio | 0.57 | 0.48 | 0.07 | ||||||
| T1: 0.0016 ± 0.0001 | 31.7 | 32.3 | 31.8 | 27.0 | 40.2 | 30.4 | |||
| T2: 0.0063 ± 0.0001 | 34.4 | 41.6 | 35.7 | 35.1 | 27.2 | 33.1 | |||
| T3: 0.0417 ± 0.0042 | 33.9 | 26.1 | 32.5 | 37.9 | 32.6 | 36.5 | |||
| n–3 PUFA:n–6 PUFA ratio | 0.73 | 0.66 | 0.0104 | ||||||
| T1: 0.0778 ± 0.0009 | 35.7 | 31.2 | 34.9 | 34.0 | 48.6 | 37.7 | |||
| T2: 0.1053 ± 0.0004 | 31.6 | 38.0 | 32.8 | 30.2 | 30.6 | 30.3 | |||
| T3: 0.1571 ± 0.0045 | 32.7 | 30.7 | 32.3 | 35.8 | 20.7 | 31.9 | |||
| Other dietary factors, daily intakes | |||||||||
| Energy, kcal | 2490 ± 68 | 2540 ± 214 | 2500 ± 68 | 1780 ± 62 | 1810 ± 83 | 1790 ± 51 | <0.0013 | 0.12 | 0.64 |
| Total carotenoids, mg/1000 kcal | 3.50 ± 2.16 | 3.43 ± 5.34 | 3.48 ± 2.01 | 4.91 ± 3.04 | 3.84 ± 3.84 | 4.64 ± 2.49 | 0.0193 | 0.15 | 0.0084 |
| Vitamin A, RE/1000 kcal | 302 ± 24 | 323 ± 31 | 305 ± 20 | 354 ± 19 | 321 ± 40 | 345 ± 18 | 0.08 | 0.83 | 0.41 |
| Vitamin C, mg/1000 kcal | 39.3 ± 2.3 | 42.7 ± 5.7 | 39.9 ± 2.1 | 46.1 ± 2.5 | 31.6 ± 2.7 | 42.4 ± 2.1 | 0.0293 | 0.84 | 0.07 |
| Vitamin E, mg/1000 kcal | 3.2 ± 0.1 | 3.0 ± 0.2 | 3.1 ± 0.1 | 3.9 ± 0.2 | 3.1 ± 0.2 | 3.7 ± 0.2 | <0.0013 | 0.20 | 0.06 |
| Vitamin B-6, mg/1000 kcal | 0.95 ± 0.03 | 0.95 ± 0.05 | 0.95 ± 0.03 | 0.95 ± 0.03 | 0.85 ± 0.03 | 0.93 ± 0.03 | 0.95 | 0.28 | 0.0194 |
| Vitamin B-12, μg/1000 kcal | 3.2 ± 0.3 | 3.1 ± 0.5 | 3.2 ± 0.2 | 2.9 ± 0.2 | 2.8 ± 0.4 | 2.9 ± 0.2 | 0.85 | 0.97 | 0.27 |
| Folate, μg/1000 kcal | 181 ± 6 | 184 ± 10 | 182 ± 5 | 198 ± 10 | 176 ± 7 | 192 ± 5 | 0.0163 | 0.77 | 0.30 |
Values are percentages or means ± SEMs. CES-D, Center for Epidemiologic Studies–Depression Scale; HANDLS, Healthy Aging in Neighborhoods of Diversity across the Life Span; HS, high school; HUFA, highly unsaturated fatty acid; PIR, poverty-income ratio; RE, retinol equivalents; T, tertile.
P values are based on 1-factor ANOVA for continuous variables and on design-based F test for categorical variables.
Different from corresponding men, P < 0.05.
Different from corresponding CES-D score <16, P < 0.05.
n–3 HUFAs included DHA + EPA + n–3 docosapentaenoic acid (DPA). n–6 HUFAs included arachidonic acid (AA). n–3 PUFAs included DHA + EPA + n–3 DPA + α-linolenic acid. n–6 PUFAs included AA + linoleic acid.
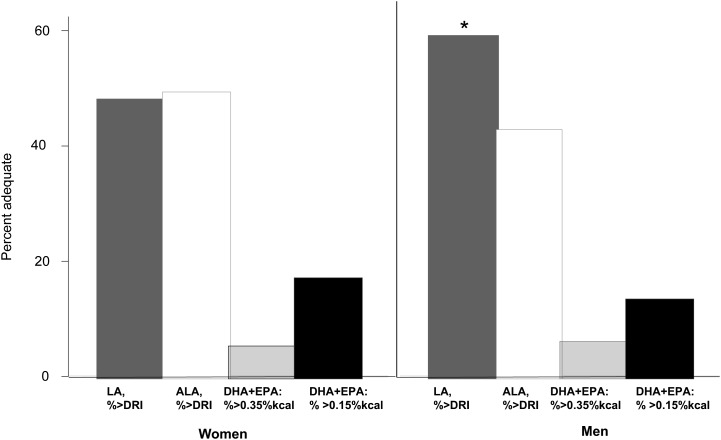
Figure 1 shows that LA and ALA intakes were adequate only in 42.7–59.3% (weighted percentages) of men and women in our sample, with a substantially higher proportion with AI observed for LA among men compared with women (59.3% vs. 48.3%). However, AI for EPA+DHA (% of energy as % kcal) for the prevention of major, postpartum, or bipolar depression (46, 47) ranged between 5.2% (percent > 0.35% kcal among women) and 17.2% (percent > 0.15% kcal among women) with no substantial sex differences.
