Abstract
A group of bioactive steroidal glycosides (pregnanes) with anorectic activity in animals was isolated from several genera of milkweeds including Hoodia and Asclepias. In this study, we investigated the effects, structure-activity relationships, and mechanism of action of pregnane glycosides on steroidogenesis in human adrenocortical H295R cells. Administration of pregnane glycosides for 24 h suppressed the basal and forskolin-stimulated release of androstenedione, corticosterone, and cortisone from H295R cells. The conversion of progesterone to 11-deoxycorticosterone and 17-hydroxyprogesterone to either androstenedione or 11-deoxycortisol was most strongly affected, with 12-cinnamoyl-, benzoyl-, and tigloyl-containing pregnanes showing the highest activity. Incubation of pregnane glycosides for 24 h had no effect on mRNA transcripts of CYP11A1, CYP21A1, CYP11B1 cytochrome enzymes and steroidogenic acute regulatory protein (StaR) protein, yet resulted in twofold decrease in HSD3B1 mRNA levels. At the same time, pregnane glycosides had no effect on the CYP1, 2, or 3 drug and steroid metabolism enzymes and showed weak Na+/K+ ATPase and glucocorticoid receptor binding. Taken together, these data suggest that pregnane glycosides specifically suppress steroidogenesis through strong inhibition of 11β-hydroxylase and steroid 17-alpha-monooxygenase, and weak inhibition of cytochrome P450 side chain cleavage enzyme and 21β-hydroxylase, but not 3β-hydroxysteroid dehydrogenase/isomerase.
Despite the apparent lack of extensive clinical research and toxicological evaluations, Hoodia-containing remedies remain one of the most popular dietary pills on the loosely regulated US botanical dietary supplement market (Rader et al., 2007). P57AS3 pregnane glycoside is the only reported active constituent from H. gordonii that acts as an appetite suppressant in rodents (van Heerden et al., 2007) by possibly increasing ATP content of hypothalamic neurons (MacLean and Luo, 2004). In a single, double blind, placebo controlled trial with 50 healthy volunteers receiving 1 g of Caralluma extract per day for 60 days, waist circumference and hunger showed a significant decline in the experimental group when compared to the placebo after 2 months of treatment (Kuriyan et al., 2007). However, the 15 days repeated consumption study in healthy overweight women receiving two 1,110 mg servings of pregnane glycoside-enriched H. gordonii extract reported no significant mean effects on energy intake and body weight, and raised a safety concern as H. gordonii extract was less well tolerated than placebo because of episodes of nausea, emesis, and increased levels of plasma bilirubin and alkaline phosphatase associated with the treatment (Blom et al., 2011).
Recently, we showed that consumption of the pregnane glycoside-enriched extract from the fast-growing swamp milkweed Asclepias incarnata (Warashina and Noro, 2000a,b) produced melanocortin receptors-mediated suppression of food intake in rats in the absence of adverse outcomes during a 90 days subchronic toxicity study (Komarnytsky et al., 2013a). While no-observable adverse effect level for pregnane glycoside-enriched extract when administered daily via oral gavage to rats was estimated at 40 mg/kg/day, we have observed a transitional increase in incidence and severity of vacuolization of the adrenal cortex in males given 50 mg/kg/day of the extract that returned to normal during the recovery phase (Komarnytsky et al., 2013a). This data raised an intriguing possibility that pregnane glycosides might affect steroidogenesis similar to cardiac glycosides digioxin, digitoxin (Pu et al., 2006), and oubain (Kau et al., 2005); however, no studies have examined their cellular and molecular targets or specific mechanism by which pregnane glycosides modulate steroid biosynthesis. H295R cells, cultured from a human adrenocortical carcinoma, are capable of producing all corticosteroids and expressing all the major pathways of steroidogenesis, including the main steroidogenic enzymes (Gazdar et al., 1990). The present study therefore examined the effects of pregnane glycoside-enriched extracts from H. gordonii (Hge) or A. incarnata (incarnatin), and individual pregnane glycosides isolated from A. incarnata roots, on corticosteroid secretion from H295R cells as well as enzymatic activity and mRNA transcript levels of key steroidogenesis-regulating proteins.
Materials and Methods
Plant source, extraction, and compound identification
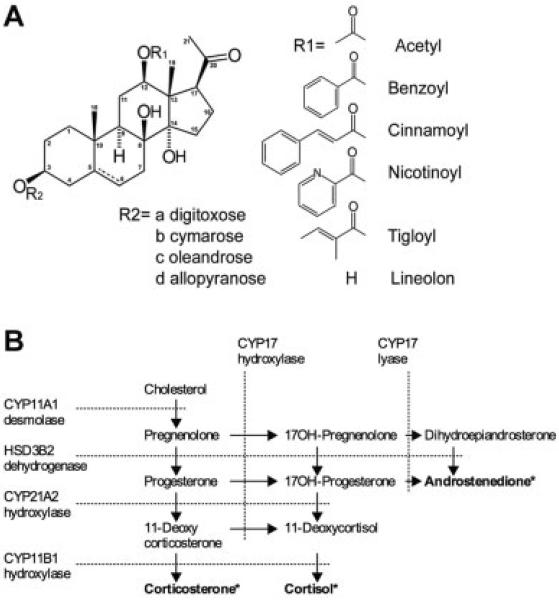
Pregnane glycoside-enriched extract from Rutgers University greenhouse-grown H. gordonii plants was prepared as described previously using methanol–dichloromethane extraction followed by solvent–solvent partitioning (van Heerden et al., 2007) and contained 25% (w:w) pregnane glycosides. Pregnane glycoside-enriched extract from Rutgers University greenhouse-grown A. incarnata plants was prepared using two-step process that included defatting and methanol extraction, resulting in 30% (w:w) total pregnane glycoside content and 3.5% (w:w) of major pregnane glycoside, 3,8,12,14-tetrahydroxypregn-5-en-20-one; (3β,12β,14β,17α)-form, 12-cinnamoyl, 3-O-[β-d-oleandropyranosyl-(1→4)-d-digitoxopyranosyl-(1→4)-β-digitoxopyranosyl-(1→4)-β-d cymaropyranoside (ikemagenin; Komarnytsky et al., 2012a). Incarnatin extract was further used to isolate eight pregnane glycosides to at least 85% purity, as shown on Figure 1A. Total pregnane content and individual pregnane glycosides were estimated by a xanthydrol-based colorimetric assay (Aitova et al., 1973), HPLC, and LCMS/ESI analysis (Komarnytsky et al., 2012a). Absence of cardiac glycosides from the pregnane glycoside-enriched extracts was confirmed by a negative Kedde reaction (Stahl, 1973).
