Abstract
Purpose
The strategy of definitive chemoradiation with selective surgical salvage in locoregionally advanced esophageal cancer was evaluated in a Phase II trial in Radiation Therapy Oncology Group (RTOG)-affiliated sites.
Methods and Materials
The study was designed to detect an improvement in 1-year survival from 60% to 77.5% (α= 0.05; power = 80%). Definitive chemoradiation involved induction chemotherapy with 5-fluorouracil (5-FU) (650 mg/mg2/day), cisplatin (15 mg/mg2/day), and paclitaxel (200 mg/mg2/day) for two cycles, followed by concurrent chemoradiation with 50.4 Gy (1.8 Gy/fraction) and daily 5-FU (300 mg/mg2/day) with cisplatin (15 mg/mg2/day) over the first 5 days. Salvage surgical resection was considered for patients with residual or recurrent esophageal cancer who did not have systemic disease.
Results
Forty-three patients with nonmetastatic resectable esophageal cancer were entered from Sept 2003 to March 2006. Forty-one patients were eligible for analysis. Clinical stage was ≥T3 in 31 patients (76%) and N1 in 29 patients (71%), with adenocarcinoma histology in 30 patients (73%). Thirty-seven patients (90%) completed induction chemotherapy followed by concurrent chemoradiation. Twenty-eight patients (68%) experienced Grade 3+ nonhematologic toxicity. Four treatment-related deaths were noted. Twenty-one patients underwent surgery following definitive chemoradiation because of residual (17 patients) or recurrent (3 patients) esophageal cancer,and 1 patient because of choice. Median follow-up of live patients was 22 months, with an estimated 1-year survival of 71%.
Conclusions
In this Phase II trial (RTOG 0246) evaluating selective surgical salvage after definitive chemoradiation in locoregionally advanced esophageal cancer, the hypothesized 1-year RTOG survival rate (77.5%) was not achieved (1 year, 71%; 95% confidence interval< 54%–82%).
Keywords: Esophageal cancer, Chemotherapy, Chemoradiation, Radiation therapy, Salvage surgery
INTRODUCTION
The role of surgery in the treatment of locoregionally advanced, nonmetastatic (>T1N0M0) esophageal cancer has not been clearly defined. Some groups have advocated a predominantly nonsurgical approach with concurrent chemoradiation (cisplatin and 5-fluorouracil [5-FU] and 50.4 Gy of radiation therapy; Radiation Therapy Oncology Group [RTOG] 85-01, 5 year survival, 18%) (1). Even though the nonoperative approach of definitive chemoradiotherapy allows organ preservation in many patients, this strategy is also associated with a high rate of locally persistent or recurrent disease (≥50%) (1–4).
Another means of addressing residual or recurrent locoregional esophageal tumor after definitive chemoradiotherapy is selective surgical resection. This strategy was used successfully on an ad hoc basis in RTOG-85-01 and theoretically allows the opportunity to improve locoregional control while reserving surgical resection only for patients with residual or recurrent locoregional disease (1). The primary goal of this trial (RTOG 0246) was to assess the 1-year survival of patients treated with this selective strategy. Other goals included the feasibility of definitive chemoradiation with selective surgical salvage and evaluation of disease-free survival.
METHODS AND MATERIALS
Patient eligibility
Patients with biopsy-proven primary squamous cell or adenocarcinoma of the esophagus or gastroesophageal junction (no tumor extension beyond 2 cm into the stomach) were eligible. Adequate bone marrow, liver, and renal functions were required as well as a Zubrod performance status of 0 or 1. Patients were required to have resectable nonmetastatic locoregionally advanced carcinoma of the esophagus greater than T1N0. All patients were required to be able to tolerate surgery as determined by a panel of medical, radiation, and surgical oncologists. Celiac adenopathy (≤2 cm) or palpable supraclavicular nodes were required to have biopsy proof of “ no cancer” prior to study entry. Patients with lack of comprehension of the protocol or inability to comply with the requirements of the study were also ineligible.
Pretreatment evaluation
All patients had a complete history and physical examination and nutritional assessment and were evaluated by a medical, surgical, and radiation oncologists prior to study entry for protocol eligibility. Computed tomography (CT) of the chest and abdomen was required as well as upper gastrointestinal endoscopy (EGD) and endoscopic ultrasonography (EUS). Bronchoscopy was performed for tumors <25 cm from the incisors, and positron emission tomography (PET) was optional but strongly encouraged. All patients signed an approved informed consent, and institutional review boards of the participating institutions approved the protocol prior to patient recruitment.
