Abstract
A score based on diet and lifestyle recommendations from the AHA has been associated with cardiovascular risk factors. We aimed to assess whether the diet components alone were associated with metabolic syndrome (MetS) and allostatic load (AL; a composite measure of 10 physiologically dysregulated variables). The diet score ranged from 0 to 90 and included intake components for dietary fats, fruits and vegetables, whole grains, salt, added sugars, and alcohol and was tested in a cross-sectional analysis of 1318 Puerto Rican adults (aged 45–75 y; 72% women) living in Boston, MA. The mean ± SD diet score was 28.0 ± 9.9 for men and 30.0 ± 10.1 for women. Replicating findings from a previous study in this cohort that used both the diet and lifestyle components, we observed associations between the diet-only score and insulin, waist circumference, and HDL cholesterol. We found novel significant associations between the continuous diet score and AL components, namely an inverse association with urinary cortisol and a positive association with serum dehydroepiandrosterone sulfate in women as well as an inverse association with urinary norepinephrine in men (all P < 0.05). In multinomial logistic regression, every 10 AHA diet score units were associated with 22% (95% CI: 1, 38; P = 0.043) lower odds of having ≥6 (vs. ≤2) dysregulated AL components in women. In men, every 10 diet score units were associated with lower odds of MetS (OR: 0.69; 95% CI: 0.52, 0.93; P = 0.016). Following AHA recommendations for a healthy diet may protect against the development of components of MetS and AL in Puerto Rican adults.
Introduction
In 2006, the AHA put forth a statement on diet and lifestyle recommendations based on the latest authoritative evidence, with the goal of reducing the risk of cardiovascular disease (CVD)7 in the general population (1). The recommendations included goals for an overall healthy, energy-balanced diet with specific guidelines for intake of fruits and vegetables, whole grains, fish, saturated fat, trans fat, dietary cholesterol, added sugars, and salt. The AHA also encouraged other lifestyle goals such as maintaining healthy body weight, engaging in physical activity, consuming alcohol in moderation (if at all), and avoiding tobacco. The statement concluded by asserting that these messages should be particularly directed at ethnic minorities, to reduce the CVD-related disparities that they present.
One such group is Puerto Ricans, the second largest Latino ethnic group in the United States (2). Puerto Ricans experience the poorest health in the nation (3, 4), with excessive prevalence of hypertension, diabetes, obesity, hyperglycemia, and dyslipidemia (5–9). We documented that 65% of men and 77% of women in a cohort of Puerto Rican adults living in the Boston area had metabolic syndrome (MetS) (10), a cluster of biological risk factors that, together, double the risk of eventual CVD events and mortality (11). A potential approach to help decrease CVD-related disparities among this group may be to consider lifestyle recommendations such as those proposed by the AHA, and to evaluate whether these suggestions may affect cardiometabolic risk factors for this group in particular.
To this end, a recent study by Bhupathiraju et al. (12) showed that higher adherence to the AHA diet and lifestyle recommendations was associated with lower insulin, C-reactive protein (CRP), and waist circumference and with higher HDL cholesterol (HDL-C) in this Boston Puerto Rican cohort. Subsequently, a study among Japanese men adapted the score to include only the dietary components and found that the AHA diet score was associated with different biomarkers than those found in Bhupathiraju et al., but also with increased odds of MetS (13). The differences in results may have been a result of population characteristics, sex, or actual mechanistic variations for diet alone vs. all lifestyle factors. MetS, as well as individual physiological biomarkers, involves diverse pathways, with an unclear unifying pathophysiological mechanism (14, 15). The discrepancies between studies remain to be evaluated. Moreover, although several studies have shown associations between other diet scores based on national dietary guidelines and MetS (16–18), the AHA-recommended diet score has not been exclusively evaluated for this outcome in both sexes.
MetS is not the only cluster of risk factors with a link to diet. We have previously shown that a dietary pattern of meats, processed meats, and french fries was associated with higher odds of allostatic load (AL) in this cohort (19). AL refers to a cluster of physiological responses to stressors (such as diet) that operate outside normal range because of cumulative wear and tear and that culminate in an unbalanced system, disturbed functioning, and disease (20, 21). These dysregulated risk factors include primary neuroendocrine markers in the hypothalamic-pituitary-adrenal axis that trigger secondary cardiometabolic variables (22), most of which are included in the conventional definition of MetS. However, several studies have shown that AL predicts morbidity and mortality of major chronic conditions beyond the predictive value of its individual components (20, 23), and with similar or stronger association than the MetS variables (24–26). We showed that this group of Puerto Ricans had high AL, with a mean of 4 out of 10 possible AL components and with 20% of men and 14% of women having ≥6 AL components (10). Higher AL was associated with increased likelihood of type II diabetes, CVD, and arthritis, particularly for those with ≥6 components, and the associations were stronger than for MetS (10). Aside from our aforementioned study, reports of dietary contributors to AL are scarce. Thus, we aimed to assess whether dietary components of the AHA recommendations were associated with cardiometabolic risk factors, MetS, and AL in Puerto Rican men and women. We subsequently discussed how our sex-specific results using diet components only for the AHA score juxtapose with those from previous studies applying diet and lifestyle components (12, 13).
Participants and Methods
Study participants.
Data from participants of the baseline cycle of the Boston Puerto Rican Heart Study, a longitudinal cohort of Puerto Rican adults in Boston, was used for this analysis. Recruitment strategies and baseline characteristics of the participants have been described in detail previously (8). Briefly, 1500 participants were recruited in the Boston metro area using door-to-door enumeration based on 2000 census data that identified census tracks of at least 25 Puerto Rican adults and randomly selected census blocks of 10 or more Hispanics. Only 1 participant per household could participate. Supplemental recruitment strategies, such as community events, media advertisement, and referrals, were also used.
