Abstract
Background
Nosocomial pneumonia is among the most common types of infection in hospitalized patients. The increasing prevalence of multi-drug resistant organisms (MDROs) in recent years points to the need for an up-to-date clinical guideline.
Methods
An interdisciplinary S3 guideline was created on the basis of a systematic literature review in the PubMed and Cochrane Library databases, with assessment and grading of the evidence according to the GRADE system.
Results: 9097 abstracts and 808 articles were screened in full text, and 22 recommendations were issued. It is recommended that any antimicrobial treatment should be preceded by a microbiological diagnostic evaluation with cultures of blood and respiratory samples. The diagnosis of nosocomial pneumonia should be suspected in any patient with a new or worsened pulmonary infiltrate who meets any two of the following three criteria: leucocyte count above 10 000 or below 4000/µL, temperature above 38.3°C, and/or the presence of purulent respiratory secretions. The initially calculated antimicrobial treatment should be begun without delay; it should be oriented to the locally prevailing resistance pattern, and its intensity should be a function of the risk of infection with MDROs. The initial treatment should be combination therapy if there is a high risk of MDRO infection and/or if the patient is in septic shock. In the new guideline, emphasis is laid on a strict de-escalation concept. In particular, antimicrobial treatment usually should not be continued for longer than eight days.
Conclusion
The new guideline’s recommendations are intended to encourage rational use of antibiotics, so that antimicrobial treatment will be highly effective while the unnecessary selection of multi-drug-resistant organisms will be avoided.
Hospital-acquired pneumonia (HAP) is one of the most frequent infections acquired by patients during a stay in hospital. By definition, HAP is pneumonia with onset no less than 48 to 72 hours after hospital admission. The category “health care associated pneumonia” (HCAP) as a subgroup of HAP in the outpatient or day patient setting has not been adopted in Europe because of doubts about its validity (1); it is not included in this guideline. Likewise, patients with a defined immune deficiency are not the subject of this guideline. Such patients exhibit a fundamentally different range of pathogens and require different diagnostic and therapeutic strategies, irrespective of where the infection was acquired (2).
The data of the German hospital infection surveillance system (KISS, Krankenhaus-Infektions-Surveillance-System) show the incidence of HAP in invasively ventilated patients to be 5.4 per 1000 ventilation days; this translates into around 15 500 cases a year in German intensive care units (3). Including HAP in noninvasively ventilated patients and patients not in intensive care units leads to a total figure of about 40 000 cases per year. Depending on ventilation status, the mortality in ICUs is about 10% to 20% (4); what proportion of overall mortality is contributed by this infection is disputed, but there is no doubt that this complication has a considerable negative impact on outcomes in intensive care. This is even more the case because of the rise in multi-drug resistant organisms (MDROs) (5, 6), which increase the risk for inadequate initial antimicrobial treatment (7, 8). In terms of management and initially calculated antimicrobial therapy for HAP, therefore, patients with risk factors for MDROs must be distinguished from those without such factors. Box 1 shows clinical risk factors for the acquisition of MDROs, while Box 2 summarizes HAP pathogens in patients with and without these risk factors. Regionally and locally (at hospital and department level) there are considerable differences in pathogen spectrum and resistance profile (9), so knowledge of the local situation is critical in the management of HAP. For this reason, institutions that treat patients with HAP should regularly collect and analyze data about pathogens and resistance so that the results can be fed into the decision making process for calculated antibiotic therapy. Ideally, these data should relate to pathogens proven to have caused HAP, or at least to those shown to be present in respiratory samples. The authors of the guideline wish further to emphasize that, where bacteria and fungi forming part of the resident oropharyngeal flora are of no relevance to the treatment of HAP, identification at the species level should not be carried out and no antibiogram should be made, so that inappropriate therapy may be avoided. Such entities include enterococci, corynebacteria, apathogenic neisseriae, α-hemolyzing streptococci, coagulase-negative staphylococci, and Candida spp.
Box 1. Risk factors for infections with multi-drug resistant organisms (MDROs)*.
Antimicrobial therapy
Hospital stay >4 days
Invasive ventilation >4 to 6 days
Care in the intensive care unit
Malnutrition
Structural lung disease
Known colonization by MDROs
Admission from long-term care, chronic dialysis, tracheostomy, open skin wounds
*according to (11)
Box 2. Spectrum of pathogens causing hospital-acquired pneumonia (HAP)*.