FIGURE 1.
Adequate Intakes of LA, ALA, and DHA+EPA (% of energy) based on DRIs (n = 755 men and n = 991 women). Values are percentages of Adequate Intake and are as follows: among men—LA (59.3%), ALA (42.7%), DHA+EPA > 0.35% kcal (6.2%), DHA+EPA > 0.15% kcal (13.3%); among women—LA (48.3%), ALA (49.3%), DHA+EPA > 0.35% kcal (5.2%), DHA+EPA > 0.15% kcal (17.2%). *P < 0.05 based on a design-based F test for sex difference. Data from references 45–47. ALA, α-linolenic acid; DRI, Dietary Reference Intake; LA, linoleic acid.
Examining correlations between the various key fatty acids (Supplemental Fig. 1), DHA and EPA were highly correlated in the total diet (r = 0.93, P < 0.01), given their common dietary source of fish, and LA and ALA were similarly tightly linked (r = 0.87, P < 0.01), given that they are both found in various cooking oils. Other highly to moderately correlated pairs of fatty acids included EPA-DPA, DPA-DHA, and DPA-AA (r > 0.50, P < 0.05).
Associations between tertiles of n–3 fatty acid exposures and elevated depressive symptoms are presented in Table 2. Multiple logistic regression models adjusting for age, sex, race, marital status, education, PIR, smoking, drug use status, and the selected potentially confounding nutrients suggested that, among women, the uppermost tertile (tertile 3) of n–3 PUFAs (compared with tertile 1) was associated with reduced odds of a CES-D score ≥16 by 49%, with a substantial effect modification by sex. Similarly, the n–3 PUFA:n–6 PUFA ratio exhibited a clear inverse dose-response relationship with elevated depressive symptoms among women (tertile 2 vs. tertile 1, OR: 0.74; 95% CI: 0.41, 1.32; tertile 3 vs. tertile 1, OR: 0.47; 95% CI: 0.27, 0.83). A similar inverse association with a CES-D ≥16 was found for the n–3 HUFA:n–6 HUFA ratio among women (tertile 2 vs. tertile 1, OR: 0.75; 95% CI: 0.40, 1.40; tertile 3 vs. tertile 1, OR: 0.52; 95% CI: 0.27, 0.99), with a substantial sex difference. The 2 other n–3 HUFA exposures [n–3 HUFAs (% of energy) and n–3 HUFA:n–6 PUFA) were suggestive of an inverse association with a CES-D ≥16 among women, particularly when comparing tertile 2 with tertile 1, although without a substantial dose-response relationship.
TABLE 2.
Association between elevated depressive symptoms (CES-D ≥16) and n–3 fatty acid absolute and relative exposures: HANDLS study1
| n–3 Fatty acid absolute exposures (% of energy) |
n–3 Fatty acid relative exposures (ratio) |
||||
| n–3 HUFAs | n–3 PUFAs | n–3 HUFA:n–6 HUFA | n–3 HUFA:n–6 PUFA | n–3 PUFA:n–6 PUFA | |
| All (n = 1746) | |||||
| T2 vs. T1 | 0.72 (0.44, 1.17) | 0.70 (0.43, 1.16) | 0.81 (0.51, 1.31) | 0.72 (0.43, 1.19) | 0.91 (0.56, 1.48) |
| T3 vs. T1 | 0.78 (0.48, 1.27) | 0.79 (0.48, 1.29) | 0.79 (0.48, 1.30) | 0.69 (0.42, 1.13) | 0.66* (0.42, 1.04) |
| Men (n = 755) | |||||
| T2 vs. T1 | 1.16 (0.51, 2.66) | 0.86 (0.39, 1.88) | 0.85 (0.42, 1.72) | 1.11 (0.47, 2.63) | 1.37 (0.60, 3.12) |
| T3 vs. T1 | 0.99 (0.47, 2.07) | 1.40 (0.66, 2.97) | 1.40 (0.64, 3.02) | 0.78 (0.35, 1.73) | 1.24 (0.60, 2.56) |
| Women (n = 991) | |||||
| T2 vs. T1 | 0.51** (0.29, 0.90) | 0.60 (0.31, 1.14) | 0.75 (0.40, 1.40) | 0.52**b (0.30, 0.91) | 0.74 (0.41, 1.32) |
| T3 vs. T1 | 0.66 (0.34, 1.28) | 0.51**2 (0.27, 0.95) | 0.52**2 (0.27, 0.99) | 0.66 (0.35, 1.24) | 0.47***2 (0.27, 0.83) |
Values are adjusted ORs with 95% CIs based on multiple logistic regression models. All models adjusted for age, sex (where applicable), race/ethnicity, marital status, education, poverty-income ratio, smoking and drug use status, measured BMI (kg/m2), and selected nutrients (expressed per 1000 kcal) as well as total energy intake, namely B-vitamins (vitamins B-6 and B-12 and total folate), total carotenoids, and vitamins A, C, and E. Each micronutrient was divided by total energy intake and multiplied by 1000 to obtain per 1000 kcal values. For fatty acids, the percentage of energy value was obtained by multiplying by 900 to account for 9 kcal/g energy density of fat. See Table 1 for means ± SEMs within each tertile for fatty acid exposures. n–3 HUFAs included DHA + EPA + n–3 docosapentaenoic acid (DPA). n–6 HUFAs included arachidonic acid (AA). n–3 PUFAs included DHA + EPA + n–3 DPA + α-linolenic acid. n–6 PUFAs included AA + linoleic acid. *P < 0.10, **P < 0.05, ***P < 0.01 for null hypothesis that loge(OR) = 0 (Wald test). CES-D, Center for Epidemiologic Studies–Depression Scale; HANDLS, Healthy Aging in Neighborhoods of Diversity across the Life Span; HUFA, highly unsaturated fatty acid; T, tertile.