To be eligible, an individual had to be of self-identified Puerto Rican descent, able to answer questions in English or Spanish, aged 45–75 y, and living in the Boston area at the time of the study. All data were collected by trained, bilingual interviewers following standardized protocols. The study was approved by the Institutional Review Board at Tufts Medical Center, Tufts University, and Northeastern University. Informed consent was obtained for all participants.
Dietary assessment and AHA diet score definition.
A semiquantitative FFQ previously adapted and validated for this population (27) was administered by trained bilingual interviewers. The FFQ included traditional Puerto Rican foods and was adjusted for usual portion sizes. Nutrient intakes were calculated from the Nutrition Data System for Research software (Nutrition Coordinating Center, Minneapolis, MN). Food groups for fruits, vegetables, fish, and alcohol were created, and mixed dishes were disaggregated when necessary. Participants with plausible energy intake (≥600 or ≤4,800 kcal/d) and valid FFQs (≤10 questions blank) were included for analysis (n = 1433).
The AHA diet and lifestyle score has been described in detail previously (12). Briefly, a total of 7 dietary recommendations have been put forth by the AHA for CVD risk reduction (1). These include the following: consume a diet rich in fruits and vegetables; choose whole-grain, high-fiber foods; consume fish, especially oily fish, at least twice a week; limit your intake of saturated and trans fat and cholesterol; minimize your intake of beverages and foods with added sugars; choose and prepare foods with little or no salt; and if you consume alcohol, do so in moderation. We used 11 components to reflect adherence to the dietary recommendations (fruit and vegetable intake, fruit and vegetable variety, whole grains, fish, saturated fats, trans fat, total fat, dietary cholesterol, added sugars, sodium, and alcohol). Participants received scores for each of the components, with each score ranging from a 0 to a maximum of 4, 6, or 10. The total possible points for the AHA diet score was 90, with higher values reflecting greater adherence to the dietary recommendations. The scoring system for all dietary components was based on quantitative recommendations provided by the AHA or by other national agencies (i.e., Centers for Disease Control and Prevention) when specific guidelines from AHA were not available. For each of the 11 components (except for fruit and vegetable variety), scores were prorated linearly between 0 and the maximum possible to reflect varying amounts of intake. Scores for fruit and vegetable variety were based on the sex-specific distribution of the variety score in the population. Those in the highest tertile received the maximum possible points of 10, those in the middle tertile received 5 points, and those in the lowest tertile received 0 points.
Outcome measures.
Outcomes included MetS as a dichotomous variable, AL as a composite measure, and their individual physiological components. Detailed protocols for data collection and laboratory analyses are described elsewhere (8). Briefly, blood pressure (BP) was the mean of the second and third readings from 3 duplicate measures taken during the interview using an electronic sphygmomanometer (model 8260, Dinamap). Waist circumference was measured in duplicate, and the mean value was used. Twelve-hour fasting blood samples were collected and analyzed for serum dehydroepiandrosterone sulfate (DHEA-S; Immulite 1000 LKDS1 kit), serum CRP (Immulite 1000 LKCRP1 kit), serum insulin (Immulite 1000 LKIN1 kit), serum glucose (reagents OSCR6121 on Olympus AU400e), plasma total cholesterol (reagents OSR6116 on Olympus AU400e), plasma HDL-C (reagents OSR6195 on Olympus AU400e), plasma TG (reagents OSR6133 on Olympus AU400e), LDL cholesterol (LDL-C; Friedewald formula), and glycosylated hemoglobin (HbA1c; Roche Unimate HbA1c kit on Cobas FARA). A 12-h morning urine sample was analyzed for cortisol, epinephrine, and norepinephrine using a direct immunoenzymatic colorimetric method with an ALPCO assay for cortisol, and 2-CAT enzyme immunoassay for epinephrine and norepinephrine, read on a Dynex MRX 96-well plate reader.
The 2005 AHA/National Heart, Lung, and Blood Institute Scientific Statement guidelines (28) were used to classify participants with MetS if they had ≥3 of the following conditions: waist circumference ≥102 cm in men or ≥88 cm in women; fasting glucose ≥5.6 mmol/L or use of glucose-lowering medication; elevated blood pressure (systolic BP ≥130 or diastolic BP ≥85 mm Hg) or use of hypertension medication; high TG (≥1.7 mmol/L); low HDL-C (<1.0 mmol/L in men or <1.3 mmol/L in women).
The composite measure of AL has been defined previously (10). In summary, 10 biomarkers representing various physiological systems were included in the score: urinary cortisol, urinary norepinephrine, urinary epinephrine, serum DHEA-S, HDL-C, total cholesterol, systolic BP, diastolic BP, plasma HbA1c, and waist circumference. One point was given for each component outside predefined cutoff points. If a component was within normal values, but medication for hypertension, diabetes, lipid-lowering, or testosterone was used, a point was assigned to reflect artificial regulation of blood pressure, HbA1c, total cholesterol, or DHEA-S, respectively. Points were added for a total of 10 as highest possible AL score. Five categories for AL were assigned as ≤2, 3, 4, 5 or ≥6 variables (10).
Other covariates.
A sociodemographic questionnaire was administered to collect data on age, sex, smoking behavior, educational attainment, household income, and medication use. A categorical variable for education was created as <8th grade, 9th–12th grade, and some college or higher. Poverty status was defined as a total household income below the poverty thresholds established by the U.S. Census Bureau, accounting for age of the head of household, participant‘s family size, and year of interview (8). Smoking was categorized as never, former, or current smoker. A language-based acculturation scale was developed from 10 questions on language use in various everyday activities (29). The Perceived Stress Scale was used to assess perceptions of one‘s life as stressful (30). A physical activity score was calculated as the total of hours spent on heavy, moderate, light, or sedentary activities in 24 h (based on a modified Paffenbarger questionnaire) multiplied by weighing factors that parallel the rate of oxygen consumption associated with each activity (31). BMI was calculated as weight (kg) divided by height (m) squared.