Fig. 1.
Pregnane glycosides and steroidogenesis. A: Chemical structure of pregnane glycosides used in this study showing a common steroid core, C12 side chain moieties, deoxysugar moieties forming polysaccharide glycosylation pattern at the C3 carbon, and an optional double bond between C5 and C6 carbons. B: Schematic illustration of steroidogenesis in human adrenal H295R cells showing end-point steroidogenic metabolites (*), metabolite conversion pathways (arrows), and respective enzyme that catalyze them (dashed lines).
Cell culture
The human adrenocarcinoma H295R cell line that expresses all the major pathways of steroidogenesis was obtained from ATCC (Manassas, VA). Cells were routinely passaged every 3–4 days and maintained in DMEM-F12 containing 2.5% Nu-serum, 1% ITS Plus Premix, and 1% penicillin–streptomycin at 37°C and 5% CO2. Cells were sub-cultured into 6-well dishes at a density of 4×105 cells/well and, once sub-confluent, exposed to fresh medium containing vehicle (0.1% ethanol) or various concentrations of pregnane glycosides added to a set of 2 wells per dose for 24 h. When indicated, steroidogenesis was stimulated by simultaneous addition of 10 μM adenylyl cyclase activator forskolin (Pu et al., 2006). Ketoconazole (10 μM) was used as a positive control (Loose et al., 1983). Cell viability was estimated using the MTT assay (Mosmann, 1983) by absorbance read at 550 nm on a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA).
Steroidogenic studies
H295R cells were prepared as described above, pre-treated with 10 μM forskolin and respective pregnane glycosides as indicated for 1 h, and supplemented with 10 μM either 25-hydroxycholesterol (Cho), pregnenolone (Pre), progesterone (Pro), or deoxycorticosterone (Doc) for total incubation time of 24 h. At the end of incubation, the medium was collected, centrifuged at 200g for 10 min and extracted three times with ethyl acetate. The ethyl acetate fractions were combined, concentrated to dryness, and dissolved in 70% methanol for steroid release analysis with HPLC/ESI-MS method using the appropriate retention time, molecular ion size, and standard curves based on synthetic steroids (Sigma, St. Louis, MO). Cortisol (Cos, 20.5 min, m/z=363 M+1), corticosterone (Cor, 23.5 min, m/z=347 M+1), 11-deoxycortisol (Dec, 24 min, m/z=347 M+1), 11-deoxycorticosterone (Doc, 27 min, m/z=331 M+1), 17-hydroxyprogesterone (17Pro, 28.5 min, m/z=331 M+1), androstenedione (And, 28.5 min, m/z=287 M+1), pregnenolone (Pre, 32 min, m/z=317 M+1), and progesterone (Pro, 33 min, m/z=315 M+1) were quantified with a Dionex Ultimate 3000 RSLC UPLC system and Varian 1200L (Varian, Palo Alto, CA) triple quadrupole mass detector with electrospray ionization (ESI) operated in a positive mode, interface using a Dionex Acclaim RSLC 120 C18 reverse phase column (150mm×2.1 mm, 2.2 μM). Schematic diagram illustrating adrenal steroidogenesis pathways is shown on Figure 1B.
Gene expression analysis
Total RNA was isolated from the scraped cell cultures using Trizol reagent (Invitrogen, Carlsbad, CA) and quantified by absorption measurements at 260 and 280 nm using the NanoDrop system (NanoDrop Technologies, Inc., Wilmington, DE). Quality of RNA was assessed by gel electrophoresis. RNA was then treated with DnaseI (Invitrogen) to remove traces of DNA contamination and the cDNAs were synthesized with 2.5 μg of RNA using Stratascript reverse transcriptase (Stratagene) according to the manufacturers protocols. Quantitative PCR was performed in duplicate essentially as described (Komarnytsky et al., 2011) using the following gene-specific primers (IDT, Coralville, IA) selected using the Primer Express 2.0 software (Applied Biosystems, Foster City, CA). Samples were subjected to a melting curve analysis to confirm the amplification specificity. The relative change in the target gene with respect to the endogenous control gene was determined using 2ΔΔCT method (Winer et al., 1999).
Enzyme assays
Na+/K+ ATPase (from pig heart) assay was performed by MDS Pharma Services (Taipei, Taiwan, Study No. 1069269) with 100 μM ATP as the substrate and 1% DMSO as vehicle. The reaction mixture was pre-incubated for 15 min at 37°C and then incubated for 1 h at 37°C in the incubation buffer consisting of 50 mM Tris–HCl pH 7.0, 5 mM MgCl2, 100 mM NaCl, 20 mM KCl. Excess of 50% of maximum stimulation or inhibition of Pi release was quantified spectrophotometrically and considered significant.