In patients without risk factors for multi-drug resistant organisms (MDROs):
-
Enterobacteriaceae
Escherichia coli
Klebsiella spp.
Enterobacter spp.
Haemophilus influenzae
Staphylococcus aureus (MSSA)
Streptococcus pneumoniae
In patients with risk factors for MDROs, also:
Staphylococcus aureus (MRSA)
ESBL-forming Enterobacteriaceae
Pseudomonas aeruginosa
Acinetobacter baumannii
Stenotrophomonas maltophilia
* according to (11)
MRSA, methicillin-resistant S. aureus;
MSSA, methicillin-sensitive S. aureus;
ESBL, extended-spectrum beta-lactamases
Methods
This guideline replaces the only currently available version in the German-speaking countries, dated 2003 (10). This revision was needed because of the considerable changes that have taken place in the epidemiology, pathogen spectrum, and resistance patterns. In addition, since publication of the previous version, studies on diagnosis and treatment have been published that have significance for the management of HAP.
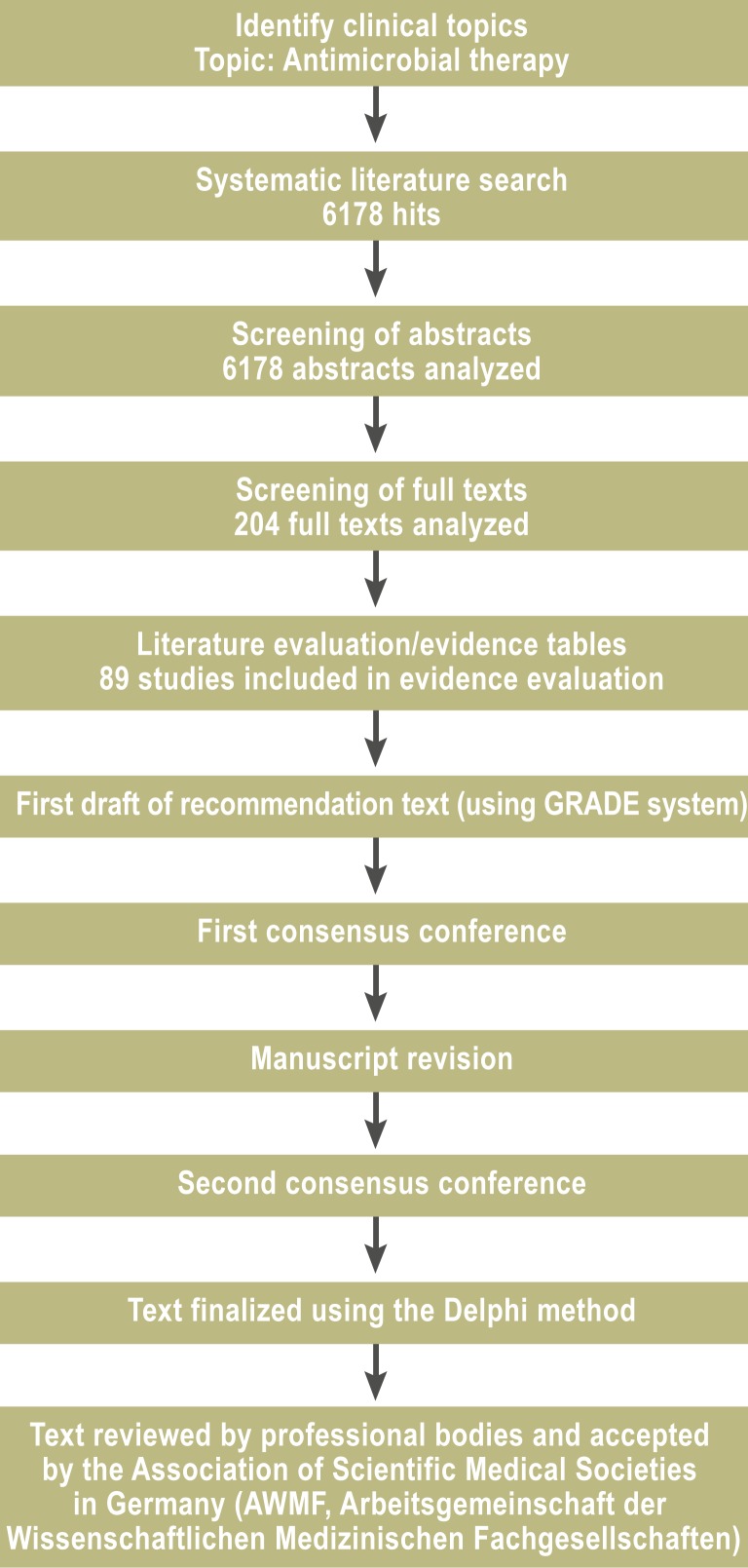
The guideline development group was made up of representatives of the participating medical societies together with the Association of Scientific Medical Societies in Germany (AWMF, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften) (Acknowledgments Box). Representatives were present from anesthesiology, internal medicine, surgery, intensive medicine, clinical infectiology, medical microbiology, hygiene, and pneumology. The guideline was produced in a two-stage process (eFigure). In the first, questions were identified that are of central importance to the management of HAP. The systematic literature search was carried out using the PubMed and Cochrane Collaboration databases, and included original articles published in German and English from 1 January 1990 to 31 December 2009. Reference lists in meta-analyses and systematic reviews were also searched. Studies published after 31 December 2009 were included if the guideline development group believed that they had significantly influenced the management of HAP; these are specially marked for identification. The literature was sifted and evaluated in several work groups, and draft recommendations were produced. The findings were discussed and reworked in two consensus conferences under the chairmanship of a representative of the AWMF, and were then accepted in a nominal group process. The recommendations were graded and evidence levels were assigned to the literature on which the guideline was based using the GRADE system (Table 1). This evaluation system includes, in addition to quality of evidence, a weighing up of benefits against risks and of the costs of the suggested therapeutic measures. The studies on which the evaluation was based are listed in evidence tables together with commentary on the evaluation (11). The formulation of the recommendations is in accordance with the National Disease Management Guideline standards (12).
eFigure.
Flow chart of the methodology followed to develop the guideline (in this case, for antimicrobial therapy)