Therapy
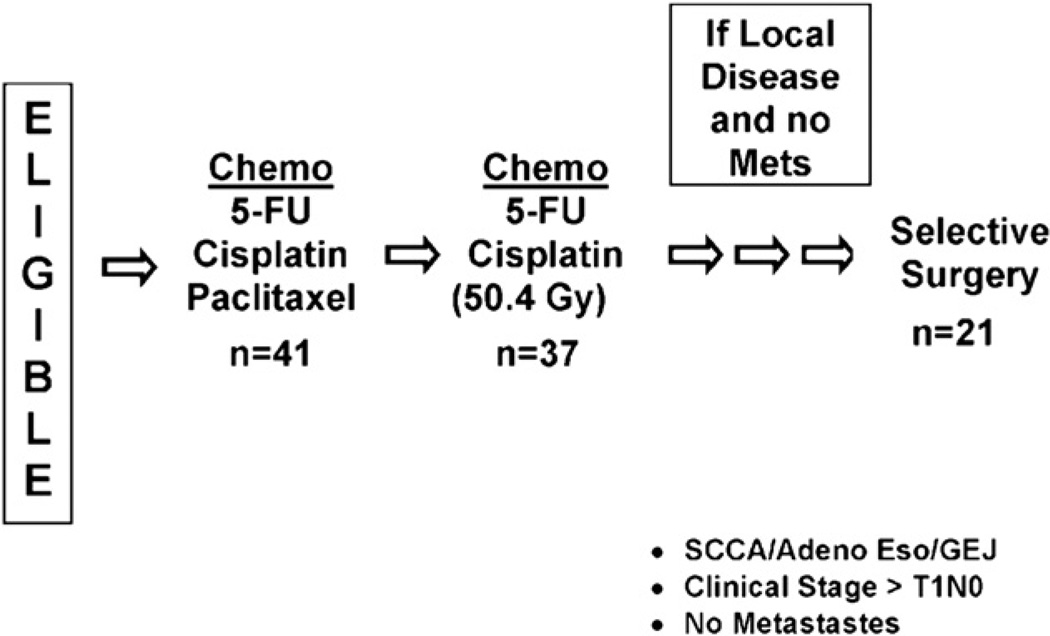
The treatment scheme is shown in Fig. 1. Patients received two cycles of induction chemotherapy prior to concurrent chemoradiotherapy. Following completion of chemoradiation, patients were assessed for residual cancer with EGD, EUS, and CT of the chest and abdomen, with PET scan optional but strongly encouraged. Clinical suspicion even without pathologic proof of residual locoregional cancer without systemic disease allowed consideration of the patient for selective salvage resection (residual disease). If there was no clinical suspicion of residual cancer, patients were followed closely (twice every 3 months, then three times every 6 months, and then yearly) with serial EGD, EUS, CT scans of the chest and abdomen and PET scan (optional but strongly encouraged).
Fig. 1.
The RTOG 0246 treatment scheme is shown.
Induction chemotherapy
Chemotherapy consisted of a combination of 5-FU, cisplatin, and paclitaxel. 5-Fluorouracil was administered at 650 mg/m2/day as continuous infusion on Days 1 to 5 and 29 to 33. Cisplatin was administered at 15 mg/m2/day as a 1-h infusion on Days 1 to 5 and 29 to 33. Paclitaxel was administered at 200 mg/m2/day as a 2-h infusion on Days 1 and 29. PEG-G-CSF (6 mg administered subcutaneously on Days 6 and 34) or G-CSF (300 or 480 µg on Days 6–15 and 34–42) was also administered.
Chemoradiotherapy
Upon completion of induction chemotherapy, patients began receiving concurrent chemoradiotherapy on Day 58 of the protocol. A total radiotherapy dose of 50.4 Gy was delivered by external beam radiation. The daily fraction size was 1.8 Gy, and 28 fractions were delivered 5 days/week. Patients underwent simulation by standard methods utilizing EGD, esophagography, and CT information to determine the exact boundaries of the carcinoma. Three-dimensional conformal radiotherapyor intensity-modulated radiotherapy was optional but not mandatory for this study, to allow inclusion of all RTOG sites. The superior and inferior borders of the field were 3 cm beyond the edges of the grossly visible carcinoma, based on preinduction chemotherapy simulation esophagram films. The lateral borders of the field were 2 cm beyond the edges of the grossly visible tumor, as defined by EUS, esophagram, or CT scan. The involved periesophageal nodes were included, and every effort was made to reduce exposure to lungs, heart, spinal cord, kidney, and liver.