Receptor-radioligand binding assays
Cannabinoid CB1 receptor binding assay was performed by MDS Pharma Services (Taipei, Taiwan, Study No. 1069269) in human recombinant HEK-293 cells using 0.5 nM [3H] CP-55,940 as a ligand (Kd 13 nM, Bmax 0.7 pmol/mg protein, specific binding 60%) and 10 μM R(+)-WIN-55,212-2 as a non-specific ligand.
Cholecystokinin CCK1 receptor binding assay was performed by MDS Pharma Services (Taipei, Taiwan, Study No. 1069269) in human recombinant NIH-3T3 cells using 0.8 nM [3H] devazepide as a ligand (Kd 0.2 nM, Bmax 0.13 pmol/mg protein, specific binding 80%) and 1 μM devazepide as a non-specific ligand.
Histamine H3 receptor binding assay was performed by MDS Pharma Services (Taipei, Taiwan, Study No. 1069269) in human recombinant CHO-K1 cells using 3 nM [3H] R(−)-α-methylhistamine as a ligand (Kd 2.4 nM, Bmax 4.2 pmol/mg protein, specific binding 95%) and 1 μM R(−)-α-methylhistamine as a non-specific ligand.
Melanocortin MC4 receptor binding assay was performed by MDS Pharma Services (Taipei, Taiwan, Study No. 1069269) in human recombinant HEK-293 cells using 0.02 nM [125I] NDP-α-MSH as a ligand (Kd 0.5 nM, Bmax 3.9 pmol/mg protein, specific binding 90%) and 3 μM NDP-α-MSH as a non-specific ligand.
Serotonin HT3 receptor binding assay was performed by MDS Pharma Services (Taipei, Taiwan, Study No. 1092673 and 1092674) in human recombinant HEK-293 cells using 0.69 nM [3H] GR-65630 as a ligand (Kd 0.2 nM, Bmax 11 pmol/mg protein, specific binding 90%) and 10 μM MDL-72222 as a non-specific ligand.
Glucocorticoid receptor binding assay was performed by MDS Pharma Services (Taipei, Taiwan, Study No. 1028624 and 1028625) in human Hela S3 cells using 6 nM [3H] dexamethasone as a ligand (Kd 5 nM, Bmax 61,000 R/cell, specific binding 75%) and 20 μM dexamethasone as a non-specific ligand. Excess of 50% of maximum ligand displacement was considered significant for all radioligand binding assays.
Inhibition of drug and steroid metabolism enzymes
CYP-based drug–drug interaction and inhibition/activation profile assays were performed by Cerep (Redmond, WA, Study No. 14894) in human liver microsomes at a concentration of 10 μM in duplicate for the following enzymes: CYP1A (phenacetin substrate), CYP2B6 (bupropion), CYP2A6 (coumarin), CYP2C8 (paclitaxel), CYP2C9 (diclofenac), CYP2C19 (omeprazole), CYP2D6 (dextromethorphan), CYP2E1 (chlorzoxazone), CYP3A (midazolam and testosterone). Excess of 50% of maximum CYP inhibition was considered significant for all assays.
Statistics
Data are represented as mean±SEM. Statistical analyses were performed using GraphPad Prism 4.0 (San Diego, CA) using Student's t-test or one-way ANOVA (as appropriate). IC50 values were determined by nonlinear, least square regression analysis. P-values of less than 0.05 were considered significant.
Results
Pregnane glycoside-enriched extracts inhibit steroid secretion from adrenal cells
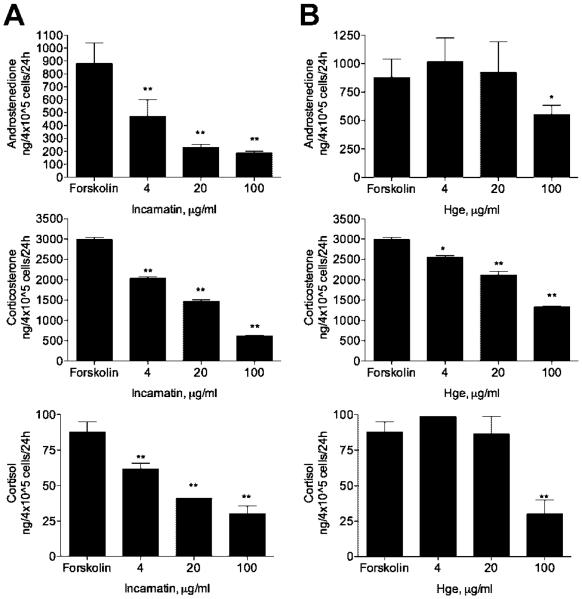
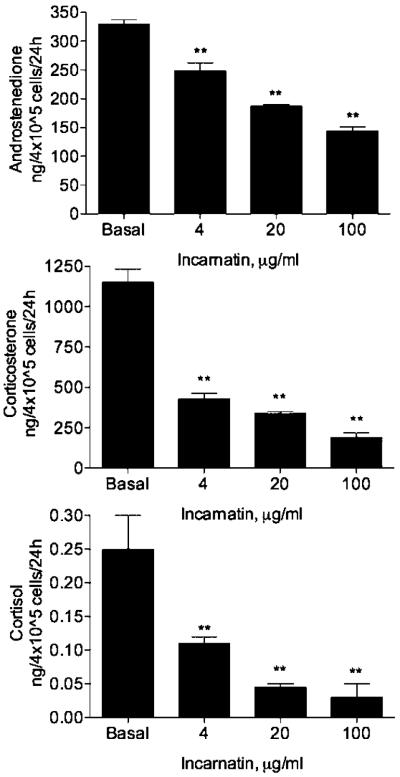
Forskolin produced a significant increase in androstenedione, corticosterone, and cortisol secretion by H295R cells. Incubation of preganane glycoside-enriched extracts incarnatin from a swamp milkweed A. incarnata and Hge from a desert succulent H. gordonii (4–100 μg/ml) for 24 h produced an inhibitory effect on forskolin-stimulated (P < 0.01, Fig. 2) and basal (P < 0.01, Fig. 3) steroid release in a dose-dependent manner. The 50% inhibition concentration values IC50 were estimated to be in the range of 5–20 μg/ml for incarnatin and 75–90 μg/ml for Hge. Ikemagenin, the major pregnane glycoside in the incarnatin extract, markedly reduced androstenedione, corticosterone, and cortisol secretion by H295R cells with the IC50 values of 0.2, 0.1, and 0.1 μM, respectively (P < 0.01, Fig. 4). Incubation with 0.2–20 μM pregnane glycosides did not produce significant cytotoxic effect in H295R cells (data not shown) as estimated using the MTT assay (Mosmann, 1983).