The association between elevated CES-D score and n–3 fatty acid exposure differed by sex on the basis of a separate model with a sex × exposure interaction term added in addition to the main effect of sex, P < 0.10.
Table 3 shows adjusted associations between the 5 n–3 fatty acid exposures and the 4 subscales of the CES-D. Multiple linear regression suggested that n–3 PUFAs (% of energy) were inversely related to somatic complaints, whereas the positive affect subscale of the CES-D was directly related to n–3 HUFAs (% of energy; both sexes and among women), n–3 HUFA:n–6 HUFA (women), and n–3 HUFA:n–6 PUFA (both sexes and among women).
TABLE 3.
Association between the 4 CES-D subscales and n–3 fatty acid absolute and relative exposures: HANDLS study1
| n–3 Fatty acid absolute exposures (% of energy) |
n–3 Fatty acid relative exposures (ratio) |
||||
| CES-D component2 | n–3 HUFAs | n–3 PUFAs | n–3 HUFA:n–6 HUFA | n–3 HUFA:n–6 PUFA | n–3 PUFA:n–6 PUFA |
| Component 1: somatic complaints | |||||
| All (n = 1745) | |||||
| T2 vs. T1 | −0.60 (0.43) | −0.17 (0.44) | −0.13 (0.42) | −0.58 (0.41) | +0.35 (0.43) |
| T3 vs. T1 | −0.67* (0.41) | −0.41 (0.42) | −0.63 (0.41) | −0.91** (0.42) | −0.21 (0.38) |
| Men (n = 755) | |||||
| T2 vs. T1 | −0.24 (0.63) | +0.48 (0.51) | −0.02 (0.97) | −0.43 (0.61) | +0.64 (0.55) |
| T3 vs. T1 | −0.55 (0.55) | +0.34 (0.61) | −0.08 (0.88) | −0.68 (0.55) | +0.76 (0.47) |
| Women (n = 990) | |||||
| T2 vs. T1 | −0.91 (0.56) | −0.73 (0.66) | −0.17 (0.60) | −0.79 (0.55) | +0.13 (0.60) |
| T3 vs. T1 | −0.84 (0.59) | −1.12**3 (0.57) | −1.13* (0.59) | −1.09* (0.60) | −1.00*3 (0.54) |
| Component 2: depressive affect | |||||
| All (n = 1742) | |||||
| T2 vs. T1 | −0.48 (0.41) | −0.28 (0.43) | −0.32 (0.39) | −0.58 (0.43) | −0.06 (0.43) |
| T3 vs. T1 | −0.38 (0.42) | −0.38 (0.41) | −0.20 (0.41) | −0.55 (0.45) | −0.45 (0.25) |
| Men (n = 753) | |||||
| T2 vs. T1 | +0.10 (0.62) | +0.05 (0.54) | −0.20 (0.49) | −0.25 (0.63) | −0.30 (0.54) |
| T3 vs. T1 | +0.12 (0.52) | +0.30 (0.53) | +0.65 (0.54) | −0.27 (0.59) | −0.07 (0.51) |
| Women (n = 989) | |||||
| T2 vs. T1 | −1.06 (0.55) | −0.61 (0.64) | −0.51 (0.58) | −0.84 (0.53) | +0.13 (0.59) |
| T3 vs. T1 | −0.92 (0.63) | −1.03*3 (0.60) | −1.10*3 (0.59) | −0.86 (0.62) | −0.72 (0.53) |
| Component 3: positive affect | |||||
| All (n = 1741) | |||||
| T2 vs. T1 | +0.14 (0.23) | +0.40*1 (0.22) | +0.13 (0.22) | +0.32 (0.24) | +0.28 (0.21) |
| T3 vs. T1 | +0.48** (0.22) | +0.47** (0.21) | +0.34 (0.23) | +0.52** (0.23) | +0.29 (0.21) |
| Men (n = 752) | |||||
| T2 vs. T1 | −0.22 (0.32) | +0.35 (0.31) | +0.11 (0.29) | +0.26 (0.32) | +0.24 (0.28) |
| T3 vs. T1 | +0.13 (0.27) | +0.37 (0.30) | +0.04 (0.32) | +0.26 (0.34) | +0.22 (0.29) |
| Women (n = 989) | |||||
| T2 vs. T1 | +0.46 (0.30) | +0.45 (0.30) | +0.14 (0.31) | +0.34 (0.29) | +0.30 (0.28) |
| T3 vs. T1 | +0.79**3 (0.30) | +0.54* (0.29) | +0.63** (0.31) | +0.70** (0.30) | +0.30 (0.28) |
| Component 4: interpersonal problems | |||||
| All (n = 1745) | |||||
| T2 vs. T1 | −0.06 (0.11) | −0.11 (0.12) | +0.00 (0.11) | −0.03 (0.11) | +0.05 (0.14) |
| T3 vs. T1 | +0.19 (0.13) | +0.13 (0.13) | +0.20 (0.13) | +0.20 (0.13) | +0.02 (0.12) |
| Men (n = 754) | |||||
| T2 vs. T1 | −0.24 (0.14) | +0.13 (0.15) | +0.17 (0.16) | −0.04 (0.16) | −0.18 (0.17) |
| T3 vs. T1 | +0.13 (0.16) | +0.24 (0.17) | +0.28 (0.16) | +0.14 (0.16) | +0.00 (0.18) |
| Women (n = 991) | |||||
| T2 vs. T1 | +0.07 (0.15) | −0.31 (0.18) | −0.15 (0.16) | −0.05 (0.15) | +0.23 (0.19) |
| T3 vs. T1 | +0.18 (0.18) | +0.01 (0.18) | +0.08 (0.19) | +0.21 (0.19) | +0.03 (0.15) |