The concurrent outpatient chemotherapy regimen consisted of 5-FU and cisplatin. Cisplatin was administered at 15 mg/m2/day as a 1-h infusion on Days 1 to 5 of radiotherapy administration. Patients received premedication and hydration before cisplatin. 5-Fluorouracil was administered at 300 mg/m2/day as a continuous infusion for 5 days per week during radiotherapy. Dose modifications (only reductions) were based on guidelines already established. Chemotherapy and acute radiotherapy toxicities as defined by National Cancer Institute Common Toxicity Criteria version 2.0 (http://ctep.info.nih.gov/) were recorded. Late radiation therapy effects (>90 days from the start of radiotherapy) were recorded by scored using the RTOG/European Organization for Research and Treatment of Cancer Late Radiation Morbidity Scoring Schema (http://www.rtog.org/).
Follow-up evaluations
A history and physical examination, serum chemistry profile, CT scan of the chest and abdomen, endoscopic biopsy, and EUS and PET scan (optional but encouraged) were performed 6 to 8 weeks following the completion of chemoradiotherapy and serially thereafter until disease progression (every 3 months for 2 years, and every 6 months for 2 years, then yearly).
Selective surgical resection
Patients were evaluated in a planned fashion with EUS, EGD, CTof the chest and abdomen, and optional but encouraged PET scan after definitive chemoradiation for evidence of residual esophageal tumor at locoregional and distant sites (6–8 weeks after, then every 3 months for 2 years, then every 6 months for 3 years, then yearly). If the patient’s condition was clinically suspicious for “residual” at initial clinical evaluation immediately after completion of chemoradiation or “recurrent” locoregional esophageal tumor on subsequent follow-up evaluations (pathologic proof not required) without systemic disease, then the patient was eligible for selective salvage esophageal resection as determined by the medical, surgical, and radiation oncologists. The type of surgery was chosen at the discretion of the treating surgeon according to the location and extent of the primary tumor. Operative procedures included Ivor-Lewis esophagectomy (abdominal-right thoracic approach), three-field or McKeown esophagectomy (right thoracic abdominal-cervical approach), or transhiatal esophagectomy (abdominal-cervical approach). Complete intrathoracic and abdominal nodal staging of all stations including 7, 8, 9, 15, 16 and 17 was encouraged. Overlying pleura and adjacent soft tissues were included when feasible to ensure an adequate radial margin. Nutritional support was emphasized throughout chemoradiation, and a jejunostomy tube was placed for postoperative nutritional support.
Statistical analysis
The primary endpoint of the study was 1-year overall survival for all patients eligible for analysis. Secondary endpoints included disease-free survival (failure included local, regional, and distant failure, as well as death due to any cause) and feasibility of a non-operative approach with induction chemotherapy, concurrent chemoradiation, and selective surgical salvage. On the basis of a 1-year survival rate of 60% from the RTOG esophageal database, it was decided that a 1-year survival rate of 77.5% or better was needed for the trial to be deemed promising enough for study in a Phase III protocol (≈hazard reduction of 50% with type I error of 0.05 and type II error of 0.20). Adjusting this figure by 10% to account for patient ineligibility or loss, a total sample size of 42 patients was estimated to be required for this study. Failure of overall survival was death as a result of any cause, and time to overall survival was measured from date of study entry to date of death. Overall survival rates were estimated univariately with the Kaplan-Meier method (5).
RESULTS
Eighteen RTOG institutions accrued 43 patients between September 2003 and March 2006. Of the 43 patients entered, 41 patients were eligible. The 2 patients excluded were ineligible because 1 patient never had a bronchoscopy performed, and the histology was unable to be verified for the other patient.
Patient characteristics
The pretreatment characteristics are listed in Table 1 and demonstrate a predominantly male population (n = 34, 83%) with adenocarcinoma histology (n = 30, 73%). EUS demonstrated the tumors were predominantly clinical stage T3/T4 (n = 31, 76%) and N1 (n = 29, 71%).