Statistical analyses.
Of the 1433 participants with available dietary data, 1318 had complete dietary information (components of diet score) and AL data. Log-transformed variables for cortisol, epinephrine, norepinephrine, DHEA-S, TG, plasma glucose, insulin, and CRP were used to normalize data distributions. Differences in baseline characteristics by sex were performed using chi-square analyses for categorical variables and t tests for continuous variables. The same statistical procedures were used to determine differences by sex in percent of participants meeting AHA recommendations and mean intake for each dietary component in the score.
Based on discrepancies in previous findings from studies with both sexes vs. men only (12, 13), and on metabolic differences by sex (32), the associations between the AHA diet score and each outcome were assessed separately by sex in these analyses. General linear models were used to determine associations between each 10-unit increase in diet score as continuous exposure, and the physiological risk factors, as outcomes, adjusting for potential confounders, including age; smoking status; education; poverty level; acculturation; perceived stress; energy intake; physical activity score; multivitamin use; use of lipid-lowering, diabetes, or hypertension medication; and BMI. For DHEA-S as outcome, we additionally adjusted for use of hormones. The model for HDL-C as outcome was additionally adjusted for LDL-C, and the model for LDL-C as outcome was additionally adjusted for HDL-C. The CRP model was additionally adjusted for white blood cell count.
Associations between each 10-unit increase of the AHA diet score with MetS as a dichotomous outcome, and the categorical variable of AL (with ≤2 variables as reference group), were tested with multinomial logistic regression models, fitted to estimate OR and 95% CI, controlling for age, smoking status, education, poverty level, acculturation, perceived stress, energy intake, physical activity score, multivitamin use, and BMI.
Statistical analyses were conducted using SAS version 9.3 (SAS Institute). A significance level of P < 0.05 was used. Results for linear regression models are reported as beta-coefficient (β) and SE, and for logistic regression results as OR (95% CI), both for each 10-unit increase in AHA diet score.
Results
Baseline characteristics of the 1318 Puerto Rican adults (Table 1) showed that women were significantly less likely to be current smokers than men (20.0 vs. 33.2%) but more likely to live below the poverty level (61.7 vs. 51.6%). Women had significantly lower acculturation (22.1 vs. 29.5) and physical activity (31.1 vs. 32.4) scores but higher perceived stress (24.2 vs. 21.9) and BMI (32.9 vs. 29.8 kg/m2) than men. The physiological biomarkers norepinephrine, HDL-C, total cholesterol, LDL-C, and CRP were higher among women than men, whereas the opposite was observed for DHEA-S, systolic and diastolic BP, and plasma TG. Women had a significantly higher prevalence of MetS than men (70.5% vs. 60.2%) but lower mean ± SD AL score (3.8 ± 1.7 vs. 4.3 ± 1.9) and were less likely to have ≥6 dysregulated AL components (15.0% vs. 22.9%).
TABLE 1.
Characteristics of participants of the Boston Puerto Rican Health Study, by sex1
| Men (n = 367) | Women (n = 951) | |
| Age, y | 56.9 ± 7.9 | 57.4 ± 7.4 |
| Current smoker, % | 33.2 | 20.0* |
| Education, % | ||
| Less than 8th grade | 44.1 | 47.7 |
| 9–12 grade (or GED) | 42.2 | 36.8 |
| Some college or higher | 13.6 | 15.5 |
| Diabetes, % | 42.0 | 39.6 |
| Use of diabetes medication, % | 34.5 | 32.6 |
| Hypertension, % | 71.6 | 68.9 |
| Use of hypertension medication, % | 52.9 | 56.3 |
| Use of lipid-lowering medication, % | 41.1 | 41.8 |
| Use of multivitamin supplement, % | 19.2 | 20.2 |
| Below poverty level, % | 51.6 | 61.7** |
| Perceived stress score | 21.9 ± 9.6 | 24.2 ± 9.3* |
| Acculturation score | 29.5 ± 22.9 | 22.1 ± 21.7* |
| Physical activity score | 32.4 ± 5.7 | 31.1 ± 4.0** |
| BMI, kg/m2 | 29.8 ± 5.1 | 32.9 ± 6.9* |
| Urinary cortisol, μg/g creatinine | 33.8 ± 25.6 | 30.6 ± 27.7 |
| Urinary epinephrine, μg/g creatinine | 4.2 ± 3.7 | 3.8 ± 3.3 |
| Urinary norepinephrine, μg/g creatinine | 37.1 ± 30.8 | 41.3 ± 28.7† |
| Serum DHEA-S, μmol/L | 3.25 ± 2.39 | 1.90 ± 1.46* |
| HDL-C, mmol/L | 1.04 ± 0.31 | 1.22 ± 0.32* |
| Total cholesterol, mmol/L | 4.49 ± 1.10 | 4.86 ± 1.05* |
| Systolic blood pressure, mm Hg | 138 ± 19 | 134 ± 19** |
| Diastolic blood pressure, mm Hg | 82.9 ± 11.1 | 80.2 ± 10.4* |
| Glycosylated hemoglobin, % | 7.0 ± 1.9 | 7.0 ± 1.7 |
| Waist circumference, cm | 102 ± 13 | 102 ± 15 |
| Plasma triglycerides, mmol/L | 1.97 ± 1.37 | 1.78 ± 1.11† |
| Serum glucose, mmol/L | 6.69 ± 2.76 | 6.66 ± 2.96 |
| Serum insulin, pmol/L | 127 ± 216 | 126 ± 144 |
| LDL-C, mmol/L | 2.49 ± 0.91 | 2.85 ± 0.89* |
| CRP, mg/L | 4.9 ± 9.5 | 6.7 ± 7.9** |
| Metabolic syndrome, % | 60.2 | 70.5** |
| Allostatic load score | 4.3 ± 1.9 | 3.8 ± 1.7** |
| Number of allostatic load components, % | ||
| ≤2 | 24.3 | 30.0 |
| 3 | 16.4 | 16.9 |
| 4 | 18.5 | 20.1 |
| 5 | 18.0 | 18.0 |
| ≥6 | 22.9 | 15.0† |
Values are means ± SDs or percentages. Difference between sexes: *P < 0.001, **P < 0.01, †P < 0.05. CRP, C-reactive protein; DHEA-S, dehydroepiandrosterone sulfate; GED, general equivalency diploma; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol.