Fig. 2.
Effect of pregnane glycoside-enriched extracts from A. incarnata (A) and H. gordonii (B) on forskolin-induced androstenedione, corticosterone, and cortisol secretion by H295R cells. All values are means ± SEM (n = 3). *P < 0.05, **P < 0.01 as compared with forskolin-induced controls.
Fig. 3.
Effect of pregnane glycoside-enriched extract from A. incarnata on basal androstenedione, corticosterone, and cortisol secretion by H295R cells. All values are means ± SEM (n = 3). *P < 0.05, **P < 0.01 as compared with forskolin-induced controls.
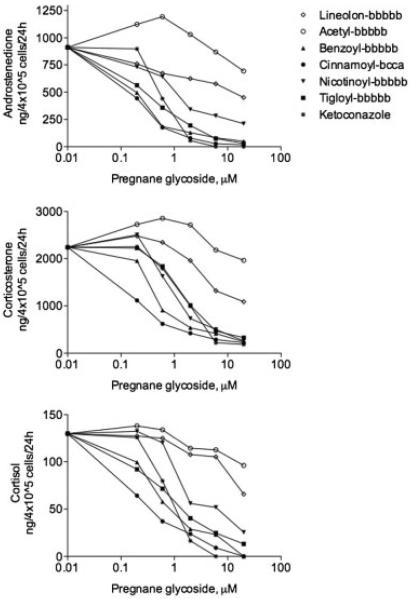
Fig. 4.
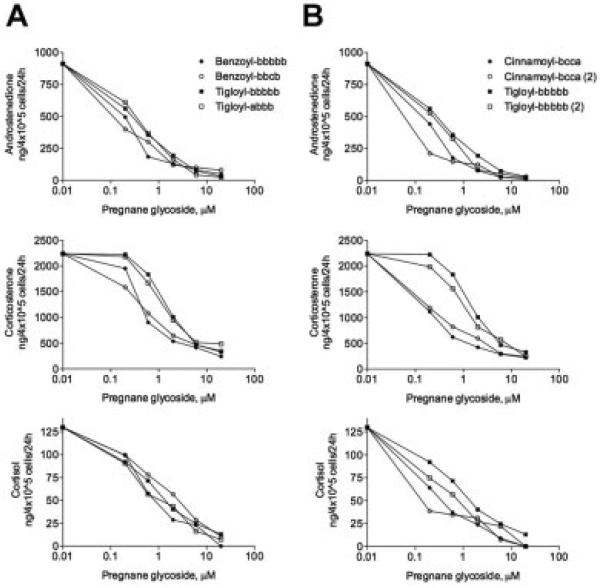
Dose-dependent effect of pregnane glycosides with different C12 moeities on corticosteroid production by H295R cells. Individual compounds contained various C12 side moieties (null, acetyl, benzoyl, cinnamoyl, nicotinoyl, or tigloyl) and shared a bbbbb (five cymarose units) glycosylation pattern with the exception of ikemagenin that contained a bcca (one cymarose, two oleandrose, and one digitoxose unit) glycosylation side chain.
Structure-activity requirements for anti-steroidogenesis effects
Swamp milkweed is an unusually rich source of pregnane glycosides (Warashina and Noro, 2000a,b) containing a diverse natural library of pregnanes that differ by the side chain modification at the C12 position, glycosylation at the C3 carbon, and presence of a double bond between carbons C5 and C6 (Fig. 1). Pregnane glycosides carrying a bulky hydrophobic modification at the C12 position (benzoyl, cinnamoyl, or nocitinoyl side chain) showed the strongest anti-steroidogenic activity (Fig. 4). When tigloyl group was present at the C12 carbon, the steroidogenic inhibitory activity of the pregnanes was reduced eightfold. Presence of 12-acetyl group decreased inhibitory effects of pregnane glycosides on androstenedione, corticosterone, and cortisol secretion 100-fold, while lineolon, a pregnane glycoside containing no modification at the C12 carbon, was inactive in this assay.
To test the effect of glycosylation at the C3 position, we compared anti-steroidogenic activity of two 12-benzoyl-containing pregnanes carrying bbbbb (five cymarose units) and bbcb (three cymarose and one oleandrose unit) glycosylation and two 12-tigloyl-containing pregnanes carrying bbbbb (five cymarose units) and abbb (one digitoxose and three cymarose units) glycosylation. Both compounds produced an inhibitory effect on androstenedione, corticosterone, and cortisol release with the IC50 values of 0.5–0.8 μM irrespective of their glycosylation patterns (Fig. 5A).
Fig. 5.
