Summary
Background
Babies with low birthweight (<2500 g) are at increased risk of early mortality. However, low birthweight includes babies born preterm and with fetal growth restriction, and not all these infants have a birthweight less than 2500 g. We estimated the neonatal and infant mortality associated with these two characteristics in low-income and middle-income countries.
Methods
For this pooled analysis, we searched all available studies and identified 20 cohorts (providing data for 2 015 019 livebirths) from Asia, Africa, and Latin America that recorded data for birthweight, gestational age, and vital statistics through 28 days of life. Study dates ranged from 1982 through to 2010. We calculated relative risks (RR) and risk differences (RD) for mortality associated with preterm birth (<32 weeks, 32 weeks to <34 weeks, 34 weeks to <37 weeks), small-for-gestational-age (SGA; babies with birthweight in the lowest third percentile and between the third and tenth percentile of a US reference population), and preterm and SGA combinations.
Findings
Pooled overall RRs for preterm were 6·82 (95% CI 3·56–13·07) for neonatal mortality and 2·50 (1·48–4·22) for post-neonatal mortality. Pooled RRs for babies who were SGA (with birthweight in the lowest tenth percentile of the reference population) were 1·83 (95% CI 1·34–2·50) for neonatal mortality and 1·90 (1·32–2·73) for post-neonatal mortality. The neonatal mortality risk of babies who were both preterm and SGA was higher than that of babies with either characteristic alone (15·42; 9·11–26·12).
Interpretation
Many babies in low-income and middle-income countries are SGA. Preterm birth affects a smaller number of neonates than does SGA, but is associated with a higher mortality risk. The mortality risks associated with both characteristics extend beyond the neonatal period. Differentiation of the burden and risk of babies born preterm and SGA rather than with low birthweight could guide prevention and management strategies to speed progress towards Millennium Development Goal 4—the reduction of child mortality.
Funding
Bill & Melinda Gates Foundation.
Introduction
An estimated 20 million infants every year are born with low birthweight (LBW; <2500 g),1 and these infants have an increased risk mortality in the first year of life. The primary causes of LBW are preterm birth, intrauterine growth restriction (IUGR), or a combination of the two. Of 135 million children born in low-income and middle-income countries (LMICs) in 2010, an estimated 29·7 million were born both term and small-for-gestationalage (SGA), 10·9 million were born preterm and appropriate-for-gestational-age, and 2·8 million were born preterm and SGA.2 Risk factors and interventions to reduce the number of babies born SGA might differ from those to reduce the number of babies born preterm. The survival and growth patterns of preterm or growth-restricted newborn babies are not well described in LMICs and the contribution to mortality of non-LBW babies (≥2500 g) who are preterm or those with IUGR in such settings is unknown.
Few studies in LMICs have investigated differences in mortality by extent of prematurity, IUGR, or the two in combination,3,4 or mortality risk in infants who are SGA stratified by gestational age.5-10 Examination of the mortality risk by degree of prematurity and SGA as a proxy for IUGR might be crucial in understanding the attributable disease burden, especially because regions such as south Asia have a reported SGA prevalence of about 40%.11,12 Such mortality risk estimates and attributable burden could enable the specific targeting of these disorders with appropriate interventions to more effectively save lives.
The Child Health Epidemiology Reference Group (CHERG) previously examined the risk of infant mortality associated with term-LBW as a proxy for IUGR.13 However, term-LBW excludes growth-restricted infants weighing more than 2500 g and high risk infants born both preterm and SGA, and such associations between mortality and SGA-non-LBW or SGA-preterm have not been well described in LMICs. With more population-based studies in LMICs now collecting data for gestational age in addition to birthweight, the CHERG identified an opportunity to assess the mortality risk of SGA and preterm on early neonatal, late neonatal, neonatal, post-neonatal, and infant mortality.
Methods
Dataset identification
We searched Medline, WHO regional databases (African Index Medicus, LILAS, EMRO), bibliographies of sentinel articles and reviews, and grey literature to identify potential datasets from low-income and middle-income countries that recorded data for gestational age and birthweight, and systematically recorded vital status from delivery through at least 28 days of life. The most recent search was done on Feb 22, 2010. We applied no no date or language restrictions. Search terms included “preterm or SGA”, “neonatal or infant mortality”, and “developing country” (see appendix for detailed search terms). CHERG investigators also identified additional datasets that were not retrieved in our search. We excluded datasets for the following reasons: greater than 25% missing data for birthweight or gestational age, or loss to follow-up; measured weight only after the first week of life; did not have systematic follow-up of vital status in the first month of life; determined gestational age in months or by fundal height; or when we deemed gestational age determination inaccurate or poorly linked to birthweight (appendix). We aimed to include only datasets that were population-based, representing all deliveries arising from specific geographical or catchment areas, whether home-based or facility-based. We excluded stillbirths from the analyses of these cohorts. We approached principal investigators to do a set of standard analyses themselves, or to share their data with the CHERG working group to do the analyses. Datasets that were shared with the working group did not contain personal identifiers and were therefore deemed exempt by the Johns Hopkins Bloomberg School of Public Health institutional review board. Datasets analysed by the original study investigators were covered under existing institutional review board approvals.
Exposure definitions
If more than one gestational age measurement was available we used estimates in the following order of preference: ultrasound, best obstetric estimate (combination of ultrasound, date of last menstrual period, and neonatal clinical exam), last menstrual period, or clinical examination of the infant within 72 h of birth. Prematurity was defined as a gestational age of less than 37 completed weeks. We categorised prematurity into less than 32 weeks (early preterm), 32 weeks to less than 34 weeks (moderate preterm), and 34 weeks to less than 37 weeks (late preterm).14
Weight was analysed if measured within 72 h. SGA was defined as birthweight below the tenth percentile of a standard optimal reference population for a given gestational age and sex. Appropriate-for-gestational-age (AGA) was defined as above the tenth percentile. WHO previously recommended data in a study by Williams and colleagues (collected in California from 1972 to 1976) as the reference.15 We chose the more recent and commonly cited Alexander reference,16 which includes 3 134 879 nationally representative, multi-ethnic births in the USA in 1991. Because that dataset provided data at only the tenth percentile, we identified another dataset (Oken and colleagues17) based on US data from 1999–2000 (6 690 717 births) that provided Z scores. We created two categories of SGA using a combination of these two reference populations:16,17 the tenth percentile of Alexander16 to the third percentile of Oken and colleagues,17 and the lowest third percentile of Oken and colleagues. AGA included large-for-gestational-age (LGA) infants (≥90% birth weight for gestational age). However, in these datasets, the prevalence of LGA was very small and their inclusion in the reference population likely had very little effect on mortality risk.