Energy intake was significantly lower for women than for men (Table 2). Women, however, had a higher mean AHA diet score than men (30.0 ± 10.1 vs. 28.0 ± 9.9). There were important differences by sex on the percent of participants meeting the AHA recommendations for all distinct dietary components of the score, except for amount of fruits and vegetables and saturated fat. More women than men followed the AHA recommendations for variety of fruits and vegetables, whole grains, total fat, trans fat, dietary cholesterol, and sodium, whereas the opposite was observed for fish, added sugars, and alcohol. In general, total fat intake was the dietary component for which the most participants met the AHA recommendations (25–35% from energy), followed by moderate alcohol intake. The rest of the components were met by <25% of participants. After saturated fat, for which no one followed the guidelines, the least followed AHA recommendations by both men and women were for whole grains, sodium, and trans fat.
TABLE 2.
Means of intake and percent of participants meeting the recommendations of the AHA diet score components for Puerto Rican men and women1
| Men | Women | |
| Energy, kcal/d | 2410 ± 850 | 1980 ± 850* |
| Total AHA diet score (range 0–90) | 28.0 ± 9.9 | 30.0 ± 10.1** |
| Fruits and vegetables, servings/d | 2.8 ± 1.7 | 3.1 ± 1.8** |
| ≥5 servings/d, % | 8.7 | 12.4 |
| Fruit and vegetable variety score | 24.9 ± 8.8 | 26.3 ± 8.0** |
| Top quartile, % | 19.6 | 22.4** |
| Whole grains, % total grain | 10.4 ± 9.7 | 14.1 ± 12.6* |
| ≥50% of total grain, % | 0.82 | 2.2** |
| Fish, servings/wk | 1.3 ± 1.5 | 1.2 ± 1.5 |
| ≥2 servings/d, % | 18.5 | 17.0† |
| Total fat, % from energy | 32.5 ± 5.5 | 31.5 ± 5.6** |
| 25–35% from energy, % | 55.0 | 59.7† |
| Saturated fat, % from energy | 9.7 ± 2.2 | 9.3 ± 2.2† |
| ≤3.5% from energy, % | 0 | 0 |
| Trans fat, % from energy | 1.2 ± 0.4 | 1.1 ± 0.4** |
| ≤0.5% from energy, % | 2.5 | 2.9** |
| Dietary cholesterol, mg/d | 359 ± 191 | 271 ± 158* |
| ≤150 mg/d, % | 9.8 | 23.5* |
| Added sugars, g/d | 61.4 ± 44.6 | 55.9 ± 46.9 |
| ≤Upper limit, % | 18.3 | 8.1* |
| Sodium, mg/d | 5600 ± 2280 | 4270 ± 2140* |
| ≤1.5 g/d, % | 0.54 | 3.2* |
| Alcohol, servings/d | 1.3 ± 2.1 | 0.55 ± 1.8** |
| ≤2 drinks/d men; ≤1 drink/d women, % | 40.1 | 30.2** |
Values are means ± SDs or percentages. Difference between sexes: *P < 0.001, **P < 0.01, †P < 0.05.
Associations between each 10 units of the AHA diet score and cardiometabolic risk factors and other physiological components of AL were determined by sex (Table 3). Among men, the diet score showed a significant inverse association with norepinephrine (β ± SE) = −0.126 ± 0.053, P = 0.018) and positive association with HDL-C (1.604 ± 0.709, P = 0.024). Among women, each 10 units of diet score were associated with lower urinary cortisol (−0.088 ± 0.028, P = 0.002), waist circumference (−0.883 ± 0.296, P = 0.003), and insulin (−0.055 ± 0.023, P = 0.016) and with higher DHEA-S (0.053 ± 0.026, P = 0.045). No other biomarker showed significant association with the AHA diet score.
TABLE 3.
Cross-sectional associations of an AHA diet score and components of metabolic syndrome and allostatic load and additional cardiometabolic risk factors in Puerto Rican men and women1
| Men |
Women |
|||
| β ± SE | P value | β ± SE | P value | |
| Log cortisol,2 μg/g creatinine | −0.058 ± 0.046 | 0.20 | −0.088 ± 0.028 | 0.002 |
| Log epinephrine,2 μg/g creatinine | −0.099 ± 0.053 | 0.06 | −0.025 ± 0.037 | 0.50 |
| Log norepinephrine,2 μg/g creatinine | −0.126 ± 0.053 | 0.018 | −0.028 ± 0.026 | 0.29 |
| Log DHEA-S,3 nmol/L | 0.040 ± 0.046 | 0.39 | 0.053 ± 0.026 | 0.045 |
| HDL-C,4 mmol/L | 1.604 ± 0.709 | 0.024 | 0.502 ± 0.421 | 0.23 |
| Total cholesterol,2 mmol/L | 2.832 ± 2.523 | 0.26 | 1.710 ± 1.380 | 0.22 |
| Systolic BP,2 mm Hg | 1.074 ± 1.097 | 0.33 | −0.812 ± 0.643 | 0.21 |
| Diastolic BP,2 mm Hg | 0.432 ± 0.648 | 0.52 | −0.290 ± 0.360 | 0.42 |
| Glycosylated hemoglobin,2 % | −0.024 ± 0.099 | 0.81 | −0.042 ± 0.047 | 0.37 |
| Waist circumference,2 cm | −0.015 ± 0.320 | 0.96 | −0.883 ± 0.296 | 0.003 |
| Log triglycerides,2 mmol/L | 0.008 ± 0.033 | 0.80 | 0.017 ± 0.018 | 0.31 |
| Log serum glucose,2 mmol/L | −0.028 ± 0.018 | 0.13 | −0.005 ± 0.010 | 0.59 |
| Log insulin,2 pmol/L | −0.040 ± 0.042 | 0.34 | −0.055 ± 0.023 | 0.016 |
| LDL-C,5 mmol/L | −0.793 ± 1.968 | 0.69 | −0.099 ± 1.164 | 0.93 |
| Log CRP,6 mg/L | −0.034 ± 0.063 | 0.59 | −0.015 ± 0.037 | 0.69 |
Values are beta-coefficient (β) ± SE for each 10-unit increase in diet score. BP, blood pressure; CRP, C-reactive protein; DHEA-S, dehydroepiandrosterone sulfate; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol.