Dose-dependent effect of pregnane glycosides with different glycosylation and steroid core structure on corticosteroid production by H295R cells. A: Glycosylation at the C3 carbon and (B) optional double bond between C5 and C6 carbons had no effect on the IC50 values.
The effect of optional double bond present between C5 and C6 carbons of the pregnane skeleton on steroidogenesis in the adrenal cells was tested using two pairs of 12-cinnamoyl-bcca and 12-tigloyl-bbbbb pregnane glycosides. The inhibitory effects of these compounds were nearly identical (Fig. 5B), suggesting that presence of the C5–C6 double bond had no effect on the anti-steroidogenic activity of pregnane glycosides.
Pregnane glycoside reduces activity of steroidogenic enzymes
Administration of 10 μM steroidogenic precursors such as membrane permeable 25-hydroxycholesterol, pregnenolone, progesterone, or 11-deoxycorticosterone significantly increased secretion of the end-point steroid metabolites androstenedione, corticosterone, and cortisol by H295R cells, with the exception of 11-deoxycorticosterone that had no effect on cortisol release (Table 1). The intermediate steroid metabolites progesterone, 17-hydroxyprogesterone, and 11-deoxycorticosterone were significantly upregulated by the appropriate precursor feeding, while pregnenolone was released into the medium by H295R cells only in trace amounts independent of stimulation (Table 1). Co-incubation of steroidogenic precursors with 10 μM ikemagenin (cinnamoyl-bcca pregnane glycoside) resulted in a significant decrease of all three end-point steroid metabolites (Table 2). Administration of 11-deoxycorticosterone did not restore corticosterone release indicating strong inhibition of 11β-hydroxylase (CYP11B1) by pregnane glycoside. Supplementation with progesterone had no effect on androstenedione, corticosterone, or cortisol secretion, but resulted in remarkable increase in 17-hydroxyprogesterone (140-fold) and 11-deoxycorticosterone (200-fold) release into the media by H295R cells, suggesting that CYP21A2 (weakly) and CYP11B1 (strongly), but not HSD3B2, are inhibited by pregnane glycoside. Pregnenolone feeding had similar results indicating that pregnane glycoside did not inhibit HSD3B2 activity. However, administration of 25-hydroxycholesterol had a minor effect on release of either end-point or intermediate steroid metabolites (pregnenolone or progesterone), suggesting weak inhibition of CYP11A1 activity by pregnane glycosides.
TABLE 1.
Changes in steroidogenic metabolite secretion by H295R cells in response to forskolin stimulation and precursor feeding
| Steroidogenic metabolite, ng/4×105 cells/24 h |
|||||||
|---|---|---|---|---|---|---|---|
| Treatment | Pre | Pro | 17Pro | And | 11Cor | Cor | Cos |
| Basal | Tr | 37±12 | Nd | 317±21 | Nd | 724±25 | 12±4 |
| Forskoline (F) | Tr | 82±16* | 21±4* | 720±21* | 8±1* | 2,932±185* | 82±8* |
| F+Cholesterol | Tr | 251±45** | 794±61** | 1,851±181** | 325±12** | 3,635±148** | 156±8** |
| F+Pregnenolone | Fd | 358±21** | 921±25** | 1,583±53** | 814±33** | 3,840±99** | 160±12** |
| F+Progesterone | Tr | Fd | 1,809±49** | 1,419±78** | 2,722±246** | 3,894±37** | 103±21** |
| F+Deoxycorticosterone | Tr | 107±16 | 71±47 | 748±25 | Fd | 3,692±361** | 12±4 |
Nd, not detected; Tr, trace amount; Fd, precursor feeding.
Significantly different from control value.
Significantly different from forskolin value (P<0.05).
TABLE 2.
Changes in steroidogenic metabolite secretion by H295R cells in response to forskolin stimulation and precursor feeding following the treatment with 10 μM ikemagenin (Ik)
| Steroidogenic metabolite, ng/4×105 cells/24 h |
|||||||
|---|---|---|---|---|---|---|---|
| Treatment | Pre | Pro | 17Pro | And | 11Cor | Cor | Cos |
| Forskoline (F) | Tr | 82±16 | 21±4 | 720±21 | 8±1 | 2,932±185 | 82±8 |
| F+Ik | Tr | 12±4* | 29±4* | 197±16* | 8±1 | 226±4* | 25±1* |
| F+Ik+Cholesterol | Tr | 70±4** | 333±21** | 514±37** | 66±8** | 925±95** | 33±1 |
| F+Ik+Pregnenolone | Fd | 350±185** | 2,890±259** | 461±99** | 1,028±197** | 880±378** | 21±4 |
| F+Ik+Progesterone | Tr | Fd | 4,363±596** | 234±29 | 1,908±16** | 428±148 | 20±4 |
| F+Ik+Deoxycorticosterone | Tr | 49±16 | 115±33 | 196±8 | Fd | 354±197 | 22±2 |
Tr, trace amount; Fd, precursor feeding.
Significantly different from forskolin value.
Significantly different from forskolin+Ik value (P<0.05).
Effect of pregnane glycoside on mRNA transcript levels of steroidogenic genes
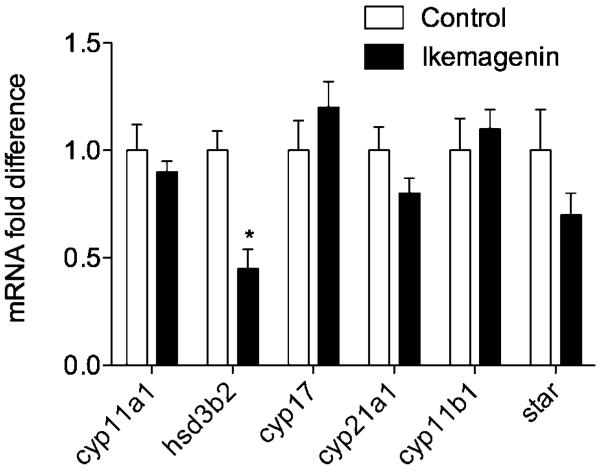
To determine whether the inhibitory effects of pregnane glycosides were cause by altered expression of steroidogenic enzymes or Star regulatory protein, their mRNA transcript levels were assesses by qPCR. Pregnane glycoside treatment had no effect on the expression levels of CYP enzymes or Star protein, but significantly (55%, P < 0.05) reduced HSD3B2 mRNA (Fig. 6).