Table 1. Quality of evidence and strength of recommendation (GRADE system)*.
| Recommendation grade | Weighing of benefit against risk/additional costs | Evidence quality |
|---|---|---|
|
“Should” or ”should not” 1 A: Strong recommendation, high-quality evidence 1 B: Strong recommendation, moderate-quality evidence 1 C: Strong recommendation, low- or very low-quality evidence |
Desired effects definitely outweigh risks/additional costs] or vice versa |
Consistent evidence from RCTs without methodological weaknesses or exceptionally strong evidence from observational studies Evidence from RCTs with methodological limitations or convincing evidence from observational studies Evidence for at least one central outcome parameter from observational studies, case series, or methodologically very limited RCTs |
|
“Should” or”should not” 2 A: Weak recommendation, high-quality evidence 2 B: Weak recommendation, moderate-quality evidence 2 C: Weak recommendation, low- or very low-quality evidence |
Desired effects probably outweigh risks/additional costs or vice versa |
Consistent evidence from RCTs without methodological weaknesses or exceptionally strong evidence from observational studies Evidence from RCTs with methodological limitations or convincing evidence from observational studies Evidence for at least one central outcome parameter from observational studies, case series, or methodologically very limited RCTs |
|
“May” or “may not” 3: No recommendation |
Inadequate evidence that benefits of the intervention outweigh risks or vice versa | No evidence for superiority/inferiority of the intervention |
RCT, randomized controlled trial * according to (11)
Results
The recommendations part of the guideline is divided into 22 separate recommendations, 10 of which relate to diagnosis and 12 to antimicrobial therapy. The background to the individual recommendations is explained in the long version (11). The present short version summarizes a series of recommendations in tabular form; issues of central importance are are commented on in this manuscript.
Clinical diagnosis, imaging
R1:How is HAP diagnosed on a clinical basis, and what should the differential diagnosis include?
Even a suspected diagnosis of HAP has implications for treatment, and should be made when a patient has new-onset or progressive infiltrate in combination with two of three further criteria:
Leukocytes >10 000 or <4000/μL
Fever >38.3 °C
Purulent secretion.
The differential diagnosis should include atelectasis, heart failure/overhydration, alveolar hemorrhage, interstitial lung disease, acute respiratory distress syndrome (ARDS), and pulmonary arterial embolism (strong recommendation, evidence level C).
R2: Which imaging procedures are indicated in the diagnostic work-up of HAP?
When HAP is suspected, a standard chest radiograph should be carried out, in two planes if possible. Immobile patients should be x-rayed supine (strong recommendation, evidence level C).