Adjusted for age; smoking status; education; poverty level; acculturation; perceived stress; energy intake; physical activity score; multivitamin use; use of lipid-lowering, diabetes, or hypertension medication; and BMI.
Additionally adjusted for use of hormones.
Additionally adjusted for LDL-C.
Additionally adjusted for HDL-C.
Additionally adjusted for white blood cell count.
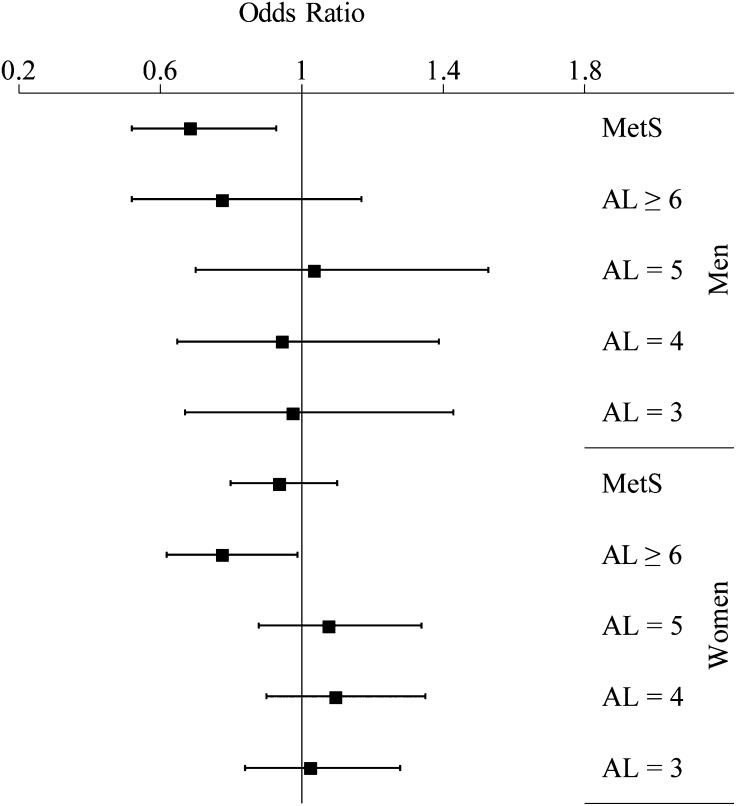
For each 10-unit increase in AHA diet score, men had 31% (95% CI: 7, 48) lower odds of having MetS but no significant association for any category of AL (Fig. 1). Among women, an additional 10 units of AHA diet score was associated with 22% (95% CI: 1, 38) lower odds of having ≥6 dysregulated AL components. Results for other categories of AL, or for MetS, were not significant among women (Supplemental Table 1).
FIGURE 1.
Odds ratio (95% CI) of metabolic syndrome and allostatic load categories (by number of components) for an increase of 10 units in AHA diet score in Puerto Rican men and women. Reference groups are no metabolic syndrome and ≤2 components of allostatic load. Adjusted for age, smoking status, education, poverty level, acculturation, perceived stress, energy intake, physical activity score, multivitamin use, and BMI. AL, allostatic load; MetS, metabolic syndrome.
Discussion
The present study showed important characteristics of diet quality in the Boston Puerto Rican community and their association with cardiometabolic outcomes. The diet in this cohort tended to be of poor quality. Fewer than 25% of the cohort members followed the recommendations for at least 9 of the 11 dietary components. The least followed recommendations were those for saturated fat, whole grains, trans fat, and sodium. Suggestions for servings of fruits and vegetables and for added sugars were also unmet by most. Although all diet components need improvement, the latter may need additional emphasis in dietary interventions and messages for this population, with special attention to the group’s traditional foods and cooking methods. Interestingly, men reported similar numbers of daily servings of fruits and vegetables as women but with more limited variety. It would be worthwhile to explore sex-specific preferences in fruits and vegetables in this population. Variety is important to ensure diverse micronutrient intake. Nutritional messages should underscore the importance of choosing from a wide variety of fruits and vegetables, particularly among men, in addition to the recommended 5 daily servings.
We report a significant inverse association between the AHA dietary score and MetS among men only. We stratified our analysis by sex a priori, given previously reported variations by sex in AL and cardiometabolic markers in this and similar populations (5, 10, 33) in diet quality, as observed here, as well as evidence of physiological and metabolic differences by sex that may influence the development of related disorders (32). In addition, we sought to explore inconsistencies between the Bhupathiraju et al. (12) article that developed the AHA diet and lifestyle score and the Kuroki et al. (13) study that applied only the AHA diet components among men.