Fig. 6.
Ikemagenin reduced mRNA expression levels of HSD but not cytochrome P450 enzymes in forskolin-induced H295R cells. Conventional RT-PCR on RNAs from individual cell samples (n = 3) was performed in duplicate using actin as an internal housekeeping gene. All values are means ± SEM. *P < 0.05 as compared with forskolin-induced controls.
Pregnane glycoside shows weak interaction with drug metabolism enzymes
To determine effect of pregnane glycosides on possible drug–drug interaction and inhibition/activation of liver cytochrome enzymes, 10 μM ikemagenin were administered to the human liver microsomes in the presence of the appropriate substrates. We observed no effect of pregnane glycoside on CYP1A, 2B6, 2C9, 2C19, 2D6, 2E1 enzymes, weak inhibition of CYP3A (25% with midozalam substrate and 19% with testosterone substrate), and a moderate activation of CYP2C8 (52% with paclitaxel substrate).
Activity of pregnane glycoside in select enzyme and receptor binding assays
Since core structure of pregnane glycosides is similar to animal steroids, we performed a set of enzyme and receptor-binding studies to understand their specificity towards activation of select signal transduction pathways. No significant responses for Na+/K+ ATPase, cannabinoid CB1, cholecystokinin CCK1, histamine H3, melanocortin MC4, and serotonin HT3 receptors were noted. Competitive binding assay to the human glucocorticoid receptor in the presence of the labeled [3H] dexamethasone was used to compare ikemagenin with dexamethasone (IC50=0.011±0μM; Ki=4.98±0.17 nM; nH=1.21±0.06). Ikemagenin produced weak binding (Fig. 7) to the glucocorticoid receptor with the IC50 of 13.2±2.36 μM (Ki=5.98±1.07 μM, nH=1.3±0.2).
Fig. 7.
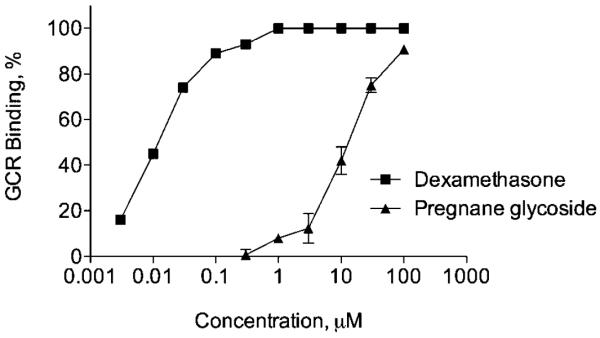
In vitro competition for specific [3H] dexamethasone binding sites by pregnane glycoside and dexamethasone. All values are means ± SEM.
Discussion
The present study demonstrates that pregnane glycosides inhibit basal and forskolin-induced secretion of androstenedione, corticosterone, and cortisol by acting directly on human H295R adrenal cells. Also, pregnane glycosides weakly inhibited conversion of 25-hydroxycholesterol to pregnenolone (catalyzed by P450scc enzyme), progesterone to 11-deoxycorticosterone and 17-hydroxyprogesterone to 11-deoxycortisol (catalyzed by 21β-hydroxylase), but strongly inhibited 11-deoxycorticosterone to corticosterone and 11-deoxycortisol to cortisol (catalyzed by 11β-hydroxylase), and 17-hydroxyprogesterone to androstenedione (second step of CYP17-mediated reaction). At the same time, administration of pregnane glycosides resulted in a twofold down-regulation of the HSD3B2 transcript. Taken together, these data suggest that pregnane glycosides exert a global suppression of steroidogenesis in H295R cells (Fig. 1). This effect might account for increased adrenal weight, adrenal-to-body weight ratio, and increased increase incidence of vacuolization of the adrenal cortex (zona reticularis) of rats gavaged daily with 50 mg/kg pregnane glycoside-enriched extract (incarnatin) from swamp milkweed roots for 90 days (Komarnytsky et al., 2012b) as body attempts to compensate decreased steroidogenesis through adrenal negative feedback loop (Briassoulis et al., 2011).