We created four mutually exclusive exposures to capture interaction between preterm (<37 weeks) and SGA (<10%): term and appropriate-for-gestational-age (as reference), term-SGA, preterm and appropriate-for-gestational-age, and preterm and SGA. We defined mortality as early neonatal (birth to 7 days), late neonatal (8–28 days), neonatal (birth to 28 days), post-neonatal (29–365 days), and infant (birth to 365 days) mortality. For late neonatal and post-neonatal infant mortality, the denominators were infants alive at the start of the interval of interest with vital status available through the end of the interval.
Analysis of individual datasets
The same analysis was done on each dataset. We used an algorithm developed by Alexander and colleagues16 to exclude infants with incompatible birthweight-gestational age combinations. Gestational ages of less than 24 weeks and more than 48 weeks were also excluded. We calculated the prevalence of LBW, preterm, SGA, and the overlap of these disorders for each dataset. We calculated relative risks (RRs) and risk differences (RDs) for early, late, neonatal, post-neonatal, and infant mortality associated with preterm and SGA categories.
RRs were adjusted for all confounders available in each dataset (including occupation of head of household, land ownership, years of maternal and paternal education or literacy, and maternal age and parity). We did adjusted analyses on 13 of the 20 datasets. Regression coefficients for the primary associations of interest were attenuated at less than 10% in all datasets except for one,32 for which the parameters were not statistically significant.
Missing exposure data
All studies had few missing gestational age data (<10%). Analyses of mortality risk by preterm categories used all available gestational age data, irrespective of missing birthweight (full gestational age cohort). There were two main reasons for missing birthweight data (most of which were from home deliveries): some infants who died soon after delivery were not weighed, and infants who were weighed were measured at different times after delivery. For risk estimates associated with SGA, we included infants with weights taken within 72 h of birth (weighed cohort). For four Asian and two African studies with missing data for birthweight or that measured some weights after 72 h, we used multiple imputation18 to impute birthweights (appendix). The imputed datasets were used to estimate the RRs and RDs.
Pooled analysis
Because multivariate adjustment did not substantially modify estimates of associations, we used the crude RRs and 95% CIs, and pooled data for the major UN Millennium Development Goal regions (Asia, Africa, and Latin America). We estimated overall and regional RRs of mortality for each exposure variable (preterm, SGA, and combinations). We used Stata (version 11) for all statistical analyses. Random effects models used the DerSimonian-Laird pooled RRs and 95% CIs, given between-study heterogeneity.19
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to most of the datasets, had access to all summary estimates from each dataset for meta-analysis, and had final responsibility for the decision to submit for publication.
Results
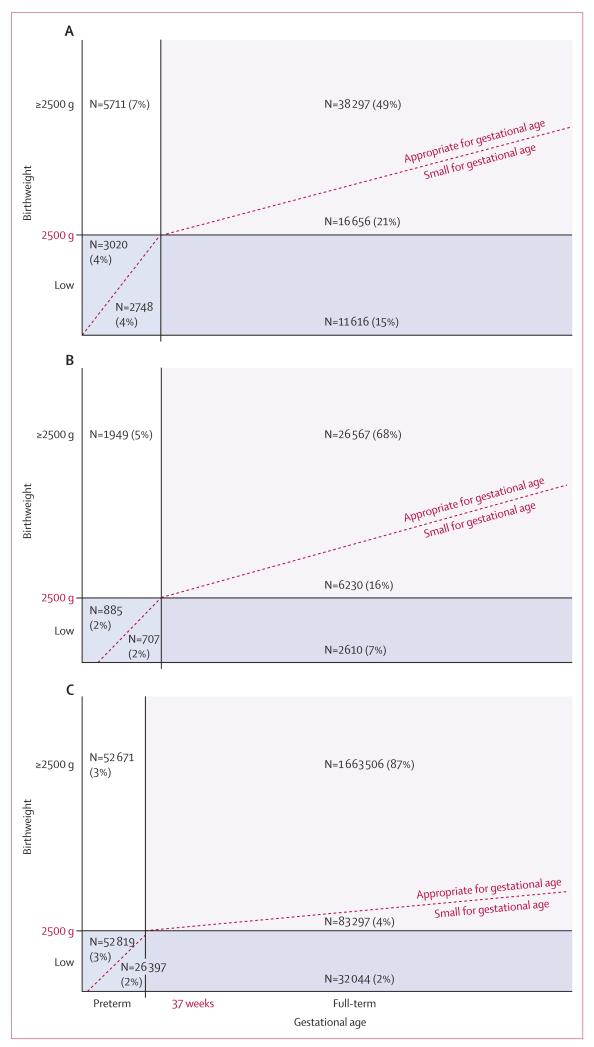
We included 20 datasets with 2 015 019 livebirths from sub-Saharan Africa, Latin America, and south and southeast Asia, with gestational age available for 2 008 675 babies and both gestational age and birthweight available for 1 996 763 babies (table and appendix).4,20-40 Study dates ranged from 1982 through to 2010. The Chilean national birth registry35 provided much of these data. The prevalence of preterm birth (<37 weeks) in the study datasets ranged from 2·7% to 28%, with varying methods of gestational age determination (see appendix for specific study methods). The proportions of preterm births before 32 weeks of gestation ranged from 0% to 4% (appendix). Comparison of preterm prevalence by method of assessment was not possible because few studies used more than one method. The prevalence of SGA was generally higher than that of preterm births, ranging from 7% in the Chile national registry to 62% in a community-based trial in south India (appendix). The proportion of LBW infants who were SGA or preterm varied by region (figure 1). In the Asian cohorts, 83% of LBW infants were SGA and 33% were preterm (67% of LBW were term and SGA). In the African cohorts, 79% of LBW infants were SGA and 38% were preterm (62% of LBW were term and SGA). In the Latin American cohorts, 53% of LBW infants were SGA and 71% were preterm (29% of LBW were term and SGA). South Asia had the highest prevalence of preterm births and number of LBW and SGA infants. A substantial proportion of SGA infants did not have LBW in Asia (54%), Africa (65%), and Latin America (59%). The proportion of children who were both SGA and preterm was small in these datasets (4% in Asia, 1% in Africa, and 2% in Latin America). In Africa and Asia, term infants were more often SGA than were preterm infants, but the opposite was true in Latin America (figure 1).