Bhupathiraju et al. (12) included BMI and physical activity guidelines in their AHA-based score and found significant associations between the score and serum insulin, waist circumference, HDL-C, and CRP. Our study showed similar associations with insulin and waist circumference, but only in women, which comprised the majority of the cohort. We observed a weaker association between the AHA diet score and HDL-C, but this observation was significant only in men. Weight and regular exercise are recognized determinants of HDL-C concentration (34), and it is possible that such components drove the stronger association in the former study. We did not see an association between the diet score and CRP. A recent review suggested that weight loss and combined diet and exercise protocols may be more beneficial in reducing inflammation than individual strategies (35). For those risk factors, the biological mechanism may involve multiple pathways, emphasizing the importance of various lifestyle strategies to reduce them.
The Japanese study (13) among male factory workers, with an adapted AHA lifestyle score using only dietary components, reported an association with MetS, as we did here, and with waist circumference, diastolic BP, and TG. We did not observe associations with any of those individual components among men. In our study, the association with MetS components in men was strongest for HDL-C, for which Kuroki et al. did not find an association. Discrepancies in results could be a result of differences in components and cutoff points, because those authors did not include trans fat because of imprecise assessment and used a simpler scoring system that was not prorated linearly. In addition, their cohort was composed of younger (25–62 y) factory workers with likely different environmental context. Further, there may be ethnic differences in underlying visceral adiposity, insulin resistance, and other metabolic or genetic markers (36).
Other studies have shown associations between diet scores based on national dietary guidelines and MetS, including the 2005 Dietary Guidelines for Americans (16, 18) and the French Nutritional Guidelines (17). These studies reported 21–36% lower odds of MetS for the American guidelines and a 9% decrease in risk of each unit of the French guidelines, similar to the 31% lower odds in men that we report here for each 10 units of the AHA diet score. None of those studies reported results by sex. Gregory et al. (37) analyzed several dietary scores in Guatemalan young adults and did not detect significant associations with MetS in either men or women but did observe differential associations by sex for individual variables. Studies that examined diet quality scores that reflect dietary guidelines (e.g., Healthy Eating Index) have also been associated with lower MetS (38, 39). Dietary recommendations proposed by United States federal agencies and by medical and professional organizations have components that overlap with those proposed by AHA (40). Thus, our results strengthened the message that adhering to general dietary recommendations may keep cardiometabolic outcomes under control.
A novel finding in our study was the significant inverse association between the AHA diet score and AL in Puerto Rican adults. In women, this result was represented by the variables of cortisol, DHEA-S, and waist circumference. The mechanism of how diet quality affects waist circumference has been previously proposed (14, 15); how neuroendocrine markers may respond to diet is less understood. Previous studies, including one conducted with our cohort, have shown that cortisol was elevated among individuals with higher intake of saturated fat and sweet foods (41) and with intake of a low-quality food pattern that included meat, junk food, soda, fried foods, fast food, and simple carbohydrates (42). The diet-cortisol mechanism may be complex and bidirectional, but the role of diet on dysregulation of the hypothalamic-pituitary-adrenal axis seems to be supported by the collective evidence.
DHEA-S is an androgen secreted by the adrenal cortex that affects endothelial function and inflammation, and low concentrations are correlated with adrenal and cardiovascular dysfunction. One study among healthy centenarians showed that, among men only, poor adherence to a Mediterranean diet was associated with low DHEA-S, but the effect was lessened in men who consumed red wine (43). Several studies substantiate positive association between light-to-moderate or moderate alcohol drinking and DHEA-S concentrations among men and women (44–46). Moderate alcohol consumption was a component of our AHA score. Although the mechanism of action is not fully elucidated, the previous studies suggested potential pathways through stimulation of adrenal or liver androgens, changes in enzyme activity in the liver that lead to altered androgen metabolism, or production of inflammatory markers, among others. In addition, we previously found an association between a meat, processed meat, and french fries dietary pattern and odds of having low DHEA-S in this Puerto Rican cohort (19). A lower AHA diet score may denote consumption of these foods and nutrients of lower quality, in agreement with the positive association of the score with DHEA-S observed here.
We did not observe an association with AL among men, maybe because there were fewer male than female participants. In addition to HDL-C, the AHA diet score was inversely associated with norepinephrine in men. Our previous study showed a protective association of a traditional rice, beans, and oils dietary pattern and this variable (19), one of the few published reports on the role of diet on norepinephrine. Other studies showed lower norepinephrine with higher energy-adjusted carbohydrate consumption (47) and with supplementation of omega-3 fatty acids during mental stress stimulation (48). Because norepinephrine is secreted under low blood sugar conditions, it is possible that a healthy diet that comprises whole grains and high fiber content helps maintain constant blood glucose, thus blunting catecholamine response. The omega-3 content in a healthier AHA diet may regulate norepinephrine’s response in the nervous system, a suggestion that requires further examination.
Several studies have reported associations between guideline-based diet scores and total cholesterol (38), blood pressure (13, 16, 18, 38), TG (13, 16), plasma glucose (18), LDL-C (38), and CRP (38) in either men, women, or both. However, inconsistencies remain within these studies. Lack of replication in our study could be a result of lower sample size to detect such associations or of different intrinsic factors of our Puerto Rican cohort. Analyses from large, multi-ethnic longitudinal studies in men and women may help elucidate these discrepancies. It is important to note that we did not test individual dietary components because we intended to assess diet holistically. Previous studies in our cohort reported an inverse association between an omega-3 fatty acid/fish intake pattern and MetS (49), a positive association between a rice, beans, and oil pattern and MetS (50), and a positive association between a meat, processed meat, and french fries pattern and AL (19). The current results, along with previous reports, supported the contribution of overall diet to these outcomes in the Puerto Rican population.