Values are adjusted β regression coefficients (SEs) based on multiple ordinary least-squares regression models. All models adjusted for age, sex (where applicable), race/ethnicity, marital status, education, poverty-income ratio, smoking and drug use status, measured BMI (kg/m2), and selected nutrients (expressed per 1000 kcal) as well as total energy intake, namely B-vitamins (vitamins B-6 and B-12 and total folate), total carotenoids, and vitamins A, C, and E. Each micronutrient was divided by total energy intake and multiplied by 1000 to obtain per 1000 kcal values. For fatty acids, the percentage of energy value was obtained by multiplying by 900 to account for 9 kcal/g energy density of fat. See Table 1 for means ± SEMs within each tertile for fatty acid exposures. n–3 HUFAs included DHA + EPA + n–3 docosapentaenoic acid (DPA). n–6 HUFAs included arachidonic acid (AA). n–3 PUFAs included DHA + EPA + n–3 DPA + α-linolenic acid. n–6 PUFAs included AA + linoleic acid. *P < 0.10, **P < 0.05, ***P < 0.01 for null hypothesis that loge(OR) = 0 (Wald test). CES-D, Center for Epidemiologic Studies–Depression Scale; HANDLS, Healthy Aging in Neighborhoods of Diversity across the Life Span; HUFA, highly unsaturated fatty acid; T, tertile.
CES-D components were computed as the sum of scores of the following individual items: CES-D component 1, somatic complaints; CES-D component 2, depressive affect; CES-D component 3, positive affect; CES-D component 4, interpersonal problems.
The association between elevated CES-D score and n–3 fatty acid exposure differed by sex on the basis of a separate model with a sex × exposure interaction term added in addition to the main effect of sex, P < 0.10.
Discussion
This study is one of very few large population surveys and among the first conducted in United States adults to show a substantial association between n–3 fatty acids (absolute and relative to n–6 fatty acids) and self-reported depressive symptoms (and their components). Our findings generally supported our hypothesis that both n–3 HUFA and n–3 PUFA intakes as well as their related ratios are linked to lower amounts of depressive symptoms. The observed association was substantial and stronger only among women and remained substantial after controlling for many of the sociodemographic, lifestyle, and health-related potential confounders, including dietary intakes of specific nutrients.
One domain of the CES-D, namely positive affect, was more sensitive to n–3 HUFA exposures than others among women. In contrast, also among women, somatic complaints were mostly linked to n–3 PUFA intakes. It is worth noting that among HANDLS study participants, fish was the major contributor of DHA and EPA (which are highly correlated in the total diet; data not shown) and that DHA+EPA (% of energy) was adequate at the 0.15% cutoff only (46) in 17.2% of women and 13.3% of men.
Our findings are consistent with other studies, including case-control (1–5), cross-sectional (6–10, 18), and prospective cohort (11–15) studies, although a number of these studies measured fatty acid concentration in tissues, particularly plasma phospholipids and RBCs. Among studies examining dietary intakes, Tanskanen et al. (10) conducted a cross-sectional population-based survey in 3204 adults in Finland. Depressive symptoms were measured by using the Beck Depression Inventory, and fish consumption was measured by using an FFQ. After adjustment for confounders, the likelihood of having depressive symptoms was substantially higher among infrequent fish consumers than among frequent consumers and among participants who reported consuming vegetable oils. Among prospective studies, Sanchez-Villegas et al. (12) used an established cohort of 7902 participants to examine the relationship between long-chain n–3 fatty acid intake, fish consumption, and mental disorders. ORs (95% CI) of mental disorders for successive quintiles of energy-adjusted n–3 fatty acid intake were as follows: 1 (reference), 0.72 (0.52–0.99), 0.79 (0.58–1.08), 0.65 (0.47–0.90), and 1.04 (0.78–1.40). Participants with a moderate consumption of fish (third and fourth quintiles of consumption: median of 83.3 and 112 g/d, respectively) had a relative risk reduction >30%. Findings from the first NHANES Epidemiology Follow-up Study (NHEFS), which used 24-h dietary recall data and a CES-D cutoff of 22, were consistent with the findings reported here: greater intake of LA was associated with as much as a 2.34 increased risk of severe depressive symptoms (14). The results of the Nurses’ Health Study, which used food-frequency data, likewise support the hypothesis that higher ALA and lower LA intakes (but not long-chain n–3 from fish) are associated with decreased risk of first episodes of depression over 10 y (15).