Table. Study characteristics of 20 included datasets.
| Setting | Primary study design | Study population | N (original cohort) |
N (analysed cohort for preterm/for SGA) |
NMR (deaths per 1000 livebirths) |
IMR (deaths per 1000 livebirths) |
Systematic follow-up period |
% LBW |
% preterm |
% SGA |
% facility delivery |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asia | ||||||||||||
|
| ||||||||||||
| Bangladesh (2005)21 |
Rural Sylhet | Cluster RCT of community sepsis treatment |
Population-based recruitment of all pregnant women in study area |
10 585 | 10 585/10 550* | 31 | · · | 1 month | 30% | 19% | 50% | 6% |
| India (2000)26 |
Rural Tamil Nadu |
RCT of newborn vitamin A supplementation |
Population-based recruitment of all pregnant women in study area |
13 294 | 12 693/12 693* | 38 | · · | 6 months | 33% | 14% | 62% | 63% |
| Nepal (1999)22 |
Rural Sarlahi | Cluster RCT of multiple micronutrient supplementation |
Recruitment of all pregnant women in study area |
4130 | 4122/4094* | 42 | 67 | 1 year | 39% | 22% | 56% | 6% |
| Nepal (2003)25 |
Peri-urban/rural Dhanusha |
RCT of antenatal micronutrient supplementation |
Antenatal clinic- based recruitment of pregnant women in study area |
1106 | 1106/1052 | 25 | · · | 1 month | 22% | 7% | 53% | 53% |
| Nepal (2004)29 |
Rural Sarlahi | Cluster RCT of newborn skin-umbilical cord cleansing with chlorhexidine |
Population-based recruitment of all pregnant women in study area |
23 662 | 23 650/22 723* | 32 | · · | 6 months | 30% | 18% | 52% | 10% |
| Pakistan (2003)39 |
Rural Sindh | Cluster RCT of maternal micronutrient supplementation |
Population-based recruitment of all pregnant women in study villages |
1548 | 1464/1434 | 18 | · · | 1 month | 19% | 28% | 28% | 100% |
| Philippines (1983)20 |
Urban Cebu | Longitudinal health- nutritional survey of infant feeding patterns |
Population-based, random cluster sample of census |
3080 | 3050/2785 | 14 | 36 | 2 years | 11% | 18% | 25% | 34% |
| Thailand (2001)24 |
Urban Bangkok | Prospective follow-up of birth cohort |
Longitudinal birth cohort of all births in four districts |
4245 | 4032/3860 | 5 | 6 | 1 year | 8% | 9% | 22% | 99% |
|
| ||||||||||||
| Sub-Saharan Africa | ||||||||||||
|
| ||||||||||||
| Burkina Faso (2004)27 |
Rural Hounde | RCT of multiple micronutrient supplementation |
Prospective, community-based cohort |
1373 | 1311/1212* | 21 | 67 | 1 year | 17% | 16% | 35% | 77% |
| Burkina Faso (2006)36 |
Rural Hounde | RCT of maternal fortified food supplementation |
Prospective, community-based cohort |
1316 | 1261/1067 | 20 | · · | 1 month | 16% | 18% | 29% | 84% |
| South Africa (2004)34 |
Urban Soweto | RCT of birth canal and newborn skin cleansing with chlorhexidine |
Facility-based delivery, tertiary-care hospital |
8113 | 8113/8098 | 7 | · · | 1 month | 8% | 4% | 16% | 100% |
| Tanzania (1998)32 |
Rural Mwanza | Maternal syphilis treatment, observational cohort |
Facility-based recruitment; urban, antenatal clinics |
1496 | 1425/1172 | 16 | · · | 3 months | 10% | 3% | 25% | 98% |
| Tanzania (2001)23 |
Urban Dares Salaam |
RCT of mutivitamin supplementation |
Facility-based, antenatal clinics |
7752 | 7740/7557 | 28 | · · | 6 weeks | 8% | 17% | 20% | 97% |
| Tanzania (2008)38 |
Rural Korogwe | Observational malaria study |
Facility-based recruitment, antenatal clinics, community follow-up. |
915 | 820/731 | 33 | · · | 28 days | 11% | 5% | 22% | 88% |
| Uganda (2005)37 |
Rural Kabale district |
RCT intermittent preventive malaria therapy and insecticide-treated nets |
Facility-based recruitment ANC clinics; only include facility births |
1561 | 1553/1477 | 17 | · · | 1 month | 7% | 6% | 10% | 100% |
| Zimbabwe (1997)33,40 |
Urban Harare | RCT of maternal- neonatal vitamin A supplementation |
Facility-based recruitment, 14 maternity clinics and hospitals |
14 110 | 13 960/13 914 | 12† | 93 | 1 year | 14% | 8% | 33% | 100% |
|
| ||||||||||||
| Latin America | ||||||||||||
|
| ||||||||||||
| Brazil (1982)30 |
Urban Pelotas city, Rio Grande do Sul, southern Brazil |
Longitudinal birth cohort survey |
Population-based, all births in Pelotas hospitals (100% facility delivery) |
5914 | 4675/4670 | 11 | 28 | 1 year | 7% | 6% | 17% | 100% |
| Brazil (1993)31 |
Urban Pelotas city, Rio Grande do Sul, southern Brazil |
Longitudinal birth cohort survey |
Population-based, all births in Pelotas hospitals (100% facility delivery) |
5279 | 4707/4632 | 7 | 14 | 1 year | 9% | 11% | 19% | 100% |
| Brazil (2004)28 |
Urban Pelotas city, Rio Grande do Sul, southern Brazil |
Longitudinal birth cohort survey |
Population-based, all births in Pelotas hospitals (100% facility delivery) |
4287 | 3903/3837 | 10 | 17 | 1 year | 11% | 16% | 15% | 100% |
| Chile (2000)35 |
Chilean national birth registry |
Birth registry | Population-based registry |
1 901 611 | 1 89 8 250/ 1 898 250 |
5 | · · | 1 month | 6% | 7% | 7% | 98% |
RCT=randomised controlled trial. SGA=small for gestational age. NMR=neonatal mortality rate. IMR=infant mortality rate. LBW=low birthweight.