For patients with refractory infiltrates and where the differential diagnosis is proving difficult, further diagnostic imaging should be considered (weak recommendation, evidence level C).
R3: What is the role of scoring in the diagnosis and risk assessment of HAP?
Clinical diagnosis of HAP is not improved through the use of pneumonia scoring systems such as the Clinical Pulmonary Infection Score (CPIS). In patients with severe sepsis, sepsis scores should be used (strong recommendation, evidence level C).
Diagnostic laboratory and microbiological tests
The recommendations for diagnostic laboratory tests are summarized in the eTable. The important thing is to investigate respiratory samples, which should be taken before antimicrobial therapy is started. According to the findings of a recent randomized controlled trial, noninvasive diagnostic testing carried out on tracheobronchial aspirate (TBAS) obtained in a sterile manner has the same diagnostic yield as invasive diagnostic bronchoscopy using bronchoalveolar lavage (BAL) (13).
eTable. Recommendations for diagnosis of hospital-acquired pneumonia*1.
| Recommendation | Remark | GRADE | |
|---|---|---|---|
| Biomarkers (CRP, PCT) | Limited | If pneumogenic sepsis is suspected, PCT testing is indicated | 1B |
| Blood culture | Yes | Bacteremia, identification of extrapulmonary foci | 1C |
| Legionella antigen in urine | Limited | If nosocomial infection is suspected | 1C |
| Respiratory culture (TBAS; BAL) | Yes | Quantitative culture; sample must be taken before start of antibiotic treatment | 1B |
| Fungus diagnosis | Limited | Yeast fungus: no targeted diagnosis; Aspergillus: in at-risk patients*2 | 1B |
*1Source: (11)
*2Bronchiectasis, liver cirrhosis, rheumatological diseases; CRP, C-reactive protein; PCT, procalcitonin; TBAS, tracheobronchial aspirate; BAL, bronchoalveolar lavage
R5: When should blood cultures be taken?
In HAP, blood cultures should be taken to detect bacteremia. They also help to guide therapy and to reveal the existence of extrapulmonary sources of infection (strong recommendation, evidence level C).
R7: What microbiological tests should be carried out on respiratory samples?
In HAP, quantitative cultures should be obtained from good quality lower airway matter such as tracheobronchial aspirate or bronchoalveolar lavage (BAL). The resulting bacterial counts give helpful clues; however, they should not be regarded as independent predictors of the presence of pneumonia, but need to be interpreted in the light of clinical data (strong recommendation, evidence level B).
In addition, a diagnostic smear test should be done to validate the sample. The results of a Gram stain have no predictive value in terms of the species isolated later, but a negative Gram stain in patients not previously treated with antibiotics has a high negative predictive value. For this reason, a Gram stain should be carried out especially when antibiotic therapy does not appear to be indicated or is to be stopped early (weak recommendation, evidence level B).
R8: When should an invasive diagnostic technique be preferred to noninvasive sampling?
In ventilator-associated pneumonia (VAP), invasive diagnostic tests are not superior to noninvasive tests, so the decision for or against invasive diagnostic techniques should be taken depending on local logistics, or for reasons related to the differential diagnosis or any possible therapeutic aspects of endoscopic examination. Contraindications for performing bronchoscopy with BAL should be respected (strong recommendation, evidence level A).
Antimicrobial therapy
The core of the treatment section is formed by the recommendations for the choice of antimicrobial therapy, which are given below. Table 2 lists recommended drugs and dosages. These recommendations are based on randomized studies, although most of these were designed as equivalence studies aimed at the licensing of new substances. This means that in general there is no evidence of superiority, especially in terms of hard endpoints such as mortality; for the same reason, a valid assessment of effect sizes is impossible (14). Criteria for the initial use of combination therapy are defined in E14. A randomized study of the Canadian Critical Care Trial Group (15) and a meta-analysis (14) both showed that in general combination therapy is not superior to monotherapy in VAP; there was no difference in 28-day mortality between the study arms. This is the reason why initial combination therapy is restricted in recommendation E14 to patients at high risk of MDROs or those in septic shock. Regarding duration of therapy (Table 3), there is also a well designed randomized study underlying the recommendation to limit the duration of therapy as a rule to 8 days (16); exceptions to this are given in the table. Regarding targeted therapy, there are hardly any randomized studies with sufficient patient numbers; an exception is a study published in 2012 comparing linezolid and vancomycin in MRSA pneumonia, which showed a difference in response but not in mortality (17).