Table 1.
Pretreatment characteristics
| Characteristic | No. of eligible patients (n = 41) |
% of total |
|---|---|---|
| Age | ||
| Median | 59 | |
| Minimum–maximum | 42–81 | |
| Gender | ||
| Male | 34 | 83 |
| Female | 7 | 17 |
| Zubrod performance status | ||
| 0 | 21 | 51 |
| 1 | 20 | 49 |
| Distance from incisor to proximal tumor margin | ||
| <25cm | 5 | 12 |
| ≥25cm | 36 | 88 |
| Histology | ||
| Squamous | 11 | 27 |
| Adenocarcinoma | 30 | 73 |
| T stage* | ||
| T1 | 1 | 2 |
| T2 | 9 | 22 |
| T3 | 29 | 71 |
| T4 | 2 | 5 |
| N stage* | ||
| N0 | 12 | 29 |
| N1 | 29 | 71 |
Clinical stage was determined prior to chemoradiation and/or surgery.
Treatment characteristics
As Fig. 1 shows, 41 patients were treated with induction chemotherapy. Chemotherapy reviews were carried out per protocol for all patients for the induction phase. Thirty-seven patients were treated with concurrent chemoradiation, of which all 37 patient had per-protocol chemotherapy and radiotherapy reviews. At the completion of chemoradiation, 23 patients (56%) did not undergo esophageal resection, whereas 18 patients (44%) underwent selective surgical resection. Table 2 shows the reasons surgical resection was not performed. Seventeen patients underwent selective surgical resection because of clinical suspicion of residual locoregional cancer and 1 patient because of patient request. Seventeen of the 18 patients (94%) demonstrated residual cancer in their resected pathologic specimens. The 1 patient who did not have residual cancer was the patient whose condition was not clinically suspicious for cancer but who requested surgery. Three additional patients underwent esophageal resection 5 to 15 months following the completion of chemoradiotherapy because of suspicion of recurrent locoregional esophageal cancer during surveillance. All 3 patients demonstrated esophageal cancer in their resected specimens.
Table 2.
Reasons for no surgery immediatelyafterchemoradiation (n = 23*)
| Reason | No. of patients (n =23)* |
% of total |
|---|---|---|
| No clinical suspicion of residual disease | 14 | 61 |
| Distant metastases | 3 | 13 |
| Patient medically inoperable | 1 | 4 |
| Patient died† | 5 | 22 |
This number includes 3 patients who eventually underwent surgery for recurrence.
Three patients died from treatment-related causes, and 2 patients died from tumor progression.
Toxicity of treatment
The incidence of Grade 3 or higher treatment-related toxicities is listed in Tables 3 and 4. There were a total of four treatment-related deaths among the 41 patients (9.8%). Two patients died during or immediately after induction chemotherapy (1 patient from pneumonia, and 1 patient from multiorgan failure from progressive upper extremity thrombosis from a central line). One patient died 179 days after the start of concurrent chemoradiation from pneumonitis, and 1 of the 21 patients (4.8%) who underwent surgery died from complications associated with an esophageal anastomotic leak. Other than the patient with the Grade 5 anastomotic leak reported above, 1 additional patient experienced a Grade 3 anastomotic leak into the chest cavity 7 days after surgery.
Table 3.
Incidence of chemotherapy, surgery, and acute radiotherapy toxicity
| No. of instances (% of total [n = 41]) per grade | |||||
|---|---|---|---|---|---|
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Allergy/immunology | 1 | 2 | 0 | 0 | 0 |
| Auditory/hearing | 0 | 1 | 0 | 0 | 0 |
| Blood/bone marrow | 16 | 14 | 9 | 1 | 0 |
| Cardiovascular (arrhythmia) | 2 | 0 | 1 | 0 | 0 |
| Cardiovascular (general) | 6 | 3 | 2 | 1 | 1* |
| Constitutional symptoms | 7 | 28 | 4 | 0 | 0 |
| Dermatology/skin | 4 | 24 | 2 | 0 | 0 |
| Gastrointestinal | 1 | 20 | 17 | 2 | 1† |
| Hemorrhage | 2 | 0 | 0 | 0 | 0 |
| Hepatic | 7 | 8 | 3 | 0 | 0 |
| Infection/febrile neutropenia | 1 | 6 | 2 | 1 | 0 |
| Lymphatics | 0 | 1 | 0 | 0 | 0 |
| Metabolic/laboratory | 19 | 12 | 6 | 0 | 0 |
| Musculoskeletal | 1 | 1 | 0 | 0 | 0 |
| Neurology | 13 | 14 | 3 | 0 | 0 |
| Ocular/visual | 1 | 0 | 0 | 0 | 0 |
| Pain | 5 | 16 | 8 | 0 | 0 |
| Pulmonary | 7 | 6 | 2 | 2 | 2‡ |
| Renal/genitourinary | 7 | 5 | 1 | 0 | 0 |
| Worst nonhematologic | 0 (0) | 13 (32) | 21 (51) | 3 (7) | 4 (10) |
| Worst overall | 0 (0) | 12 (29) | 21 (51) | 4 (10) | 4 (10) |
Patient died 27 days after the start of induction chemotherapy of multiorgan failure from progressive upper extremity thrombosis from a central line.