Weights imputed for datasets that met criteria described in appendix.
Enrolment of newborn babies occurred up to 96 h after birth, and the study might have missed neonatal deaths that happened before enrolment.
Figure 1. The relation between birthweight and gestational age in Asia (A), Africa (B), and Latin America (C).
For Asian cohorts (A), percentages are for 78 048 infants. For African cohorts (B), percentages are for 38 948 infants. For Latin American cohorts (C), percentages are for 1 910 734 infants.
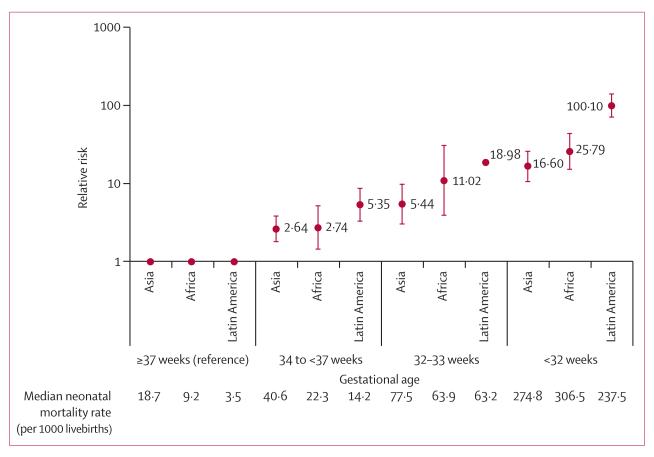
Neonatal mortality rates and relative risks increased with decreasing gestational age across studies and regions (figure 2). Although the highest relative risks (RRs) were seen in Latin America (mainly due to the very low mortality in the reference group), the risk differences were similar across regions. The overall pooled RRs across all regions were 6.82 (3·56–13·07) for neonatal mortality and 2·50 (1·48-4·22) for post-neonatal mortality. The overall pooled RRs across all regions for late preterm (34 weeks to <37 weeks), when most preterm births occur, were 3·05 (2·02–4·60; figure 2 and appendix). The RDs per 1000 livebirths for late preterm ranged from nine (95% CI 0–18) in Africa to 18 (9–28) in Asia, with an overall RD per 1000 livebirths of 13 (9–18; appendix). The overall pooled RRs for early preterm (<32 weeks) were 28·82 (15·51-53·56; figure 2 and appendix), although the RDs per 1000 livebirths ranged from 196 (95–297) in Asia to 350 (189–511) in Latin America, with an overall RD of 245 per 1000 livebirths (192–297; appendix). Early and late neonatal and post-neonatal infant mortality followed similar patterns, but the RRs were more attenuated the longer the follow-up period extended (appendix), except for early preterm in Latin America, when mortality risk decreased substantially across the early-post-neonatal to late-post-neonatal periods. However, a statistically significantly increased mortality risk persisted into the post-neonatal infant period in all regions for preterm compared to term.
Figure 2. Relative risk of neonatal mortality associated with gestational age.
Error bars are 95% CI.
Imputation of missing birthweights did not alter the prevalence of SGA, which was already high, although it did increase the risk of mortality associated with SGA (figure 3 and appendix). Neonatal mortality rates in creased with severity of SGA (figure 3). The overall pooled RRs for SGA across all regions were 1·83 (1·34–2·50) for neonatal mortality and 1·90 (1·32–2·73) for post-neonatal mortality. The highest RR for SGA in neonates below the third percentile was in Latin America, compared with 1·91 (1·40–2·60) in Asia, although the CIs overlapped. The overall RR was 2·41 (1·66–3·50). Regional pooled RDs per 1000 livebirths for SGA below the third percentile ranged from 12 (10–15) in Asia to 23 (17–29) in Latin America, with an overall RD per 1000 livebirths of 14 (9–19; appendix). The RRs associated with SGA were of similar magnitude in the early-neonatal, late-neonatal, and post-neonatal periods across regions (appendix). The RRs associated with SGA were overall of smaller magnitude than preterm, and the association with increased mortality did not attenuate beyond the neonatal period and persisted through the first year of life.
Figure 3. Relative risk of neonatal mortality associated with small for gestational age.
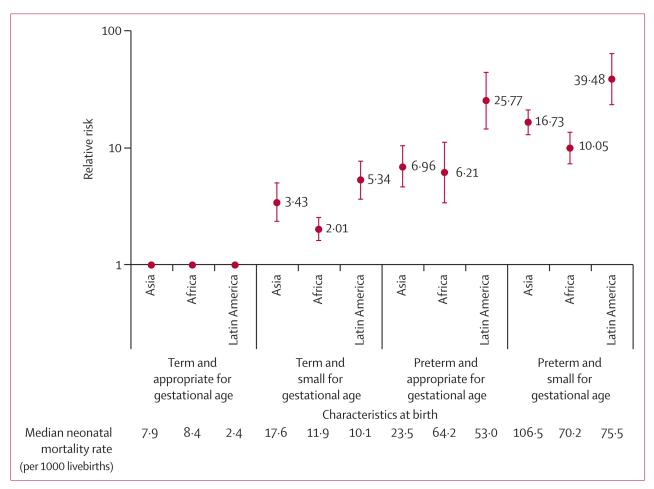
Relative to term and AGA, the increased risk of neonatal mortality was lowest in those born term-SGA (overall RR 2·44, 1·67–3·57) and highest in preterm-SGA infants (15·42, 9·11–26·12). RRs were similar for Asia and Africa, but higher in Latin America (figure 4). These higher RRs in Latin America were probably driven by the low mortality rate (2·4 per 1000) in the reference group. Pooled RDs were similar across regions (appendix). The RRs for term-SGA babies did not vary by timing of mortality (figure 5). For preterm-AGA and preterm-SGA babies, RRs were highest in the early neonatal period and lowest in the post-neonatal period, although the CIs largely overlapped (ie, no statistically significant between-group differences; figure 5 and appendix). This pattern of attenuated risk by length of follow-up was probably driven by prematurity and not SGA.