Both pregnane-glycoside enriched extracts from a swamp milkweed Asclepias incarnata and a desert succulent H. gordonii inhibited steroidogenesis in a similar fashion (Figs. 2 and 3). While H. gordonii plants produce two major pregnane glycosides carrying a single core aglycone structure with a 12-tigloyl moiety (van Heerden et al., 2007), swamp milkweed contains a vast natural library of pregnane glycosides including six core aglycones with null, acetyl, benzoyl, cinnamoyl, nicotinoyl, and tigloyl moieties at the C12 position and more than 60 individual pregnane glycosides that differ in hydroxylation patterns and glycosylation moieties (Warashina and Noro, 2000a,b). While major swamp milkweed pregnane glycoside ikemagenin is analogous to the major bioactive P57AS3 pregnane glycoside isolated from H. gordonii (van Heerden et al., 2007), it possesses several unique structural properties. These include a bulky lipophilic cinnamoyl moiety attached at C12 position, additional hydroxylation at C18 position, and a different set of rare deoxysugar moieties (oleandrose, digitoxose, and cymarose) attached at the C3 position (Komarnytsky et al., 2012a). A number of other bioactive compounds possess deoxysugars attached to their aglycones; in many cases, these deoxysugars are essential for their biological activity as in case of cardiac glycosides (Schoner and Scheiner-Bobis, 2007), antibiotics (Salah-Bey et al., 1998), or antitumor agents (Otten et al., 1997). Cardiac glycosides isolated from foxglove are administered to patients with congestive heart failure to increase intracellular accumulation of calcium ions after Na+/K+-ATPase inhibition as occurs in myocardium (Hougen and Smith, 1978). The endogenous digitalis-like glycosides have been found in healthy adults serum (Valdes and Graves, 1985) and adrenal glands (Hamlyn et al., 1991), however their physiological role has not been elucidated. Previously, it has been reported that cardiac glycosides inhibited steroidogenesis in adrenal cells with IC50 value of 0.3μM (Kau et al., 2005; Pu et al., 2006). In this study, pregnane glycosides induced a decrease in basal and stimulated corticosteroid secretion with IC50 values from 0.2–1μM. Bulky C12 moiety was necessary for preserving the anti-steroidogenic activity, while the double bond between C5–C6 carbons and type of deoxysugars in the glycoside side chain had no effect on the activity (Fig. 4).
Pregnane glycosides, however, lack the lactone ring characteristic of cardiac glycosides that is responsible for cardiac stimulating activity (Gobbini and Cerri, 2005). Since both types of steroid glycosides are often present in the same plant, a therapeutic range for cardiac glycosides in serum is 1–3 nM (Clark et al., 1992), and 50% emetic dose ED50 for cardenolides in the range of 50μg (Martin et al., 1992), one must ensure that botanical extracts enriched with pregnane glycosides are free from contaminating steroid glycosides with the use of the Kedde reaction (Stahl, 1973). Otherwise, low-level contamination with cardiac glycosides or cardenolides may be responsible for the respective side effects (changes in blood pressure, pulse, heart rate, nausea, or emesis) observed during toxicological and pharmacological evaluations.
Taken collectively, our results suggested that the inhibitory action of pregnane glycosides on corticosteroid release is primarily associated with the suppressed effect on steroidogenic cytochrome P450 enzymes activity rather than the decreased expression (Fig. 5). Interestingly, they showed little interaction with cytochrome P450 drug and steroid metabolism enzymes with the exception of weak inhibition of CYP3A and moderate activation of CYP2C8 in the human liver microsomes. Metabolic instability of steroids in the presence of CYPs not only affects the magnitude and duration of their actions but may also alter the profiles of their physiological, pathological, pharmacological and toxicological effects in relevant organs (Zhang and Yang, 2009). At the same time, pregnane glycosides did not inhibit Na+/K+ ATPase activity, suggesting minimal or no cardiotonic and cardiotoxic effects. Pregnane glycosides did not bind to a set of selected receptors with the exception of weak binding to the glucocorticoid receptor (Fig. 7). Ikemagenin (IC50 value of 13.2 μM) was 15 times more effective than glycyrrhetinic acid and three times more effective than carbenoloxone, but approximately 300-fold less effective than corticosterone and 10,000-fold less effective than dexamethasone in competing for [3H] dexamethasone binding sites (Soro et al., 1997). It is not clear whether this affinity accounts for some or any of the direct in vitro effects on steroidogenesis observed in this study, increased vacuolization of the adrenal cortex in vivo (Komarnytsky et al., 2012b), or reductions in food intake and weight gain associated with pregnane glycoside administration (Komarnytsky et al., 2012a).
Acknowledgments
Contract grant sponsor: National Center for Complementary and Alternative Medicine (NCCAM) (NIH Botanical Research Center); Contract grant number: 2P50AT002776-06.
Literature Cited
- Aitova R, Maslennikova V, Abubakirov N. Determination of 2-deoxyaldoses and their natural glycosides with the aid of xanthydrol. Chem Nat Compounds. 1973;9:605–609. [Google Scholar]
- Blom WA, Abrahamse SL, Bradford R, Duchateau GS, Theis W, Orsi A, Ward CL, Mela DJ. Effects of 15-d repeated consumption of Hoodia gordonii purified extract on safety, ad libitum energy intake, and body weight in healthy, overweight women: A randomized controlled trial. Am J Clin Nutr. 2011;94:1171–1181. doi: 10.3945/ajcn.111.020321. [DOI] [PubMed] [Google Scholar]
- Briassoulis G, Damjanovic S, Xekouki P, Lefebvre H, Stratakis CA. The glucocorticoid receptor and its expression in the anterior pituitary and the adrenal cortex: A source of variation in hypothalamic-pituitary-adrenal axis function; implications for pituitary and andrenal tumors. Endocr Pract. 2011;17:941–948. doi: 10.4158/EP11061.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WG, Brater DC, Johnson AR. Digitalis. Goth's medical pharmacology. Mosby; St. Louis, MO: 1992. pp. 408–420. [Google Scholar]