Patient died from an esophageal leak 24 days after surgery.
Both patients died from pneumonitis; 1patient died 57 days after the start of induction chemotherapy (no chemoradiotherapy or surgery), and the other patient died 179 days after the start of chemoradiotherapy (no surgery).
Table 4.
Late radiotherapy toxicity†
| No. of instances (% of total [n = 40]) per grade | |||||
|---|---|---|---|---|---|
| Site | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Esophagus | 3 | 1 | 0 | 0 | 0 |
| Lung | 2 | 0 | 0 | 0 | 1* |
| Other | 1 | 0 | 0 | 0 | 0 |
| Worst overall | 6 (15) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
The same patient reported in Table 4 who died from pneumonitis; toxicity was attributed to both chemotherapy and late radiotherapy.
>90 days from the start of radiotherapy.
Survival
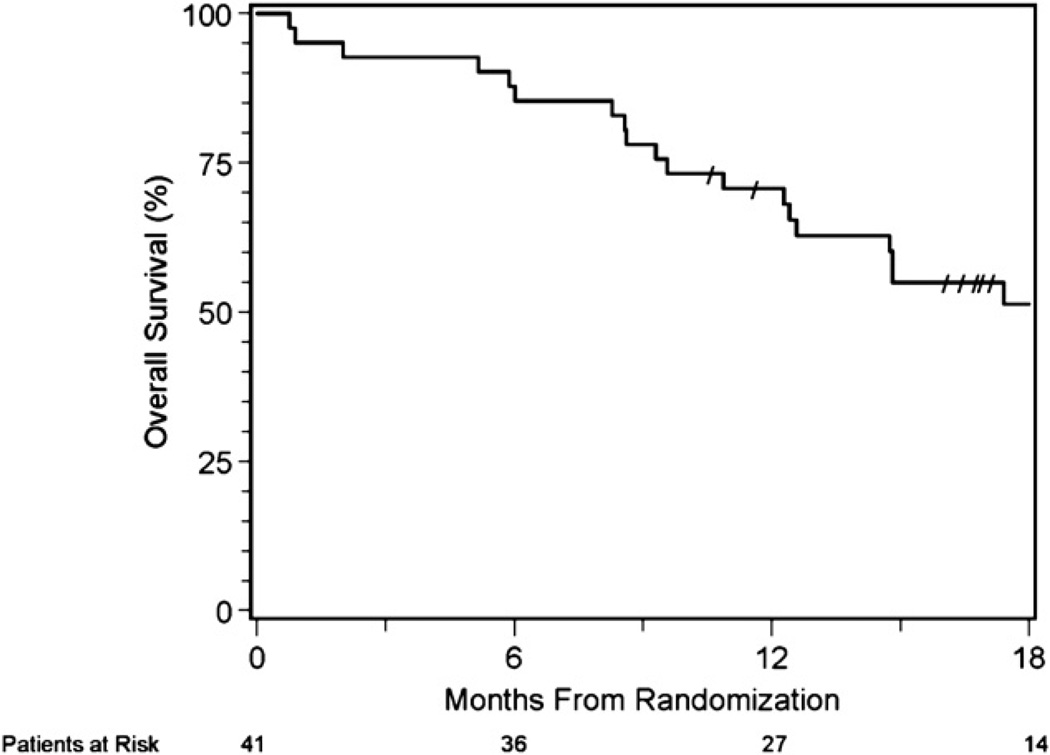
The median follow-up time was 16.1 months (0.76 months minimum; 35.1 months maximum) for all patients and 22 months (10.6 months minimum; 35.1 months maximum) for the 21 patients who are still alive. The estimated 1-year survival for all evaluable patients (n = 41) is 71% (95% confidence interval [CI], 54%–82%) (Fig. 2). The hypothesized 1-year survival rate of 77.5% or better was not achieved. At the time of this analysis, 21 patients remained alive (51%), of whom 13 patients received chemoradiation and selective surgery, and 8 patients received chemoradiation alone. Thirteen patients remain alive and free of disease (5 patients after chemoradiation alone and 8 patients after chemoradiation and selective salvage resection). The 1-year survival rate for the 15 patients who underwent surgery after chemoradiation for residual disease is 83% (95% CI,56%–94%).