Figure 4. Relative risk of neonatal mortality associated with preterm and size for gestational age.
Figure 5. Relative risk of early-neonatal, late-neonatal, and post-neonatal infant mortality associated with preterm and size for gestational age.
Discussion
We identified a large percentage of infants who were SGA but not LBW or preterm (21% in Asia, 16% in Africa, and 4% in Latin America), although their mortality rates were lower than preterm infants or SGA-LBW infants. Although most LBW was in term and SGA babies in Asia and Africa, the majority of babies with LBW in Latin America were preterm. Preterm mortality risk associations were generally higher at all gestational age categories (late, moderate, and early preterm) than SGA (3% to <10% or <3%). However, the attributable mortality risk for SGA infants is substantial, because many infants in LMICs are born SGA, especially in south Asia. The predominant increased mortality risk associated with preterm birth occurred in the first week of life, with statistically significant but attenuated risk remaining in the late-neonatal and post-neonatal periods. Although CIs overlapped, mortality risks associated with SGA were slightly higher in the late than early neonatal period, with persistent risk in the post-neonatal period.
The highest neonatal and infant mortality rates were detected in the Asian and African studies. The highest RRs for all exposures were seen in Latin America, although absolute risk differences were more comparable across regions. This higher RR in Latin America is due to very low mortality in the reference group in Latin America compared with Africa and Asia. In particular, the Latin America analysis was dominated by national registry data from Chile in which term neonatal mortality was 1·7 per 1000 livebirths. By comparison with these data, the average term neonatal mortality was 11 per 1000 livebirths in the African datasets and 19 per 1000 livebirths in the Asian ones. Alternately, preterm infants might be more severely preterm and survive delivery in Latin America but have delayed mortality.
Preterm birth was associated with an increased mortality risk compared with term birth. The proportion of infants born within 32 weeks of gestation was low but these infants had substantially higher mortality risks than did term infants in the first week of life. Late preterm birth comprised 50–96% of preterm births, and was associated with a smaller but statistically significant neonatal mortality risk ranging from 2·52 in Asia to 5·58 in Latin America (risk differences per thousand livebirths were nine in Africa, 11 in Latin America, and 18 in Asia). Evidence-based, low-cost interventions are feasible for LMICs and could reduce mortality related to preterm birth complications, such as antenatal corticosteroids for preterm labour, Kangaroo mother care, and treatment of neonatal infections.41-43 Our findings suggest that simple interventions to target the improved care of late and moderate preterm infants could have a large effect on the reduction of mortality burden in preterm infants. In areas with a large proportion of facility deliveries and much post-delivery care, clinical interventions such as surfactant administration and continuous positive airway pressure might also improve survival.
Using a common US birthweight reference population, the prevalence of SGA and term-SGA babies was very high (higher than 50%) in many of the community-based South Asian cohorts.11,12 In statistical modelling to estimate national and regional prevalences of SGA, we estimated that 42% of babies were term-SGA and that 3% of babies were preterm-SGA.2 These findings contrast with a much lower prevalence of SGA in Africa, despite the high prevalence of risk factors such as malaria and HIV infection in pregnancy. SGA neonates had roughly double the mortality risk of appropriate-for-gestational-age infants, with slightly higher risk in those with more severe SGA. A large proportion of SGA infants weighed 2500 g or more, although their mortality risk was similar to that of term-AGA babies. The very high rates of SGA in South Asia might be explained by higher rates of adolescent pregnancy, chronic maternal malnutrition, low pre-pregnancy body-mass index, low weight gain in pregnancy, and low maternal height.44-46
The mortality risk associated with being preterm-SGA was substantially higher than for either alone. An estimated 2·8 million infants are born both preterm and SGA in LMICs annually2 and these infants are at a 10–40 times increased risk of dying in the first month of life compared with term and appropriate-for-gestational-age infants. These children are key targets for public health interventions.
A strength of this analysis was the large number of representative livebirths analysed from a wide range of studies and geographical regions, producing internal validity and reduced variance of estimates. We adjusted for several covariates but noted that they did not alter the risk estimates. Although the cohort sizes varied substantially, with the Chilean national registry data dominating in absolute numbers, the use of random effects models to do meta-analyses downweights the effect of such large datasets. Although it constitutes 92% of the data, its contribution to the Latin American mortality risk estimate is about 50% and its contribution to the global estimate is 9·7%. While Chile is proably a good representation of a middle-income country in Latin America, it does not represent other low-income countries in the region. The earliest Brazilian cohort30 might be more representative of such countries because it was done in a low-income setting.
We re-analysed individual data to estimate SGA prevalence and risk, applying a common SGA reference population. This reduced the variation associated with the use of different SGA reference populations commonly seen across studies. Although the use of a common reference population is a controversial issue, with arguments made about whether it is appropriate to apply one standard to all populations, we selected one reference population to be able to better compare our analyses across populations. Our use of a birthweight rather than fetal growth standard might have systematically under-represented SGA in preterm infants because preterm infants might have more pathological IUGR than fetuses that remain in the womb for longer. The Intergrowth Study is collecting data for common fetal growth reference using healthy populations from many countries. The use of this standard will help resolve this limitation in the future.
The large sample size allowed us to stratify preterm and SGA into severity categories and to pool mortality risk associated with each category by re-analysing primary data. Available covariates varied by study and residual con founding could have occurred. However, these findings might not be generalisable to countries or regions as a whole because they are derived from cohorts or randomised trials in which the geographical areas might have been selected for specific risk characteristics as evidenced by the range of preterm prevalence across studies (table). However, the use of meta-analyses with random effects of 20 cohorts probably reduced the bias and increased the precision of regional and global risk estimates. Although the prevalence of preterm and SGA births seemed quite variable across these datasets, the mortality differences associated with these characteristics were stable within regions. Hence these estimates are likely to be valid for use in attributable risk calculations if representative estimates of preterm and SGA prevalence are used and residual confounding was minimal.