Because of the cross-sectional design of this study, we could not establish causality or directionality of the associations. Another limitation is that most of the evidence used to determine the diet components of the AHA recommendations resulted from population studies comprised mostly of non-Hispanic white individuals. The AHA recognizes the complex and multifactorial factors for CVD disparities among racial and ethnic groups but maintains that the recommended diet and lifestyle changes are generalizable to ethnic minorities (1). Although our validated FFQ and extensive nutrient database likely assessed typical intake comprehensively, there could be some error in capturing the diet score. Also, other nutrients or foods that were not included here (e.g., supplements, phytochemicals) may have speculative influence on cardiometabolic risk factors (1). We did not apply weights to the diet components, although it has been shown that weighted scores may be useful in some cases (51). A strength of our analysis was that the components of the diet score were prorated linearly to account for a wide range of intake. Participants in our study with missing data for AL were less likely to have MetS or diabetes (10), which may reflect different dietary behaviors and introduce bias. We did not adjust for multiple comparisons given that we conducted the analysis under the hypotheses that each variable operates under an exclusive mechanism. Still, caution should be used when interpreting marginally significant findings. Physiological responses to diet may entail complex interactive or synergistic mechanisms that we did not test here and that warrant further examination.
In conclusion, we report significant associations between an AHA diet score and physiological variables of MetS and AL, with distinct results by sex. Following the recommendations for a healthy, high-quality diet, as stated by the AHA, may help Puerto Ricans keep cardiometabolic risk factors under control and reduce the likelihood of developing eventual chronic diseases. Dietary messages based on comprehensive, evidence-based guidelines, and that incorporate traditional foods, may be meaningful for public health applications in this and similar populations.
Supplementary Material
Acknowledgments
J.M. formulated the study questions, conducted statistical analysis, interpreted the results, and wrote the manuscript draft; S.B. contributed portions of the manuscript and statistical modeling and provided comments on the discussion; and K.L.T. oversaw data collection and processing and provided substantial intellectual feedback for dietary analysis and data interpretation. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AL, allostatic load; BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; DHEA-S, dehydroepiandrosterone sulfate; HbA1c, glycosylated hemoglobin; HDL-C, HDL cholesterol; LDL-C, LDL-cholesterol; MetS, metabolic syndrome.
Literature Cited
- 1.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 2.Collazo SG, Ryan CL, Bauman KJ. Profile of the Puerto Rican population in United States and Puerto Rico: 2008. Dallas, TX: U.S. Census Bureau, Housing and Household Economic Statistics Division; 2010. Available from: www.census.gov/hhes/socdemo/education/data/acs/paa2010/Collazo_Ryan_Bauman_PAA2010_Paper.pdf.
- 3.Hajat A, Lucas JB, Kington R. Health outcomes among Hispanic subgroups: data from the National Health Interview Survey, 1992–95. Adv Data. 2000;15(Supp. 1):1–14. [PubMed] [Google Scholar]
- 4.Li C, Balluz LS, Okoro CA, Strine TW, Lin JM, Town M, Garvin W, Murphy W, Bartoli W, Valluru B. Surveillance of certain health behaviors and conditions among states and selected local areas—Behavioral Risk Factor Surveillance System, United States, 2009. MMWR Surveill Summ. 2011;60:1–250. [PubMed] [Google Scholar]
- 5.Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pabon-Nau LP, Cohen A, Meigs JB, Grant RW. Hypertension and diabetes prevalence among U.S. Hispanics by country of origin: the National Health Interview Survey 2000–2005. J Gen Intern Med. 2010;25:847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Rompay MI, Castaneda-Sceppa C, McKeown NM, Ordovas JM, Tucker KL. Prevalence of cardiovascular disease risk factors among older Puerto Rican adults living in Massachusetts. J Immigr Minor Health. 2011;13:825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitman S, Silva A, Shah AM. Disproportionate impact of diabetes in a Puerto Rican community of Chicago. J Community Health. 2006;31:521–31. [DOI] [PubMed] [Google Scholar]
- 10.Mattei J, Demissie S, Falcon LM, Ordovas JM, Tucker K. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Soc Sci Med. 2010;70:1988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–14. [DOI] [PubMed] [Google Scholar]
- 12.Bhupathiraju SN, Lichtenstein AH, Dawson-Hughes B, Tucker KL. Adherence index based on the AHA 2006 diet and lifestyle recommendations is associated with select cardiovascular disease risk factors in older Puerto Ricans. J Nutr. 2011;141:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuroki Y, Kanauchi K, Kanauchi M. Adherence index to the American Heart Association Diet and Lifestyle Recommendation is associated with the metabolic syndrome in Japanese male workers. Eur J Intern Med. 2012;23:e199–203. [DOI] [PubMed] [Google Scholar]
- 14.Bremer AA, Mietus-Snyder M, Lustig RH. Toward a unifying hypothesis of metabolic syndrome. Pediatrics. 2012;129:557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogli-Cawley JJ, Dwyer JT, Saltzman E, McCullough ML, Troy LM, Meigs JB, Jacques PF. The 2005 Dietary Guidelines for Americans and risk of the metabolic syndrome. Am J Clin Nutr. 2007;86:1193–201. [DOI] [PubMed] [Google Scholar]
- 17.Kesse-Guyot E, Fezeu L, Galan P, Hercberg S, Czernichow S, Castetbon K. Adherence to French nutritional guidelines is associated with lower risk of metabolic syndrome. J Nutr. 2011;141:1134–9. [DOI] [PubMed] [Google Scholar]
- 18.Hosseini-Esfahani F, Jessri M, Mirmiran P, Bastan S, Azizi F. Adherence to dietary recommendations and risk of metabolic syndrome: Tehran Lipid and Glucose Study. Metabolism. 2010;59:1833–42. [DOI] [PubMed] [Google Scholar]