Fig. 2.
Overall survival is shown for the 41 eligible patients.
DISCUSSION
RTOG 85-01 demonstrated the superiority of concurrent chemoradiation (cisplatin, 5-FU, and 50.4 Gy) over radiotherapy alone (50.4 Gy) in treating locoregionally advanced esophageal cancer (1, 4). RTOG 85-01 also demonstrated that long-term survival (5 years, 18%) could be achieved in a population with predominantly squamous cell carcinoma with an initially nonoperative approach (1). The high rate of locoregional failure (52%) led to several subsequent trials. Intergroup (INT) 0122 attempted to improve locoregional control through the addition of three cycles of induction chemotherapy (5-FU, cisplatin) and intensification of radiotherapy (5-FU, cisplatin, and 64.8 Gy) (2). Unfortunately, 6 patients (13%) died during treatment, and locoregional control was not improved. In an effort to reduce toxicity, RTOG 94-05 abandoned the induction chemotherapy and compared a higher radiation dose (5-FU, cisplatin, 64.8 Gy) with the standard radiation dose (5-FU, cisplatin, 50.4 Gy) (3). The high-dose arm demonstrated increased treatment-related mortality (12% vs. 2%, respectively) without any improvement in locoregional failure (56% vs. 52%, respectively), leading to the lower dose being accepted as the RTOG standard (5-FU, cisplatin, 50.4 Gy).
This trial (RTOG 0246) evaluated the organ-preserving strategy of selective surgery following definitive chemoradiation. Because of encouraging Phase II safety and activity data at a single institution utilizing paclitaxel, the definitive chemoradiation regimen consisted of two cycles of induction chemotherapy (cisplatin, 5-FU, and paclitaxel) prior to concurrent chemoradiation (5-FU, cisplatin, and 50.4 Gy) (6). Treatment-related toxicity in this multicenter trial was high during induction chemotherapy, with two treatment-related deaths either during (1 death from progressive upper extremity thrombosis) or immediately after (1 death from pneumonia) induction chemotherapy. As INT 0122 suggested, induction chemotherapy prior to concurrent chemoradiation may be too difficult to deliver in a multi-institutional setting (2). RTOG 0113 also recently reported a high number of treatment-related deaths (six deaths, 8.3%) in 72 locoregionally advanced esophageal cancer patients treated with induction chemotherapy followed by concurrent chemoradiation (7). Concurrent chemoradiation alone without prior induction chemotherapy has been utilized in two randomized trials with predominantly squamous cell carcinoma populations, comparing concurrent chemoradiation alone versus concurrent chemoradiation and surgery (8, 9). In those studies, treatment-related mortality was significantly higher on the trimodality arm than on the chemoradiation-alone arm (9.3% vs. 0.8% [9], respectively; 12% vs. 1% [8], respectively) but was due in large part to increased perioperative mortality. Selective esophageal resection in our study, however, was not associated with increased operative mortality, with only one death (4.8%) occurring because of complications following an anastomotic leak. Furthermore, there were no deaths among the 3 patients undergoing salvage esophageal resection for recurrent esophageal cancer 5 to 15 months after the completion of chemoradiotherapy, despite the higher operative mortality reported by some studies with salvage esophagectomy (10). The strategy of selective esophageal resection for treating residual or recurrent locoregional esophageal cancer may therefore be feasible in the future as an organ-preserving nonoperative strategy, especially if induction chemotherapy is avoided.