A limitation of our community-based datasets was the exclusion of early deaths in infants who were not weighed. We used multiple imputation to address the potential effect on mortality associations. Nevertheless, we might have underestimated the mortality risks associated with SGA given that we were missing some covariate data with which to impute birthweight. Another limitation is the variability and accuracy of gestational age measurement between datasets. Nine studies included ultrasound dating, whereas the remainder relied on maternal recall of their last menstrual period or clinical exam. We used the best gestational age determination, excluding datapoints with improbable gestational age and birthweight combinations and datasets with poor gestational age measures. Gestational age categories might have been subject to some misclassification although these were probably minimised by the active, frequent pregnancy surveillance in many studies. Stillbirths were excluded from these analyses, but it is possible that there was misclassification of stillbirths and livebirths, especially in home deliveries that relied on maternal report, but the direction of this misclassification is unclear. Although assessment of cause of death by adverse pregnancy outcome is of interest, very few studies had cause of death, and most population-based studies that did, had cause assigned by physician via verbal autopsy. Assessing causes of death associated with SGA or preterm in these contexts would be a valuable future contribution to the design of appropriate mortality reduction interventions.
Many babies are born SGA in LMICs, especially in south Asia and sub-Saharan Africa, and such babies have increased risk of mortality and poor growth.47 Preterm birth affects a smaller number of neonates but is associated with a higher mortality risk and seems to be a greater contributor to attributable risk in Latin America than in Asia. In both these regions, these risks extend beyond the neonatal period. Although few effective interventions to prevent preterm exist,48 several interventions exist to improve preterm survival. Inter ventions shown to reduce SGA have focused mainly on increasing calories and protein during pregnancy, and more recently (during the past decade) maternal micro nutrient supplementation.48 As measurement of gestational age improves in LMICs, targeting interventions and tracking outcomes that reduce the incidence and improve the survival of babies born preterm and SGA rather than with a LBW might more clearly guide progress towards the reduction of child mortality.
Supplementary Material
Acknowledgments
Funding was provided by the Bill & Melinda Gates Foundation (810-2054) by a grant to the US Fund for UNICEF to support the activities of the Child Health Epidemiology Reference Group. Financial support for analysis was offered to investigators through a subcontract mechanism administered by the US Fund for UNICEF. For funding information for the individuals studies please see appendix. We thank the additional members of the CHERG SGA-Preterm Birth working group: Siân Clarke, Simon Kariuki, John Lusingu, James Ndirangu, Marie-Louise Newell, Robert Ntozini, Heather Rosen, and Feiko O Ter Kuile. The individual studies would like to acknowledge the role played by following people in those studies: Ramesh Adhikari, Christian Coles, Anthony Costello, Gary Darmstadt, Sheela Devi, Subarna Khatry, Hermann Lanou, Steven LeClerq, Dharma Manandhar, Daniel Minja, Gernard I Msamanga, R D Thulasiraj, Willy Urassa, Helen Weiss, and Keith West. We would like to thank Joanna Schellenberg for her support of this work.
Footnotes
Conflicts of interest
We declare that we have no conflict of interest.
See Online for appendix
For The Intergrowth Study see http://www.intergrowth21.org.uk
Contributor Information
Prof Joanne Katz, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD USA.
Anne CC Lee, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD USA; Brigham and Women’s Hospital, Boston, MA, USA.
Naoko Kozuki, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD USA.
Prof Joy E Lawn, Saving Newborn Lives and Save the Children USA, Washington, DC, USA; Maternal Reproductive and Child Health Centre, London School of Hygiene and Tropical Medicine, London, UK.
Prof Simon Cousens, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, UK.
Hannah Blencowe, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, UK.
Prof Majid Ezzati, MRC-HPA Centre for Environment and Health, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK.
Prof Zulfiqar A Bhutta, Division of Women and Child Health, Aga Khan University, Karachi, Pakistan.
Tanya Marchant, Maternal Reproductive and Child Health Centre, London School of Hygiene and Tropical Medicine, London, UK; Faculty of Infectious Disease and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, UK; Malaria Centre, London School of Hygiene and Tropical Medicine, London, UK.
Barbara A Willey, Maternal Reproductive and Child Health Centre, London School of Hygiene and Tropical Medicine, London, UK; Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, UK; Malaria Centre, London School of Hygiene and Tropical Medicine, London, UK.
Prof Linda Adair, University of North Carolina School of Public Health, NC, USA.
Prof Fernando Barros, Programa de Pós-graduacao em Epidemiologia, Universidade Federal de Pelotas, Pelotas, RS, Brazil; Programa de Pós-graduação em Saúde e Comportamento, Univertsidade Católica de Pelotas, Centro, Pelotas, RS, Brazil.
Prof Abdullah H Baqui, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD USA.
Prof Parul Christian, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD USA.
Prof Wafaie Fawzi, Department of Nutrition, Harvard School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA; Department of Global Health and Population, Harvard School of Public Health, Boston, MA, USA.
Prof Rogelio Gonzalez, Pontificia Universidad Católica de Chile, School of Medicine, Santiago, Chile; Clínica Santa María, Santiago, Chile.
Prof Jean Humphrey, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD USA; Zvitambo, Borrowdale, Harare, Zimbabwe.
Lieven Huybregts, Department of Food Safety and Food Quality, Ghent University, Ghent, Belgium; Woman and Child Health Research Center, Department of Public Health, Institute of Tropical Medicine, Antwerpen, Belgium.
Prof Patrick Kolsteren, Department of Food Safety and Food Quality, Ghent University, Ghent, Belgium; Woman and Child Health Research Center, Department of Public Health, Institute of Tropical Medicine, Antwerpen, Belgium.
Aroonsri Mongkolchati, ASEAN Institute for Health Development, Mahidol University, Nakhon Pathom, Thailand.
Luke C Mullany, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD USA.
Richard Ndyomugyenyi, Vector Control Division, Ministry of Health, Kampala Uganda.