- 19.Mattei J, Noel SE, Tucker KL. A meat, processed meat, and French fries dietary pattern is associated with high allostatic load in Puerto Rican older adults. J Am Diet Assoc. 2011;111:1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, Berkman LF, Reuben DB. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004;58:1985–97. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 22.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. [DOI] [PubMed] [Google Scholar]
- 23.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation–allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med. 1997;157:2259–68. [PubMed] [Google Scholar]
- 24.Crews DE. Composite estimates of physiological stress, age, and diabetes in American Samoans. Am J Phys Anthropol. 2007;133:1028–34. [DOI] [PubMed] [Google Scholar]
- 25.Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. J Clin Epidemiol. 2002;55:696–710. [DOI] [PubMed] [Google Scholar]
- 26.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA. 2001;98:4770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–18. [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- 29.Bermúdez OI, Falcon LM, Tucker KL. Intake and food sources of macronutrients among older Hispanic adults: association with ethnicity, acculturation, and length of residence in the United States. J Am Diet Assoc. 2000;100:665–73. [DOI] [PubMed] [Google Scholar]
- 30.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 31.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–45. [DOI] [PubMed] [Google Scholar]
- 32.Iyer A, Kauter K, Brown L. Gender differences in metabolic syndrome: a key research issue? Endocr Metab Immune Disord Drug Targets. 2011;11:182–8. [DOI] [PubMed] [Google Scholar]
- 33.Bernabe-Ortiz A, Benziger CP, Gilman RH, Smeeth L, Miranda JJ. Sex differences in risk factors for cardiovascular disease: the PERU MIGRANT study. PLoS ONE. 2012;7:e35127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fletcher B, Berra K, Ades P, Braun LT, Burke LE, Durstine JL, Fair JM, Fletcher GF, Goff D, Hayman LL, et al. Managing abnormal blood lipids: a collaborative approach. Circulation. 2005;112:3184–209. [DOI] [PubMed] [Google Scholar]
- 35.Michigan A, Johnson TV, Master VA. Review of the relationship between C-reactive protein and exercise. Mol Diagn Ther. 2011;15:265–75. [DOI] [PubMed] [Google Scholar]
- 36.Forouhi NG, Sattar N. CVD risk factors and ethnicity–a homogeneous relationship? Atheroscler Suppl. 2006;7:11–9. [DOI] [PubMed] [Google Scholar]
- 37.Gregory CO, McCullough ML, Ramirez-Zea M, Stein AD. Diet scores and cardio-metabolic risk factors among Guatemalan young adults. Br J Nutr. 2009;101:1805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicklas TA, O'Neil CE, Fulgoni VL., 3rd Diet quality is inversely related to cardiovascular risk factors in adults. J Nutr. 2012;142:2112–8. [DOI] [PubMed] [Google Scholar]
- 39.Akbaraly TN, Singh-Manoux A, Tabak AG, Jokela M, Virtanen M, Ferrie JE, Marmot MG, Shipley MJ, Kivimaki M. Overall diet history and reversibility of the metabolic syndrome over 5 years: the Whitehall II prospective cohort study. Diabetes Care. 2010;33:2339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krebs-Smith SM, Kris-Etherton P. How does MyPyramid compare to other population-based recommendations for controlling chronic disease? J Am Diet Assoc. 2007;107:830–7. [DOI] [PubMed] [Google Scholar]
- 41.Laugero KD, Falcon LM, Tucker KL. Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite. 2011;56:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duong M, Cohen JI, Convit A. High cortisol levels are associated with low quality food choice in type 2 diabetes. Endocrine. 2012;41:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonini FM, Petruzzi E, Pinzani P, Orlando C, Petruzzi I, Pazzagli M, Masotti G. Effect of diet and red wine consumption on serum total antioxidant capacity (TAC), dehydroepiandrosterone-sulphate (DHEAS) and insulin-like growth factor-1 (IGF-1) in Italian centenarians. Arch Gerontol Geriatr. 2005;41:151–7. [DOI] [PubMed] [Google Scholar]
- 44.Fukui M, Kitagawa Y, Nakamura N, Kadono M, Hasegawa G, Yoshikawa T. Association between alcohol consumption and serum dehydroepiandrosterone sulphate concentration in men with type 2 diabetes: a link to decreased cardiovascular risk. Diabet Med. 2005;22:1446–50. [DOI] [PubMed] [Google Scholar]
- 45.Sierksma A, Sarkola T, Eriksson CJ, van der Gaag MS, Grobbee DE, Hendriks HF. Effect of moderate alcohol consumption on plasma dehydroepiandrosterone sulfate, testosterone, and estradiol levels in middle-aged men and postmenopausal women: a diet-controlled intervention study. Alcohol Clin Exp Res. 2004;28:780–5. [DOI] [PubMed] [Google Scholar]
- 46.Rinaldi S, Peeters PH, Bezemer ID, Dossus L, Biessy C, Sacerdote C, Berrino F, Panico S, Palli D, Tumino R, et al. Relationship of alcohol intake and sex steroid concentrations in blood in pre- and post-menopausal women: the European Prospective Investigation into Cancer and Nutrition. Cancer Causes Control. 2006;17:1033–43. [DOI] [PubMed] [Google Scholar]
- 47.Young JB, Troisi RJ, Weiss ST, Parker DR, Sparrow D, Landsberg L. Relationship of catecholamine excretion to body size, obesity, and nutrient intake in middle-aged and elderly men. Am J Clin Nutr. 1992;56:827–34. [DOI] [PubMed] [Google Scholar]
- 48.Delarue J, Matzinger O, Binnert C, Schneiter P, Chiolero R, Tappy L. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metab. 2003;29:289–95. [DOI] [PubMed] [Google Scholar]
- 49.Noel SE, Newby PK, Ordovas JM, Tucker KL. Adherence to an (n-3) fatty acid/fish intake pattern is inversely associated with metabolic syndrome among Puerto Rican adults in the Greater Boston area. J Nutr. 2010;140:1846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noel SE, Newby PK, Ordovas JM, Tucker KL. A traditional rice and beans pattern is associated with metabolic syndrome in Puerto Rican older adults. J Nutr. 2009;139:1360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arsenault JE, Fulgoni VL, 3rd, Hersey JC, Muth MK. A novel approach to selecting and weighting nutrients for nutrient profiling of foods and diets. J Acad Nutr Diet. 2012;112:1968–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.