In addition, our study found that the putative protective effect of n–3 fatty acids on depressive outcomes was restricted to women. This could either be due to differences in sample sizes between men and women in our study (n = 755 men and n = 991 women) and others with differential overall and exposure-level specific prevalence of EDS or a real difference by which this class of fatty acids may have a sex-specific influence on depressive symptoms and mental health in general. Using an approach similar to that in a previous study (49), a power analysis indicated that, in fact, sample sizes were adequate for both men and women in these analyses. Details are provided in the Power Calculation (Supplemental Text 1). Given that the difference in the association between sexes was substantial (based on testing for interaction between sex and fatty acid exposures), the alternative hypothesis of a possible sex-specific biological influence may be plausible. In general, earlier observational studies did not specifically examine effect modification by sex in the relationship between n–3 fatty acids and depression or other mood-related outcomes, whereas some restricted their sample to 1 sex, usually women. Thus, we were not able to compare our findings of sex differential in this association with previous studies, particularly those with a cross-sectional design.
Several mechanisms have been proposed to explain the association between n–3 fatty acid status and depression, and these have been discussed in detail elsewhere (17, 67). Briefly, one of the main pathways involves the serotonergic neurotransmitter system (3, 68), which was shown to be regulated by n–3 fatty acids in both human (16) and animal studies (69). Another pathway, the “macrophage theory of depression,” relates to the activation of an inflammatory response to clinical depression by overproduction of cytokines and eicosanoids (70–73). Whereas n–6 fatty acids are known to promote the formation of proinflammatory eicosanoids such as prostaglandin E2 (3), n–3 fatty acids from fish oil were shown to have the opposite effect (74). In fact, proinflammatory eicosanoids were shown to regulate the actions of cytokines and α-interferon (29), which, in turn, if administered to humans were shown to cause psychiatric disturbances and symptoms emulating major depression (75, 76). Finally, a third mechanism termed “cAMP signal transduction hypothesis” involves impaired phospholipid metabolism and impaired fatty acid–related signal transduction in the etiology of both depression and bipolar disorder and attempts to link depression to cardiovascular and autoimmune disease through these metabolic impairments (77).
Our study has notable strengths. First, to our knowledge, it is the only large observational study to assess an n–3 fatty acid–depression association among the young and middle-aged white and African American United States population using the CES-D to assess amount of depressive symptoms. Second, our study examined complex arrays of associations between various exposures including total n–3 PUFAs and n–3 HUFAs as well as n–3:n–6 ratios of interest, while examining moderating effects of sex. Third, it is among few large United States studies that included two 24-h dietary recalls, reducing measurement error and enabling dietary variables to reflect usual intake to a greater extent than a single recall. Finally, our analysis accounted for unequal probability of sampling by incorporating sampling weights and obtaining estimates of means, proportions, and regression coefficients that were representative of Baltimore City.
However, our study has several limitations. First, its cross-sectional design did not allow us to ascertain temporality, although several cohort studies have shown that fish consumption (or n–3 HUFA intake) predicted incident depression (11–13, 32). It is thus important to conduct more longitudinal studies in a United States community to verify temporality of associations. Second, measurement errors in dietary exposures are not totally avoided by having multiple 24-h recalls. Additionally, even though 24-h recalls were originally not intended to measure usual or habitual intakes, some earlier studies have shown that even a single 24-h recall is able to classify participants to almost the same extent within this distribution as other methods that were intended to measure habitual intake, including the FFQ, which has a time frame of 1 y (78). Another more recent study compared the 24-h recall with the FFQ and concluded that the Block and Diet History Questionnaire FFQs underestimate total energy and nutrient intakes compared with the AMPM (41). In addition, only two 24-h recalls from the AMPM were needed to capture the long-term intake as evidenced by a third study (79). Thus, in our study, the mean of two 24-h recalls from the AMPM may be estimating long-term intake as well. Finally, data on supplemental intakes of n–3 fatty acids in the form of fish oil tablets were not available at the time of the survey, although this form of intake is still less popular than multivitamins and other forms of supplements. Ideally, a biomarker measure of n–3 fatty acids (e.g., adipose tissue, RBCs, or serum) is currently the gold standard because it takes into account all sources of n–3 fatty acids and provides an unbiased measure of exposure.
In summary, the relationship between n–3 fatty acids and depression has been examined in a limited number of observational and experimental studies. Additional prospective observational studies are needed to strengthen evidence of a causal association in the hypothesized direction. This should possibly include supplemental n–3 fatty acid intakes in addition to their main dietary source as well as a biomarker for intake of n–3 fatty acids, while considering the balance of n–3 to n–6 fatty acids. Our study findings support the hypothesis of a protective effect of n–3 fatty acids, both HUFAs and PUFAs, against depressive symptoms, particularly among women. Whereas reverse causality cannot be ruled out, it is more likely that reduced concentrations of n–3 fatty acids may contribute to symptoms of depression, and not vice-versa. Thus, these findings support the need for Dietary Reference Intake recommendations in the US, particularly for DHA and EPA (i.e., n–3 HUFAs), given their strong impact on depressive symptoms, particularly the domain of positive affect.
