Abstract
The microtubule-associated protein tau is abnormally hyperphosphorylated in the brains of individuals with Alzheimer disease and other tauopathies, and is believed to play a critical role in the pathogenesis of these diseases. While the mechanisms leading to abnormal tau phosphorylation remain elusive, the recent demonstration of reversible tau phosphorylation during hibernation provides an ideal physiological model to study this critical process in vivo. In this study, arctic ground squirrels (AGS) during hibernation were used to study mechanisms related to tau hyperphosphorylation. Our data demonstrate that tau is hyperphosphorylated at all six sites (S199, T205, S214, S262, S396, and S404) examined in hibernating AGS. Interestingly, only three of these sites (S199, S262, and S404) are dephosphorylated in aroused animals, suggesting a reversible phosphorylation at selective sites. Summer-active AGS demonstrated the lowest tau phosphorylation at all these sites. To explore the mechanisms underlying increased tau phosphorylation during hibernation, the expression level and enzyme activity of various potential tau kinases and protein phosphatases were examined. The kinetic analysis of enzyme activity at different temperatures revealed differential changes in enzyme activity with temperature decline. Specifically, increased protein kinase A activity, decreased protein phosphatase 2A activity, as well as substantial contribution from glycogen synthase kinase-3β, likely play a key role in increased tau phosphorylation during hibernation in AGS.
Keywords: hibernation, protein kinase A, protein phosphatase 2A, reversible phosphorylation, tau
Tau is implicated as a key protein involved in the pathogenesis of Alzheimer’s disease (AD) as: (i) it is a major component of the neurofibrillary tangles (NFTs) (Grundke-Iqbal et al. 1986), (ii) number of NFTs is closely correlated with neuronal loss and the degree of dementia (Braak and Braak 1994), and (iii) there is increasing recognition of the relevance of so-called ‘tau-only’ dementia (Roder 2003). The normal function of tau is to modulate the assembly, dynamic behavior, and spatial organization of microtubules in neurons (Matsuo et al. 1994). Tau protein has more than 40 known phosphorylation sites and becomes hyperphosphorylated in NFTs in AD neurons with some sites being only phosphorylated in the AD-specific soluble form of tau termed paired helical filaments (PHF)-tau in AD (Gong et al. 2006). Phosphorylation of tau at certain sites (e.g. Ser262) impairs microtubule binding activity and severely impairs tau-tubulin binding. Site-specific phosphorylation of tau also sequesters normal tau, MAP1 and 2, inhibits assembly and disrupts preformed microtubules [reviewed in (Iqbal and Grundke-Iqbal 2007)]. As hyperphosphorylation of tau appears to be a very early event in the pathological cascade of AD (Braak and Braak 1994), it could represent a critical event leading to abnormal aggregation and disrupted function of tau in affected neurons. The level of tau phosphorylation is the consequence of the balance between protein kinases and phosphatase and several tau kinases i.e. glycogen synthase kinase (GSK)-3, cyclin dependent kinase 5 (Cdk5), microtubule associated protein (MAPK), protein kinase A (PKA), protein kinase C, or calmodulin-dependent protein kinase II (Gomez-Ramos et al. 2004) and phosphatases [i.e. PP1, protein phosphatase 2A (PP2A), and PP2B] (Tian and Wang 2002) have been described to play a role. However, how these kinases and phosphatases contribute to abnormal tau phosphorylation and aggregation in vivo is unclear largely because of the lack of a physiological model to study the regulation of tau phosphorylation and dephosphorylation.
Hibernation is a unique physiological state characterized by profound decreases in oxidative metabolism and body temperature during bouts of prolonged torpor, periodically interrupted by brief periods of rewarmings to core temperatures near 37°C, usually lasting < 24 h, known as interbout arousals (Drew et al. 2001) which consume most of the energy during hibernation cycle (Kenagy et al. 1989). In this hibernation-arousal cycle, no brain damage occurs. Interestingly, recent studies suggested that, highly phosphorylated tau is readily formed during hibernation and is fully reversible after arousal in European ground squirrels. Most importantly, PHF-like tau is increased in the entorhinal cortex, and hippocampus (Arendt et al. 2003), areas that are affected in AD. The reversible phosphorylation of tau during hibernation thus provides a unique opportunity to study physiological regulation of tau phosphorylation. In this study, we confirmed that reversible tau phosphorylation occurred during hibernation in a different species (i.e. arctic ground squirrel) and further explored the underlying mechanisms by determining the involvement of different kinases and phosphatases.