One of the difficulties of a nonoperative approach with selective surgical resection is identifying patients with microscopic residual disease after definitive chemoradiation. Several studies have suggested that endoscopic mucosal biopsies of the primary tumor after chemoradiation are not reliable for detecting residual disease, with over 75% of the negative mucosal biopsies demonstrating residual esophageal cancer at resection (11). Other studies have suggested that EUS and CT scans also have difficulty distinguishing residual cancer from fibrosis following chemoradiation (12, 13). Even PET scans are plagued with false negatives because of microscopic disease and false positives because of esophageal ulceration (14, 15). Because of these difficulties, RTOG 0246 recommended reassessment of the patient following definitive chemoradiation by a panel of medical, radiation, and surgical oncologists, with selective surgical resection allowed even if there was only clinical suspicion but no pathologic proof of residual or recurrent disease. Despite these liberal criteria, all but 1 of the patients who were taken to surgery for suspected residual or recurrent disease in RTOG 0246 were found to have tumor in the resected pathologic specimens. Following chemoradiation, there are indeed probably two subsets of esophageal cancer patients who are eligible to be considered for selective esophageal resection: those patients who have “residual” localized cancer evident immediately after treatment and those patients who have developed “recurrent” localized disease found on subsequent follow-up. Unfortunately, there are not enough patients in this small single-arm study to clearly delineate the differences between these (residual and recurrent) groups. Of the 21 patients who underwent esophageal resection for residual or recurrent esophageal cancer, 8 patients (38%) are still alive and free of disease. Since this is a preliminary report, further follow-up will be needed to determine if any of these patients are salvaged in the long-term.
Because RTOG 0246 did not achieve the 1-year hypothesized survival rate of 77.5%, this organ-preserving treatment strategy was not moved forward as the experimental arm of a randomized trial. Nevertheless, the results from this trial are worth reviewing, as the hypothesized survival rate of 77.5% was based on prior RTOG trials in which 1-year survival was found to be 60% in a predominantly squamous cell carcinoma population. This estimation may have been overly optimistic for adenocarcinoma because a subset analysis of patients in RTOG 8501 by Cooper et al. (1) demonstrated decreased survival of adenocarcinoma patients compared to squamous cell carcinoma patients, with 3-year survival rates of 17% vs. 30%, respectively, after definitive chemoradiation. While it did not meet the protocol hypothesis for pursuing a Phase III trial, RTOG 0246 did demonstrate the feasibility of definitive chemoradiation and selective surgical resection, as 1-year survival (1 year, 71%) (Fig. 2) was comparable to that of trials with planned surgical resection and preoperative chemotherapy (e.g., RTOG 8911, 1 year, 59%) or preoperative chemoradiation (1 year, 72%) (2, 16–18). The strategy of selective surgical resection may be reasonable as an organ-preserving approach as several studies have suggested that most of the benefit of surgery may come from the treatment of clinical nonresponders (8). In fact, Stahl et al. (9) observed in their randomized study that a 3-year survival of 32% was possible with surgery in clinical nonresponders in whom an R0 resection could be achieved. This compared to a 3-year survival of 9.4% for clinical nonresponders treated with chemoradiation alone (9). Perhaps a selective surgical approach based on concurrent chemoradiation that avoids induction chemotherapy might be able to reduce treatment-related mortality and target clinical nonresponders.
CONCLUSIONS
In conclusion, RTOG 0246 demonstrates the potential of an organ-preserving strategy with definitive chemoradiation and selective surgical resection in a predominantly adenocarcinoma population. The 1-year estimated survival (71%; 95% CI,54%–82%) did not achieve the RTOG hypothesized 1-year survival (77.5%), but this may have been due in part to the higher-than-expected treatment-related mortality. Current nonsurgical treatment strategies are underway in RTOG studies evaluating the benefit of the addition of biologic agents to concurrent chemoradiation. In the future, selective surgical resection may also be able to be incorporated in these organ-preserving approaches, especially if molecular markers can help identify a subset of patients at high risk for locoregional failure and chemoradiation resistance.