Supplementary Material
Acknowledgments
The authors thank Melissa H. Kitner-Triolo and Wayne Chan for their careful internal review of this manuscript. M.A.B. had full access to the data used in this manuscript and completed all of the statistical analyses, wrote and revised the manuscript, planned analysis, performed data management, and had primary responsibility for the final content; M.T.F.K. wrote and revised parts of the manuscript, participated in literature review, and participated in data acquisition and planning of analysis; H.A.B. wrote and revised parts of the manuscript, planned analysis, and participated in literature review; J.R.H. wrote and revised parts of the manuscript and participated in literature review; M.K.E. wrote and revised parts of the manuscript and participated in data acquisition; and A.B.Z. wrote and revised parts of the manuscript and participated in data acquisition and planning of analysis. All authors read and approved the final version of the manuscript.
Footnotes
AA, arachidonic acid; ALA, α-linolenic acid; AI, Adequate Intake; AMPM, Automated Multiple Pass Method; CES-D, Center for Epidemiologic Studies–Depression Scale; DPA, docosapentaenoic acid; EDS, elevated depressive symptoms; HANDLS, Healthy Aging in Neighborhoods of Diversity across the Life Span; HS, high school; HUFA, highly unsaturated fatty acid; LA, linoleic acid; PIR, poverty-income ratio.
Literature Cited
- 1.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–55. [DOI] [PubMed] [Google Scholar]
- 2.Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55:891–6. [DOI] [PubMed] [Google Scholar]
- 3.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20:4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38:35–46. [DOI] [PubMed] [Google Scholar]
- 4.Rees AM, Austin MP, Owen C, Parker G. Omega-3 deficiency associated with perinatal depression: case control study. Psychiatry Res. 2009;166:254–9. [DOI] [PubMed] [Google Scholar]
- 5.Schins A, Crijns HJ, Brummer RJ, Wichers M, Lousberg R, Celis S, Honig A. Altered omega-3 polyunsaturated fatty acid status in depressed post-myocardial infarction patients. Acta Psychiatr Scand. 2007;115:35–40. [DOI] [PubMed] [Google Scholar]
- 6.Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamaki H. Fish consumption, depression, and suicidality in a general population. Arch Gen Psychiatry. 2001;58:512–3. [DOI] [PubMed] [Google Scholar]
- 7.Appleton KM, Peters TJ, Hayward RC, Heatherley SV, McNaughton SA, Rogers PJ, Gunnell D, Ness AR, Kessler D. Depressed mood and n-3 polyunsaturated fatty acid intake from fish: non-linear or confounded association? Soc Psychiatry Psychiatr Epidemiol. 2007;42:100–4. [DOI] [PubMed] [Google Scholar]
- 8.Conklin SM, Harris JI, Manuck SB, Yao JK, Hibbeln JR, Muldoon MF. Serum omega-3 fatty acids are associated with variation in mood, personality and behavior in hypercholesterolemic community volunteers. Psychiatry Res. 2007;152:1–10. [DOI] [PubMed] [Google Scholar]
- 9.Raeder MB, Steen VM, Vollset SE, Bjelland I. Associations between cod liver oil use and symptoms of depression: the Hordaland Health Study. J Affect Disord. 2007;101:245–9. [DOI] [PubMed] [Google Scholar]
- 10.Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamaki H, Lehtonen J, Vartiainen E. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001;52:529–31. [DOI] [PubMed] [Google Scholar]
- 11.Astorg P, Couthouis A, Bertrais S, Arnault N, Meneton P, Guesnet P, Alessandri JM, Galan P, Hercberg S. Association of fish and long-chain n-3 polyunsaturated fatty acid intakes with the occurrence of depressive episodes in middle-aged French men and women. Prostaglandins Leukot Essent Fatty Acids. 2008;78:171–82. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Villegas A, Henriquez P, Figueiras A, Ortuno F, Lahortiga F, Martinez-Gonzalez MA. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur J Nutr. 2007;46:337–46. [DOI] [PubMed] [Google Scholar]
- 13.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–2. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe AR, Ogbonna EM, Lim S, Li Y, Zhang J. Dietary linoleic and oleic fatty acids in relation to severe depressed mood: 10 years follow-up of a national cohort. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:972–7. [DOI] [PubMed] [Google Scholar]
- 15.Lucas M, Mirzaei F, O'Reilly EJ, Pan A, Willett WC, Kawachi I, Koenen K, Ascherio A. Dietary intake of n–3 and n–6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr. 2011;93:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. [DOI] [PubMed] [Google Scholar]
- 17.Sontrop J, Campbell MK. Omega-3 polyunsaturated fatty acids and depression: a review of the evidence and a methodological critique. Prev Med. 2006;42:4–13. [DOI] [PubMed] [Google Scholar]
- 18.Silvers KM, Scott KM. Fish consumption and self-reported physical and mental health status. Public Health Nutr. 2002;5:427–31. [DOI] [PubMed] [Google Scholar]
- 19.Lin PY, Mischoulon D, Freeman MP, Matsuoka Y, Hibbeln J, Belmaker RH, Su KP. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry. 2012;17:1161–3; author reply, 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72:1577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klerman GL, Weissman MM. Increasing rates of depression. JAMA. 1989;261:2229–35. [PubMed] [Google Scholar]