Table 2. Calculated antimicrobial therapy in hospital-acquired pneumonia*.
| Patients not at increased risk for multi-drug resistant organisms (MDROs) | |
| Drug | Dosage (per day) |
| Aminopenicillin/beta-lactamase inhibitor | |
| Ampicillin/sulbactam | 3 × 3 g |
| Amoxicillin/clavulanic acid | 3 × 2.2 g |
| or | |
| Cephalosporin, group 3a | |
| Ceftriaxone | 1 × 2 g |
| Cefotaxime | 3 × 2 g |
| or | |
| Carbapenem | |
| Ertapenem | 1 × 1 g |
| or | |
| Fluoroquinolone | |
| Moxifloxacin | 1 × 400 mg |
| Levofloxacin | 2 × 500 mg |
| Patients at increased risk for MDROs | |
| Drug | Dosage (per day) |
| Beta-lactam drug active against Pseudomonas spp. | |
| Piperacillin/tazobactam | 3–4 × 4.5 g |
| or | |
| Cefepime | 3 × 2 g |
| Ceftazidime | 3 × 2 g |
| or | |
| Imipenem/cilastatin | 3 × 1 g |
| Meropenem | 3 × 1 g |
| Doripenem | 3 × 0.5–1 g |
| plus | |
| Fluoroquinolone | |
| Ciprofloxacin | 3 × 400 mg |
| Levofloxacin | 2 × 500 mg |
| or | |
| Aminoglycoside | |
| Gentamicin | 1 × 3–7 mg/kg body weight (trough concentration <1 µg/ml) |
| Tobramycin | 1 × 3–7 mg/kg body weight (trough concentration <1 µg/ml) |
| Amikacin | 1 × 15–20 mg/kg body weight (trough concentration <4 µg/ml) |
| If methicillin-resistant Staphylococcus aureus is suspected | |
| plus a glycopeptide or oxazolidinone | |
| Vancomycin | 2 × 15 mg/kg body weight (trough concentration: 15–20 µg/mL) |
| Linezolid | 2 × 600 mg |
*Source: (11)
Table 3. Recommendations on course of therapy (E11, E15–18)*1.
| Recommendation | GRADE | |
|---|---|---|
| Start treatment | As soon as possible; in patients with septic shock, within 1 hour | 1B |
| End treatment early | After 3 days if the probability of HAP is low | 1B |
| De-escalation | 48 to 72 hours after the start of treatment and after re-assessment of clinical parameters, microbiology, biomarkers, X-ray | 1B |
| Duration of treatment | 8 days (may be extended in the presence of special etiologies*2) | 1A |
*1Source: (11)
*2Invasive Staphylococcus aureus infection, invasive aspergillosis, individual decision in the presence of P.aeruginosa
R12: What calculated therapy options are recommended for patients with HAP who are not at increased risk of infection with MDROs?
For patients not at increased risk of MDROs, group 3a cephalosporins, aminopenicillins/beta-lactamase inhibitors, ertapenem, or the fluoroquinolones levofloxacin and moxifloxacin, which are active against pneumococci, are among the recommended therapeutic options. The choice of drug should be made in the light of the local pathogen spectrum and resistance profile (strong recommendation, evidence level C).
R13: What calculated therapy options are recommended for patients with HAP who are at increased risk of infection with MDROs?
For patients at increased risk of MDROs, piperacillin/tazobactam or carbapenems or cephalosporins effective against Pseudomonas, initially in combination with an aminoglycoside or a fluoroquinolone effective against Pseudomonas, are among the recommended therapy options. Ceftazidime should only be used in combination with another drug that is more effective against Staphylococcus aureus. The choice of drug should be made in the light of the local pathogen spectrum and resistance profile (strong recommendation, evidence level B).
If an MRSA infection is suspected, a drug effective against MRSA should be added (strong recommendation, evidence level B).
R14: When should combination therapy be chosen?
Initial combination therapy should only be chosen for patients at increased risk of harboring multi-drug resistant gram-negative organisms or those in septic shock. After 2 to 3 days, the requirement for combination therapy should be reassessed, and if a sensitive pathogen is demonstrated, or the patient becomes stabilized, therapy should be de-escalated to monotherapy (Box 3). The choice of drug should be made in the light of the local pathogen spectrum and resistance profile (strong recommendation, evidence level B).