- Gazdar AF, Oie HK, Shackleton CH, Chen TR, Triche TJ, Myers CE, Chrousos GP, Brennan MF, Stein CA, La Rocca RV. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. 1990;50:5488–5496. [PubMed] [Google Scholar]
- Gobbini M, Cerri A. Digitalis-like compounds: The discovery of the O-aminoalkyloxime group as a very powerful substitute for the unsaturated gamma-butyrolactone moiety. Curr Med Chem. 2005;12:2343–2355. doi: 10.2174/0929867054864787. [DOI] [PubMed] [Google Scholar]
- Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci USA. 1991;88:6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougen TJ, Smith TW. Inhibition of myocardial monovalent cation active transport by subtoxic doses of ouabain in the dog. Circ Res. 1978;42:856–863. doi: 10.1161/01.res.42.6.856. [DOI] [PubMed] [Google Scholar]
- Kau MM, Kan SF, Wang JR, Wang PS. Inhibitory effects of digoxin and ouabain on aldosterone synthesis in human adrenocortical NCI-H295cells. J Cell Physiol. 2005;205:393–401. doi: 10.1002/jcp.20415. [DOI] [PubMed] [Google Scholar]
- Komarnytsky S, Cook A, Raskin I. Potato protease inhibitors inhibit food intake and increase circulating cholecystokinin levels by a trypsin-dependent mechanism. Int J Obes (Lond) 2011;35:236–243. doi: 10.1038/ijo.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarnytsky S, Esposito D, Poulev A, Raskin I. Subchronic toxicological evaluation of pregnane glycoside-enriched extract from swamp milkweed Asclepias incarnata in rats. Pharmaceut Biol manuscript. 2013a ID NPHB- 2012-1571. R1. [Google Scholar]
- Komarnytsky S, Esposito D, Rathinasabapathy T, Poulev A, Raskin I. Effects of pregnane glycosides on food intake depend on stimulation of melanocortin pathway and BDNF in animal model. J Agric Food Chem. 2013b doi: 10.1021/jf3033649. DOI: 10.1021/jf3033649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyan R, Raj T, Srinivas SK, Vaz M, Rajendran R, Kurpad AV. Effect of Caralluma fimbriata extract on appetite, food intake and anthropometry in adult Indian men and women. Appetite. 2007;48:338–344. doi: 10.1016/j.appet.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Loose DS, Kan PB, Hirst MA, Marcus RA, Feldman D. Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes. J Clin Invest. 1983;71:1495–1499. doi: 10.1172/JCI110903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean DB, Luo L-G. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: Studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004;1020:1–11. doi: 10.1016/j.brainres.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Martin RA, Lynch SP, Brower LP, Malcolm SB, Van Hook T. Cardenolide content, emetic potency, and thin-layer chromatography profiles of monarch butterflies, Danaus plexippus, and their larval host-plant milkweed, Asclepias humistrata, in Florida. Chemoecology. 1992;3:1–13. [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Otten SL, Gallo MA, Madduri K, Liu X, Hutchinson CR. Cloning and characterization of the Streptomyces peucetius dnmZUV genes encoding three enzymes required for biosynthesis of the daunorubicin precursor thymidine diphospho-l-daunosamine. J Bacteriol. 1997;179:4446–4450. doi: 10.1128/jb.179.13.4446-4450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu HF, Wang SW, Tseng CI, Huang HL, Lin CW, Hsu JM, Chen MJ, Chow YC, Wang PS. Mechanisms of digoxin and digitoxin on the production of corticosterone in zona fasciculata-reticularis cells of ovariectomized rats. J Cell Biochem. 2006;97:303–313. doi: 10.1002/jcb.20603. [DOI] [PubMed] [Google Scholar]
- Rader J, Delmonte P, Trucksess M. Recent studies on selected botanical dietary supplement ingredients. Anal Bioanal Chem. 2007;389:27–35. doi: 10.1007/s00216-007-1254-7. [DOI] [PubMed] [Google Scholar]
- Salah-Bey K, Doumith M, Michel JM, Haydock S, Cortes J, Leadlay PF, Raynal MC. Targeted gene inactivation for the elucidation of deoxysugar biosynthesis in the erythromycin producer Saccharopolyspora erythraea. Mol Gen Genet. 1998;257:542–553. doi: 10.1007/s004380050680. [DOI] [PubMed] [Google Scholar]
- Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs. 2007;7:173–189. doi: 10.2165/00129784-200707030-00004. [DOI] [PubMed] [Google Scholar]
- Soro A, Panarelli M, Holloway CD, Fraser R, Kenyon CJ. In vivo and in vitro effects of carbenoxolone on glucocorticoid receptor binding and glucocorticoid activity. Steroids. 1997;62:388–394. doi: 10.1016/s0039-128x(96)00252-8. [DOI] [PubMed] [Google Scholar]
- Stahl E. Drug analysis by chromatography and microscopy. Ann Arbor Science Publishers; Ann Arbor, MI: 1973. [Google Scholar]
- Valdes RJ, Jr., Graves SW. Protein binding of endogenous digoxin-immunoactive factors in human serum and its variation with clinical condition. J Clin Endocrinol Metab. 1985;60:1135–1143. doi: 10.1210/jcem-60-6-1135. [DOI] [PubMed] [Google Scholar]
- van Heerden FR, Marthinus Horak R, Maharaj VJ, Vleggaar R, Senabe JV, Gunning PJ. An appetite suppressant from Hoodia species. Phytochemistry. 2007;68:2545–2553. doi: 10.1016/j.phytochem.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Warashina T, Noro T. Cardenolide and oxypregnane glycosides from the root of Asclepias incarnata L. Chem Pharm Bull (Tokyo) 2000a;48:516–524. doi: 10.1248/cpb.48.516. [DOI] [PubMed] [Google Scholar]
- Warashina T, Noro T. Steroidal glycosides from the aerial part of Asclepias incarnata. Phytochemistry. 2000b;53:485–498. doi: 10.1016/s0031-9422(99)00560-9. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Yang L. Interactions between human cytochrome P450 enzymes and steroids: Physiological and pharmacological implications. Expert Opin Drug Metab Toxicol. 2009;5:621–629. doi: 10.1517/17425250902967648. [DOI] [PubMed] [Google Scholar]