- 22.Leaf A, Weber PC. A new era for science in nutrition. Am J Clin Nutr. 1987;45:1048–53. [DOI] [PubMed] [Google Scholar]
- 23.Eaton SB, Konner M. Paleolithic nutrition: a consideration of its nature and current implications. N Engl J Med. 1985;312:283–9. [DOI] [PubMed] [Google Scholar]
- 24.Gibson RA, Neumann MA, Lien EL, Boyd KA, Tu WC. Docosahexaenoic acid synthesis from alpha-linolenic acid is inhibited by diets high in polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;88:139–46. [DOI] [PubMed] [Google Scholar]
- 25.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuipers RS, Luxwolda MF, Dijck-Brouwer DA, Eaton SB, Crawford MA, Cordain L, Muskiet FA. Estimated macronutrient and fatty acid intakes from an East African Paleolithic diet. Br J Nutr. 2010;104:1666–87. [DOI] [PubMed] [Google Scholar]
- 27.Horrocks LA, Yeo YK. Docosahexaenoic acid-enriched foods: production and effects on blood lipids. Lipids. 1999;34 Suppl:S313. [DOI] [PubMed] [Google Scholar]
- 28.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–79. [DOI] [PubMed] [Google Scholar]
- 29.Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc. 2009;109:668–79. [DOI] [PubMed] [Google Scholar]
- 30.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n–3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2007;85:1103–11. [DOI] [PubMed] [Google Scholar]
- 31.Beydoun MA, Kaufman JS, Sloane PD, Heiss G, Ibrahim J. n-3 Fatty acids, hypertension and risk of cognitive decline among older adults in the Atherosclerosis Risk in Communities (ARIC) study. Public Health Nutr. 2008;11:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strøm M, Mortensen EL, Halldorsson TI, Thorsdottir I, Olsen SF. Fish and long-chain n–3 polyunsaturated fatty acid intakes during pregnancy and risk of postpartum depression: a prospective study based on a large national birth cohort. Am J Clin Nutr. 2009;90:149–55. [DOI] [PubMed] [Google Scholar]
- 33.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20:267–75. [PMC free article] [PubMed] [Google Scholar]
- 34.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 35.Ramos MI, Allen LH, Haan MN, Green R, Miller JW. Plasma folate concentrations are associated with depressive symptoms in elderly Latina women despite folic acid fortification. Am J Clin Nutr. 2004;80:1024–8. [DOI] [PubMed] [Google Scholar]
- 36.Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137:1081–4. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen HT, Kitner-Triolo M, Evans MK, Zonderman AB. Factorial invariance of the CES-D in low socioeconomic status African Americans compared with a nationally representative sample. Psychiatry Res. 2004;126:177–87. [DOI] [PubMed] [Google Scholar]
- 38.Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004;104:595–603. [DOI] [PubMed] [Google Scholar]
- 39.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77:1171–8. [DOI] [PubMed] [Google Scholar]
- 40.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–32. [DOI] [PubMed] [Google Scholar]
- 41.Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J Nutr. 2006;136:2594–9. [DOI] [PubMed] [Google Scholar]
- 42.Rumpler WV, Kramer M, Rhodes DG, Moshfegh AJ, Paul DR. Identifying sources of reporting error using measured food intake. Eur J Clin Nutr. 2008;62:544–52. [DOI] [PubMed] [Google Scholar]
- 43.USDA, Agriculture Research Service Food Surveys Research Group. Food and Nutrient Database for Dietary Studies, 3.0. Beltsville (MD): USDA, 2008. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=17031.
- 44.Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, Shroff MR, Mason MA, Evans MK, Zonderman AB. The sex-specific role of plasma folate in mediating the association of dietary quality with depressive symptoms. J Nutr. 2010;140:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Academy of Science. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington: National Academies Press, 2002. [DOI] [PubMed]
- 46.Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83 Suppl:1483S–93S. [DOI] [PubMed] [Google Scholar]
- 47.Kris-Etherton PM, Grieger JA, Etherton TD. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot Essent Fatty Acids. 2009;81:99–104. [DOI] [PubMed] [Google Scholar]
- 48.Sanhueza C, Ryan L, Foxcroft DR. Diet and the risk of unipolar depression in adults: systematic review of cohort studies. J Hum Nutr Diet. 2013;26:56–70. [DOI] [PubMed] [Google Scholar]
- 49.Beydoun MA, Shroff MR, Beydoun HA, Zonderman AB. Serum folate, vitamin B-12, and homocysteine and their association with depressive symptoms among U.S. adults. Psychosom Med. 2010;72:862–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murakami K, Mizoue T, Sasaki S, Ohta M, Sato M, Matsushita Y, Mishima N. Dietary intake of folate, other B vitamins, and omega-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition. 2008;24:140–7. [DOI] [PubMed] [Google Scholar]
- 51.Morris DW, Trivedi MH, Rush AJ. Folate and unipolar depression. J Altern Complement Med. 2008;14:277–85. [DOI] [PubMed] [Google Scholar]