Box 3. De-escalating antimicrobial therapy*.
Re-evaluate after 2 to 3 days
If pathogen demonstrated, de-escalate to targeted monotherapy
Precondition: sample for microbiology (BAL, TBAS) must have been taken before start of therapy
If no pathogen demonstrated, but clinical improvement/treatment success is observed, de-escalate, usually to beta-lactam monotherapy
BAL, bronchoalveolar lavage; TBAS, tracheobronchial aspirate
*according to (11)
Recommendations on the start of therapy, de-escalation, and duration of therapy are summarized in Table 3. Special emphasis is laid on a strict de-escalation strategy, so as to limit patient exposure to antibiotics and hence reduce the selection pressure on the endogenous flora as much as possible. Therapy de-escalation should start after as little as 2 to 3 days, guided by the results of the re-assessment, so long as the patient has stabilized (Box 3). Treatment failure is not rare in HAP, occurring in 10% to 15% of cases. The guideline recommends a structured strategy for determining the reason for treatment failure, which normally includes a repeat diagnostic work-up, preferably bronchoscopic (18), to identify the etiology (eBox).
eBox. Differential diagnosis and what to do when treatment fails*.
-
If the diagnosis is correct:
Infection by bacterial or non-bacterial intrinsically resistant pathogen
Resistance developed during therapy
Antimicrobial therapy underdosed
Superinfection with a “new” pathogen
Cavitating infection or infection affecting more than one organ (e.g., lung abscess, pleural empyema)
These diagnoses can be confirmed or ruled out by appropriate microbiological diagnostic tests or by chest imaging.
-
If the diagnosis of hospital-acquired pneumonia (HAP) is incorrect:
Interstitial lung disease (e.g., cryptogenic organizing pneumonia, COP)
Drug-induced pneumonitis
Congestive heart failure
Pulmonary embolism/infarction
Alveolar hemorrhage
Aspiration syndrome
Atelectasis
Testing these diagnoses requires echocardiography, bronchoscopy with differential cytology, and/or angio-CT.
*according to (11)
Few data exist at present about ventilator-associated tracheobronchitis (VAT) and inhalational therapy of VAP, which may be considered on an individual basis as additive to systemic therapy in patients with MDRO infections. The following recommendations on this topic reflect the current state of the data:
R20: Should ventilator-associated tracheobronchitis (VAT) be treated with antimicrobial drugs?
In ventilated patients, VAT may be a risk factor for the development of ventilator-associated pneumonia (VAP). Treatment with antibiotics cannot be recommended, since not enough evidence exists to support this (no recommendation, evidence level C).
In exceptional cases, antibiotic treatment may be considered in patients at risk and/or those with MDRO colonization with increasing purulent airway secretions or recurrent respiratory infections (weak recommendation, evidence level C).
R21: When is inhalational antimicrobial treatment of VAP indicated (alone or in combination with systemic treatment)?
Inhalational antibiotic treatment cannot at present be generally recommended. In selected patients, such as those with MDROs, the addition of aerosolized colistin or tobramycin to systemic antibiotic treatment should be considered (weak recommendation, evidence level C).
The final recommendation is concerned with targeted therapy aimed at known pathogens. Detailed opinions on differential therapy are contained in the long version of the guideline (11). For the multi-drug resistant gram-negative species, in particular, the choice is very limited and the study situation unsatisfactory, so the recommendation is to plan treatment in collaboration with an infectiologist or microbiologist.
R22: What is appropriate targeted therapy for proven infections with: MRSA—Pseudomonas aeruginosa—Acinetobacter baumannii—Stenotrophomonas maltophilia—ESBL-forming enterobacteria—carbapenem-resistant enterobacteria?
In targeted therapy for HAP, the drug should be chosen in accordance with the following criteria:
MRSA strains: Tested anti-infective monotherapy drugs are vancomycin, teicoplanin, and linezolid. For very ill patients, the combination of vancomycin with rifampicin is a further option.
Pseudomonas aeruginosa: Ceftazidime, cefepim, piperacillin, doripenem, imipenem and meropenem, and ciprofloxacin and levofloxacin are effective therapeutic options. The combination of a beta-lactam antibiotic effective against Pseudomonas with an aminoglycoside (gentamicin, tobramycin, amikacin) or a fluoroquinolone effective against Pseudomonas may be considered on an individual basis in cases of severe infection. Superiority to monotherapy has not been definitely proven. If resistance is shown to all standard drugs, treatment with colistin in indicated; combination therapy should be aimed at, if possible in consultation with an infectiologist/microbiologist.