Jyh Kae Nien, Fetal Maternal Medicine Unit, Clinica, Davila, Santiago, Chile; Faculty of Medicine, Universidad de Los Andes, Santiago, Chile.
David Osrin, Institute for Global Health, UCL Institute of Child Health, London, UK.
Dominique Roberfroid, Woman and Child Health Research Center, Department of Public Health, Institute of Tropical Medicine, Antwerpen, Belgium.
Ayesha Sania, Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA.
Christentze Schmiegelow, Centre for Medical Parasitology, Institute of International Health, Immunology, and Microbiology, University of Copenhagen and Department of Infectious Diseases, Copenhagen University Hospital, Copenhagen, Denmark.
Mariangela F Silveira, Programa de Pós-graduacao em Epidemiologia, Universidade Federal de Pelotas, Pelotas, RS, Brazil.
Prof James Tielsch, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD USA; Department of Global Health, George Washington School of Public Health and Health Services, George Washington University, Washington, DC, USA.
Anjana Vaidya, Institute for Global Health, UCL Institute of Child Health, London, UK.
Sithembiso C Velaphi, Department of Paediatrics, Division of Neonatology, Chris Hani Baragwaneth Hospital, University of Witwatersrand, Soweto, South Africa.
Prof Cesar G Victora, Programa de Pós-graduacao em Epidemiologia, Universidade Federal de Pelotas, Pelotas, RS, Brazil.
Deborah Watson-Jones, Malaria Centre, London School of Hygiene and Tropical Medicine, London, UK; Mwanza Intervention Trial Unit, National Institutes of Medical Research, Mwanza, Tanzania.
Prof Robert E Black, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD USA.
References
- 1.UNICEF. WHO. Low birthweight: country, regional and global estimates. New York: 2004. [Google Scholar]
- 2.Lee ACC, Katz J, Blencowe H, et al. Born too small: national and regional estimates of term and preterm small-for-gestational-age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. doi: 10.1016/S2214-109X(13)70006-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer MS, Demissie K, Yang H, Platt RW, Sauvé R, Liston R, the Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System The contribution of mild and moderate preterm birth to infant mortality. JAMA. 2000;284:843–49. doi: 10.1001/jama.284.7.843. [DOI] [PubMed] [Google Scholar]
- 4.Marchant T, Willey B, Katz J, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. 2012;9:e1001292. doi: 10.1371/journal.pmed.1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narchi H, Skinner A, Williams B. Small for gestational age neonates—are we missing some by only using standard population growth standards and does it matter? J Matern Fetal Neonatal Med. 2010;23:48–54. doi: 10.3109/14767050903067352. [DOI] [PubMed] [Google Scholar]
- 6.Clausson B, Cnattingius S, Axelsson O. Preterm and term births of small for gestational age infants: a population-based study of risk factors among nulliparous women. Br J Obstet Gynaecol. 1998;105:1011–17. doi: 10.1111/j.1471-0528.1998.tb10266.x. [DOI] [PubMed] [Google Scholar]
- 7.Kristensen S, Salihu HM, Keith LG, Kirby RS, Fowler KB, Pass MA. SGA subtypes and mortality risk among singleton births. Early Hum Dev. 2007;83:99–105. doi: 10.1016/j.earlhumdev.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Pulver LS, Guest-Warnick G, Stoddard GJ, Byington CL, Young PC. Weight for gestational age affects the mortality of late preterm infants. Pediatrics. 2009;123:e1072–77. doi: 10.1542/peds.2008-3288. [DOI] [PubMed] [Google Scholar]
- 9.Regev RH, Reichman B. Prematurity and intrauterine growth retardation—double jeopardy? Clin Perinatol. 2004;31:453–73. doi: 10.1016/j.clp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Grisaru-Granovsky S, Reichman B, Lerner-Geva L, et al. Mortality and morbidity in preterm small-for-gestational-age infants: a population-based study. Am J Obstet Gynecol. 2012;206:150–e1-7. doi: 10.1016/j.ajog.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Wu LA, Katz J, Mullany LC, et al. The association of preterm birth and small birthweight for gestational age on childhood disability screening using the Ten Questions Plus tool in rural Sarlahi district, southern Nepal. Child Care Health Dev. 2012;38:332–40. doi: 10.1111/j.1365-2214.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 12.Osendarp SJ, van Raaij JM, Arifeen SE, Wahed M, Baqui AH, Fuchs GJ. A randomized, placebo-controlled trial of the effect of zinc supplementation during pregnancy on pregnancy outcome in Bangladeshi urban poor. Am J Clin Nutr. 2000;71:114–19. doi: 10.1093/ajcn/71.1.114. [DOI] [PubMed] [Google Scholar]
- 13.Black RE, Allen LH, Bhutta ZA, et al. the Maternal and Child Undernutrition Study Group Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 14.Barfield WD, Lee KG. [accessed July 5, 2012];Late preterm infants. 2012 http://www.uptodate.com/contents/late-preterm-infants.