- 52.Sánchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B(6) and vitamin B(12) intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009;22:122–33. [DOI] [PubMed] [Google Scholar]
- 53.Scott TM, Tucker KL, Bhadelia A, Benjamin B, Patz S, Bhadelia R, Liebson E, Price LL, Griffith J, Rosenberg I, et al. Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. Am J Geriatr Psychiatry. 2004;12:631–8. [DOI] [PubMed] [Google Scholar]
- 54.Bottiglieri T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev. 1996;54:382–90. [DOI] [PubMed] [Google Scholar]
- 55.D'Anci KE, Rosenberg IH. Folate and brain function in the elderly. Curr Opin Clin Nutr Metab Care. 2004;7:659–64. [DOI] [PubMed] [Google Scholar]
- 56.Tolmunen T, Hintikka J, Ruusunen A, Voutilainen S, Tanskanen A, Valkonen VP, Viinamaki H, Kaplan GA, Salonen JT. Dietary folate and the risk of depression in Finnish middle-aged men: a prospective follow-up study. Psychother Psychosom. 2004;73:334–9. [DOI] [PubMed] [Google Scholar]
- 57.Kivelä SL, Pahkala K, Eronen A. Depression in the aged: relation to folate and vitamins C and B12. Biol Psychiatry. 1989;26:210–3. [DOI] [PubMed] [Google Scholar]
- 58.Levitt AJ, Joffe RT. Folate, B12, and life course of depressive illness. Biol Psychiatry. 1989;25:867–72. [DOI] [PubMed] [Google Scholar]
- 59.Oishi J, Doi H, Kawakami N. Nutrition and depressive symptoms in community-dwelling elderly persons in Japan. Acta Med Okayama. 2009;63:9–17. [DOI] [PubMed] [Google Scholar]
- 60.Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H, Christophe A. Lower serum vitamin E concentrations in major depression: another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000;58:241–6. [DOI] [PubMed] [Google Scholar]
- 61.Owen AJ, Batterham MJ, Probst YC, Grenyer BF, Tapsell LC. Low plasma vitamin E levels in major depression: diet or disease? Eur J Clin Nutr. 2005;59:304–6. [DOI] [PubMed] [Google Scholar]
- 62.Cherubini A, Martin A, Andres-Lacueva C, Di Iorio A, Lamponi M, Mecocci P, Bartali B, Corsi A, Senin U, Ferrucci L. Vitamin E levels, cognitive impairment and dementia in older persons: the InCHIANTI study. Neurobiol Aging. 2005;26:987–94. [DOI] [PubMed] [Google Scholar]
- 63.Kamphuis MH, Geerlings MI, Grobbee DE, Kromhout D. Dietary intake of B(6-9-12) vitamins, serum homocysteine levels and their association with depressive symptoms: the Zutphen Elderly Study. Eur J Clin Nutr. 2008;62:939–45. [DOI] [PubMed] [Google Scholar]
- 64.Willett WC. Nutritional epidemiology. 2nd ed New York: Oxford University Press; 1998. [Google Scholar]
- 65.STATA statistics/data analysis: release 10.0. College Station, TX: Stata Corporation; 2007.
- 66.Selvin S. Statistical analysis of epidemiologic data. 3rd ed Oxford: Oxford University Press; 2004. [Google Scholar]
- 67.Logan AC. Neurobehavioral aspects of omega-3 fatty acids: possible mechanisms and therapeutic value in major depression. Altern Med Rev. 2003;8:410–25. [PubMed] [Google Scholar]
- 68.Hibbeln JR, Salem N., Jr Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr. 1995;62:1–9. [DOI] [PubMed] [Google Scholar]
- 69.Otrock ZK, Beydoun A, Barada WM, Masroujeh R, Hourani R, Bazarbachi A. Transient global amnesia associated with the infusion of DMSO-cryopreserved autologous peripheral blood stem cells. Haematologica. 2008;93:e36–7. [DOI] [PubMed] [Google Scholar]
- 70.Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. [DOI] [PubMed] [Google Scholar]
- 71.Lieb J, Karmali R, Horrobin D. Elevated levels of prostaglandin E2 and thromboxane B2 in depression. Prostaglandins Leukot Med. 1983;10:361–7. [DOI] [PubMed] [Google Scholar]
- 72.Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–6. [DOI] [PubMed] [Google Scholar]
- 73.Song C, Lin A, Bonaccorso S, Heide C, Verkerk R, Kenis G, Bosmans E, Scharpe S, Whelan A, Cosyns P, et al. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J Affect Disord. 1998;49:211–9. [DOI] [PubMed] [Google Scholar]
- 74.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. [DOI] [PubMed] [Google Scholar]
- 75.Bonaccorso S, Puzella A, Marino V, Pasquini M, Biondi M, Artini M, Almerighi C, Levrero M, Egyed B, Bosmans E, et al. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res. 2001;105:45–55. [DOI] [PubMed] [Google Scholar]
- 76.Maes M, Smith RS. Fatty acids, cytokines, and major depression. Biol Psychiatry. 1998;43:313–4. [DOI] [PubMed] [Google Scholar]
- 77.Horrobin DF, Bennett CN. Depression and bipolar disorder: relationships to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis. Possible candidate genes. Prostaglandins Leukot Essent Fatty Acids. 1999;60:217–34. [DOI] [PubMed] [Google Scholar]
- 78.Bingham SA, Gill C, Welch A, Day K, Cassidy A, Khaw KT, Sneyd MJ, Key TJ, Roe L, Day NE. Comparison of dietary assessment methods in nutritional epidemiology: weighed records v. 24 h recalls, food-frequency questionnaires and estimated-diet records. Br J Nutr. 1994;72:619–43. [DOI] [PubMed] [Google Scholar]
- 79.Stote KS, Radecki SV, Moshfegh AJ, Ingwersen LA, Baer DJ. The number of 24 h dietary recalls using the US Department of Agriculture's automated multiple-pass method required to estimate nutrient intake in overweight and obese adults. Public Health Nutr. 2011;14:1736–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.