ESBL strains: Carbapenems are effective. If resistance is shown to carbapenems as well, colistin should be used, if possible as combination therapy.
Stenotrophomonas maltophilia: If in vitro sensitivity is shown, co-trimoxazole is indicated. If resistance to co-trimoxazole is shown, testing should be done for sensitivity to ceftazidime, moxifloxacin, levofloxacin, tigecycline, and ticarcillin/clavulanic acid and one of these drugs used. The clinical relevance of the isolate should be investigated first.
Acinetobacter spp.: Imipenem or meropenem are the most usually effective. In cases of pan-resistance, colistin is indicated, if possible in combination with another drug that is effective in vitro. Tigecycline is an additional option for salvage therapy.
The necessity of a general combination therapy has not been established (strong recommendation, evidence level B).
Future prospects
The guideline development group has decided not to issue recommendations on the prevention of HAP, referring readers instead to the relevant guidelines of the Robert Koch Institute (19). One central theme when dealing with hospital-acquired infections is to prevent the selection of MDROs during treatment. One of the most important tasks, therefore, is to curb the excessive use of antibiotics. Antibiotic stewardship programs are increasingly in use in German hospitals with the aim of promoting the rational use of anti-infective drugs through guidelines such as the one presented here (20). The goal is to slow the rise in infections, especially infections with gram-negative MDROs (21).
Acknowledgements. This S3 guideline was developed by the following.
Professional bodies:
German Society of Anaesthesiology and Intensive Care Medicine
German Society of Infectious Diseases
German Society for Hygiene and Microbiology
German Respiratory Society
Paul Ehrlich Society for Chemotherapy
German Surgical Society (Deutsche Gesellschaft für Chirurgie)
German Society of Internal Medicine
German Society of Internal Intensive Care and Emergency Medicine
German Sepsis Society
Robert Koch Institute
Authors
Prof. Klaus Dalhoff, Lübeck
Prof. Dr. Dr. Marianne Abele-Horn, Würzburg
Prof. Stefan Andreas, Immenhausen
Prof. Torsten T. Bauer, Berlin
Prof. Dr. Heike von Baum, Ulm
Prof. Maria Deja, Berlin
Prof. Santiago Ewig, Bochum
Prof. Petra Gastmeier, Berlin
Prof. Sören Gatermann, Bochum
Prof. Herwig Gerlach, Berlin
Prof. Beatrice Grabein, Munich
Prof. Gert Höffken, Dresden
Prof. Winfried Kern, Freiburg
Dr. Evelyn Kramme, Lübeck
Prof. Christoph Lange, Borstel
Prof. Joachim Lorenz, Lüdenscheid
Prof. Konstantin Mayer, Giessen
Prof. Irit Nachtigall, Berlin
Prof. Matthias Pletz, Jena
Prof. Gernot Rohde, Maastricht
Dr. Simone Rosseau, Berlin
Dr. Bernhard Schaaf, Dortmund
Dr. Reiner Schaumann, Berlin
Dr. Dirk Schreiter, Dresden
Dr. Hartwig Schütte, Berlin
Prof. Harald Seifert, Cologne
Dr. Helmut Sitter, Marburg
Prof. Claudia Spies, Berlin
Prof. Tobias Welte, Hannover
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Professor Dalhoff has had travel and accommodation costs reimbursed and has received fees for the preparation of scientific educational presentations from Astra- Zeneca, Bayer Vital, Novartis, Pfizer, and MSD. He has received third-party funding for his study center from Cubist, Cigma, Johnson & Johnson, and Cerexan. He has received funding from Bayer Vital for a research project initiated by himself.
Professor Ewig declares that no conflict of interest exists.