- 15.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol. 1982;59:624–32. [PubMed] [Google Scholar]
- 16.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–68. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 17.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–89. [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Adair LS. Low birth weight and intrauterine growth retardation in Filipino infants. Pediatrics. 1989;84:613–22. [PubMed] [Google Scholar]
- 21.Baqui AH, El-Arifeen S, Darmstadt GL, et al. the Projahnmo Study Group Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet. 2008;371:1936–44. doi: 10.1016/S0140-6736(08)60835-1. [DOI] [PubMed] [Google Scholar]
- 22.Christian P, West KP, Khatry SK, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. Am J Clin Nutr. 2003;78:1194–202. doi: 10.1093/ajcn/78.6.1194. [DOI] [PubMed] [Google Scholar]
- 23.Fawzi WW, Msamanga GI, Urassa W, et al. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med. 2007;356:1423–31. doi: 10.1056/NEJMoa064868. [DOI] [PubMed] [Google Scholar]
- 24.Isaranurug S, Mo-suwan L, Choprapawon C. A population-based cohort study of effect of maternal risk factors on low birthweight in Thailand. J Med Assoc Thai. 2007;90:2559–64. [PubMed] [Google Scholar]
- 25.Osrin D, Vaidya A, Shrestha Y, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet. 2005;365:955–62. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 26.Rahmathullah L, Tielsch JM, Thulasiraj RD, et al. Impact of supplementing newborn infants with vitamin A on early infant mortality: community based randomised trial in southern India. BMJ. 2003;327:254. doi: 10.1136/bmj.327.7409.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberfroid D, Huybregts L, Lanou H, et al. the MISAME Study Group Effects of maternal multiple micronutrient supplementation on fetal growth: a double-blind randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2008;88:1330–40. doi: 10.3945/ajcn.2008.26296. [DOI] [PubMed] [Google Scholar]
- 28.Santos IS, Barros AJ, Matijasevich A, Domingues MR, Barros FC, Victora CG. Cohort profile: the 2004 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2011;40:1461–68. doi: 10.1093/ije/dyq130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tielsch JM, Darmstadt GL, Mullany LC, et al. Impact of newborn skin-cleansing with chlorhexidine on neonatal mortality in southern Nepal: a community-based, cluster-randomized trial. Pediatrics. 2007;119:e330–40. doi: 10.1542/peds.2006-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Victora CG, Barros FC. Cohort profile: the 1982 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2006;35:237–42. doi: 10.1093/ije/dyi290. [DOI] [PubMed] [Google Scholar]
- 31.Victora CG, Hallal PC, Araújo CL, Menezes AM, Wells JC, Barros FC. Cohort profile: the 1993 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2008;37:704–09. doi: 10.1093/ije/dym177. [DOI] [PubMed] [Google Scholar]
- 32.Watson-Jones D, Changalucha J, Gumodoka B, et al. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J Infect Dis. 2002;186:940–47. doi: 10.1086/342952. [DOI] [PubMed] [Google Scholar]
- 33.Malaba LC, Iliff PJ, Nathoo KJ, et al. the ZVITAMBO Study Group Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am J Clin Nutr. 2005;81:454–60. doi: 10.1093/ajcn.81.2.454. [DOI] [PubMed] [Google Scholar]
- 34.Cutland CL, Madhi SA, Zell ER, et al. the PoPS Trial Team Chlorhexidine maternal-vaginal and neonate body wipes in sepsis and vertical transmission of pathogenic bacteria in South Africa: a randomised, controlled trial. Lancet. 2009;374:1909–16. doi: 10.1016/S0140-6736(09)61339-8. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez R, Merialdi M, Lincetto O, et al. Reduction in neonatal mortality in Chile between 1990 and 2000. Pediatrics. 2006;117:e949–54. doi: 10.1542/peds.2005-2354. [DOI] [PubMed] [Google Scholar]
- 36.Huybregts L, Roberfroid D, Lanou H, et al. Prenatal food supplementation fortified with multiple micronutrients increases birth length: a randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2009;90:1593–600. doi: 10.3945/ajcn.2009.28253. [DOI] [PubMed] [Google Scholar]
- 37.Ndyomugyenyi R, Clarke SE, Hutchison CL, Hansen KS, Magnussen P. Efficacy of malaria prevention during pregnancy in an area of low and unstable transmission: an individually-randomised placebo-controlled trial using intermittent preventive treatment and insecticide-treated nets in the Kabale Highlands, southwestern Uganda. Trans R Soc Trop Med Hyg. 2011;105:607–16. doi: 10.1016/j.trstmh.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmiegelow C, Minja D, Oesterholt M, et al. Factors associated with and causes of perinatal mortality in northeastern Tanzania. Acta Obstet Gynecol Scand. 2012;91:1061–68. doi: 10.1111/j.1600-0412.2012.01478.x. [DOI] [PubMed] [Google Scholar]
- 39.Bhutta ZA, Rizvi A, Raza F, et al. A comparative evaluation of multiple micronutrient and iron-folic acid supplementation during pregnancy in Pakistan: impact on pregnancy outcomes. Food Nutr Bull. 2009;30(suppl):S496–505. doi: 10.1177/15648265090304S404. [DOI] [PubMed] [Google Scholar]
- 40.Humphrey JH, Hargrove JW, Malaba LC, et al. the ZVITAMBO Study Group HIV incidence among post-partum women in Zimbabwe: risk factors and the effect of vitamin A supplementation. AIDS. 2006;20:1437–46. doi: 10.1097/01.aids.0000233578.72091.09. [DOI] [PubMed] [Google Scholar]
- 41.Lawn JE, Mwansa-Kambafwile J, Barros FC, Horta BL, Cousens S. ‘Kangaroo mother care’ to prevent neonatal deaths due to pre-term birth complications. Int J Epidemiol. 2010;39(suppl 1):144–54. doi: 10.1093/ije/dyq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mwansa-Kambafwile J, Cousens S, Hansen T, Lawn JE. Antenatal steroids in preterm labour for the prevention of neonatal deaths due to complications of preterm birth. Int J Epidemiol. 2010;39(suppl 1):i122–33. doi: 10.1093/ije/dyq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.March of Dimes; The Partnership of Maternal, Newborn, and Child Health; Save the Children; World Health Organization. Born too soon: the global action report on preterm birth. Geneva: 2012. [Google Scholar]
- 44.Mehra S, Agrawal D. Adolescent health determinants for pregnancy and child health outcomes among the urban poor. Indian Pediatr. 2004;41:137–45. [PubMed] [Google Scholar]
- 45.Bott SJS, Shah I, Puri C, editors. Towards adulthood: exploring the sexual and reproductive health of adolescents in South Asia. World Health Organization; Geneva: 2000. [Google Scholar]
- 46.Monden CW, Smits J. Maternal height and child mortality in 42 developing countries. Am J Hum Biol. 2009;21:305–11. doi: 10.1002/ajhb.20860. [DOI] [PubMed] [Google Scholar]
- 47.Black RE, Victora CG, Walker SP, Maternal and Child Nutrition Study Group Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013 doi: 10.1016/S0140-6736(13)60937-X. published online June 6. http://dx.doi.org/10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 48.Bhutta ZA, Das JK, Rizvi A, et al. The Lancet Interventions Review Group. the Maternal and Child Nutrition Study Group Evidence based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013 doi: 10.1016/S0140-6736(13)60996-4. published online June 6. http://dx.doi.org/10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.