Acknowledgments
We thank David P. Kelsen for technical assistance; Wanda Reese and Debbie Waits for assistance with preparing the manuscript; Jaffer A. Ajani, Kathryn Winter, and Christopher G. Willett for administrative support; Kathryn Winter and Ritsuko Komaki for collection and assembly of radiotherapy QA data; Stephen G. Swisher and Kathryn Winter for data analysis and interpretation; Stephen G. Swisher, Kathryn Winters, Ritsuko R. Komaki, Jaffer A. Ajani, Tsung T. Wu, Wayne Hofstetter, A. Konski, and Christopher G. Willett for manuscript writing; and Stephen G. Swisher, Jaffer A. Ajani, Ritsuko Komaki, Tsung T. Wu, and Christopher G. Willett for conception and design.
This work was supported by National Cancer Institute grants RTOG U10 CA21661 and CCOP U10 CA3742.
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01) JAMA. 1999;281:1623–1626. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 2.Minsky BD, Neuberg D, Kelsen DP, et al. Neoadjuvant Chemo-therapy Plus Concurrent Chemotherapy and High-Dose Radiation for Squamous Cell Carcinoma of the Esophagus: A Preliminary Analysis of the Phase II Intergroup Trial 0122. J Clin Oncol. 1996;14:149–155. doi: 10.1200/JCO.1996.14.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) Phase III Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy. J Clin Oncol. 2001;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 4.Herskovic A, Martz K, Al-Sarraf M, et al. Combined Chemotherapy and Radiotherapy Compared with Radiotherapy Alone in Patients with Cancer of the Esophagus. N Engl J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan EL, Meier P. Non-parameteric estimation from in complete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 6.Ajani JA, Komaki R, Putnam JB, et al. A Three-Step Strategy of Induction Chemotherapy Then Chemoradiation Followed by Surgery in Patients with Potentially Resectable Carcinoma of the Esophagus or Gastroesophageal Junction. Cancer. 2001;92:279–286. doi: 10.1002/1097-0142(20010715)92:2<279::aid-cncr1320>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Ajani JA, Winter K, Komaki R, et al. Phase II Randomized Trial of Two Nonoperative Regimens of Induction Chemotherapy Followed by Chemoradiation in Patients with Localized Carcinoma of the Esophagus: RTOG 0113. J Clin Oncol. 2008;26:1–6. doi: 10.1200/JCO.2008.16.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;23:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 9.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 10.Swisher SG, Wynn P, Putnam JB, et al. Salvage esophagectomy for recurrent tumors after definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg. 2001;123:175–183. doi: 10.1067/mtc.2002.119070. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Cleary KR, Yao JC, et al. Significance of post-chemo-radiation biopsy in predicting residual esophageal carcinoma in the surgical specimen. Dis Esophagus. 2004;17:38–43. doi: 10.1111/j.1442-2050.2004.00355.x. [DOI] [PubMed] [Google Scholar]
- 12.Beseth BD, Bedford R, Isacoff WH, et al. Endopscopic ultra-sound does not accurate assess pathologic stage of esophageal cancer after neoadjuvant chemoradiotherapy. Am Surg. 2000;66:827–831. [PubMed] [Google Scholar]
- 13.Jones DR, Parker LA, Detterbeck FC, et al. Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer. 1999;85:1026–1032. doi: 10.1002/(sici)1097-0142(19990301)85:5<1026::aid-cncr3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Swisher SG, Maish M, Erasmus JJ, et al. Utility of PET,CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152–1160. doi: 10.1016/j.athoracsur.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Erasmus JJ, Munden RF, Truong MT, et al. Preoperative chemo-radiation-induced ulceration in patients with esophageal cancer: a confounding factor in tumor response assessment in integrated computed tomographic-positron emission tomographic imaging. J Thorac Oncol. 2006;1:478–486. [PubMed] [Google Scholar]
- 16.Urba SG, Orringer MB, Turrisi A, et al. Randomized Trial of Preoperative Chemoradiation Versus Surgery Alone in Patients With Locoregional Esophageal Carcinoma. J Clin Oncol. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 17.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy Followed by Surgery Compared with Surgery Alone for Localized Esophageal Cancer. N Engl J Med. 1998;339:1979–1984. doi: 10.1056/NEJM199812313392704. [DOI] [PubMed] [Google Scholar]
- 18.Kelsen DP, Winter KA, Gunderson LL, et al. Long-Term Results of RTOG Trial 8911 (USA Intergroup 113): A Random Assignment Trial Comparison of Chemotherapy Followed by Surgery Compared with Surgery Alone for Esophageal Cancer. J Clin Oncol. 2007;25:3719–3725. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]