References
- 1.Ewig S, Welte T, Chastre J, et al. Rethinking the concepts of community-acquired and health-care-associated pneumonia. Lancet Infect Dis. 2010;10:279–287. doi: 10.1016/S1473-3099(10)70032-3. [DOI] [PubMed] [Google Scholar]
- 2.Dalhoff K, Marxsen J, Steinhoff J. Pneumonien bei Immunsuppression. Internist. 2007;48:507–518. doi: 10.1007/s00108-007-1838-5. [DOI] [PubMed] [Google Scholar]
- 3.Meyer E, Sohr D, Gastmeier P, et al. New identification of outliers and ventilator-associated pneumonia rates from 2005 to 2007 within the German Nosocomial Infection Surveillance System. J Hosp Infect. 2009;73:246–252. doi: 10.1016/j.jhin.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis. 2010;51:120–125. doi: 10.1086/653060. [DOI] [PubMed] [Google Scholar]
- 5.Gatermann S, Kaase M. Nachweis von Carbapenemasen 2010. Epidemiol Bull. 2011;32:301–304. [Google Scholar]
- 6.Geffers C, Gastmeier P. Nosocomial infections and multidrug resistant organisms - epidemiological data from KISS. Dtsch Arztebl Int. 2011;108:87–93. doi: 10.3238/arztebl.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhary M, Shrivastava SM, Varughese L, et al. Efficacy and safety evaluation of fixed dose combination of cefepime and amikacin in comparison with cefepime alone in treatment of nosocomial pneumonia patients. Curr Clin Pharmacol. 2008;3:118–122. doi: 10.2174/157488408784293660. [DOI] [PubMed] [Google Scholar]
- 8.Garnacho-Montero J, Sa-Borges M, Sole-Violan J, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med. 2007;35:1888–1895. doi: 10.1097/01.CCM.0000275389.31974.22. [DOI] [PubMed] [Google Scholar]
- 9.Meyer E, Schwab F, Gastmeier P. Nosocomial methicillin resistant Staphylococcus aureus pneumonia - epidemiology and trends based on data of a network of 586 German ICUs (2005-2009) Eur J Med Res. 2010;15:514–524. doi: 10.1186/2047-783X-15-12-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenz J, Bodmann KF, Bauer TT, et al. Nosocomial pneumonia: prevention, diagnosis, treatment. Pneumologie. 2003;57:532–545. doi: 10.1055/s-2003-42217. [DOI] [PubMed] [Google Scholar]
- 11.Dalhoff K, Abele-Horn M, Andreas S, et al. Epidemiologie, Diagnostik und Therapie erwachsener Patienten mit nosokomialer Pneumonie S-3 Leitlinie der Deutschen Gesellschaft für Anästhesiologie und Intensivmedizin, der Deutschen Gesellschaft für Infektiologie, der Deutschen Gesellschaft für Hygiene und Mikrobiologie, der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin e. V. und der Paul-Ehrlich Gesellschaft für Chemotherapie. www.awmf.org/uploads/tx_szleitlinien/020-013l_S3_Nosokomiale_Pneumonie_Epidemiologie_Diagnostik_Therapie_2012-10.pdf. doi: 10.1055/s-0032-1325924. [DOI] [PubMed] [Google Scholar]
- 12.S3-Leitlinie. Epidemiologie Diagnostik und Therapie erwachsener Patienten mit nosokomialer Pneumonie. www.awmf.org/uploads/tx_szleitlinien/020-013l_S3_Nosokomiale_Pneumonie_Epidemiologie_Diagnostik_Therapie_2012-10.pdf. Last accessed on 4 July 2013.
- 13.The Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355:2619–2630. doi: 10.1056/NEJMoa052904. [DOI] [PubMed] [Google Scholar]
- 14.Aarts MA, Hancock JN, Heyland D, et al. Empiric antibiotic therapy for suspected ventilator-associated pneumonia: a systematic review and meta-analysis of randomized trials. Crit Care Med. 2008;36:108–117. doi: 10.1097/01.CCM.0000297956.27474.9D. [DOI] [PubMed] [Google Scholar]
- 15.Heyland DK, Dodek P, Muscedere J, et al. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med. 2008;36:737–744. doi: 10.1097/01.CCM.0B013E31816203D6. [DOI] [PubMed] [Google Scholar]
- 16.Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 17.Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 18.Wu CL, Yang Die, Wang NY, et al. Quantitative culture of endotracheal aspirates in the diagnosis of ventilator-associated pneumonia in patients with treatment failure. Chest. 2002;122:662–668. doi: 10.1378/chest.122.2.662. [DOI] [PubMed] [Google Scholar]
- 19.Kommission für Krankenhaushygiene und Infektionsprävention am Robert Koch-Institut Prävention der nosokomialen Pneumonie. Bundesgesundheitsbl. 2000;43:302–309. [Google Scholar]
- 20.Dellit TH, Owens RC, McGowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 21.Canton R, Akóva M, Carmeli Y, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect. 2012;18:413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]