Materials and methods
Animals
All procedures were performed in accordance with University of Alaska Fairbanks Institutional Animal Care and Use Committee. Arctic ground squirrels (AGS; Spermophilus parryii), were used for these experiments. Adult AGS of both sexes were trapped during mid-July in the northern foothills of the Brooks Range, Alaska, approximately 40 miles south of the Toolik Field Station of the University of Alaska Fairbanks (688380N, 1498380W; elevation 809 m) and transported to Fairbanks (permit obtained from Alaska Department of Fish & Game). Ground squirrels were housed individually at 16–18°C and fed rodent chow, sunflower seeds, and fresh carrots and apples ad libitum until mid-September when they were moved to a cold chamber set to an ambient temperature (Ta) of 2°C and a 4:20-hour light : dark cycle for hibernation. Tissue was obtained from four groups; euthermic AGS (n = 5), hibernating (or torpid) AGS (n = 5), aroused AGS (n = 5) during the winter months, and summer euthermic AGS (n = 5). Winter euthermic AGS (abbreviated euthermic or E) were housed at 2°C, but did not hibernate prior to tissue collection. Hibernating AGS (abbreviated hibernating or H) had low respiratory rates and body temperature near ambient temperature. Aroused AGS (abbreviated aroused or A) recently (5–7 hr) emerged from prolonged torpor and achieved body temperature of at least 34°C while kept at an ambient temperature of ~2°C. Summer euthermic AGS (abbreviated summer or S) were housed at 20°C and 12 : 12 light : dark until euthanized for tissue sampling in late July. Groups were matched for sex and age of animals (i.e. adult or juvenile). Before sampling euthermic and aroused AGS, animals were lightly anesthetized with halothane (5% halothane mixed with O2 delivered at 1.5 L/min) or halothane and ketamine (2 mg/kg)/xylazine (1 mg/kg); hibernators were not initially anesthetized. Rectal body temperatures were measured with a thermistor. Following decapitation, brains were removed immediately, dissected, and frozen in liquid nitrogen. Frozen tissue samples were stored at −80°C. Time from decapitation to freezing was less than 10 min.
Immunoblot
Tissue from forebrain of euthermic, hibernating, aroused AGS, and the summer group were homogenized in 10 volume of lysis buffer [50 mM Tris–HCl (pH 7.6), 0.02% sodium azide, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1% Nonidet P-40, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mg/mL aprotinin, 1 mg/mL antipain, and 1 mM sodium orthovanadate], and centrifuged at 11 742 g for 10 min at 4°C. The supernatant was used for immunoblot analysis. The protein concentration was determined by bicinchoninic acid assay (Pierce, Rockford, IL, USA). Proteins were separated by SDS–polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membrane by standard procedure as described previously (Zhu et al. 2000). Transferred blots were blocked by 10% non-fat milk (in Tris-buffered saline solution with Tween 20), and then probed with different antibodies (Table 1). Blots were developed by the enhanced chemiluminescence technique (Santa Cruz Biotechnology, Santa Cruz, CA, USA) according to the manufacturer’s instruction. Quantification of the result was performed using a digital image analysis software Quantity One (Bio-rad, Hercules, CA, USA) and expressed as optical densities.
Table 1.
Antibodies used in this study
| Name | Abbreviation | Isotype | Epitope | WB/IP dilution | Source |
|---|---|---|---|---|---|
| GSK-3β | GSK-3β | Mouse IgG1 | Rat GSK-3β1-160aa | 1 : 2500 | BD |
| pGSK3β (Ser9) | pGSK3β (9) | Rabbit polyclonal | GSK-3β phospho-serine9 | 1 : 2000 | Cell Signaling |
| pGSK3β (Y216) | pGSK3β (216) | Mouse IgG1 | Rat GSK3β (pY216) peptide | 1 : 2000 | BD |
| cdk5 | cdk5 | Mouse monoclonal | Human full-length cdk5 | 1 : 2000 | Abcam |
| p35 | p35 | Rabbit polyclonal | Recognize p35 and p25 | 1 : 2000 | Santa Cruz |
| PP2Ac | PP2Ac | Mouse IgG1 | Human PP2A 153–309aa | 1 : 5000 | BD |
| Anti-methyl-PP2A | Methyl-PP2A | Mouse IgG | Human PP2A 302–309aa | 1 : 1000 | Millipore |
| PKAc | PKAc | Mouse IgG1 | Human PKAc αunit | 1 : 2500 | BD |
| PKA RI | PKA RI | Mouse IgG2b | Mouse PKA RI 225–331aa | 1 : 2000 | BD |
| PKA RIIβ | PKA RIIβ | Mouse IgG1 | Human PKA RIIβ | 1 : 2000 | BD |
| Anti-tau[pS199] | pS199 | Rabbit polyclonal | pS199 | 1 : 1000 | Invitrogen |
| Anti-tau[pT205] | pT205 | Rabbit polyclonal | pT205 | 1 : 1000 | Invitrogen |
| Anti-tau[pS214] | pS214 | Rabbit polyclonal | pS214 | 1 : 1000 | Invitrogen |
| Anti-tau[pS262] | pS262 | Rabbit polyclonal | pS262 | 1 : 1000 | Abcam |
| Anti-tau[pS396] | pS396 | Rabbit polyclonal | pS396 | 1 : 2000 | Invitrogen |
| Anti-tau[pS404] | pS404 | Rabbit polyclonal | pS404 | 1 : 1000 | Invitrogen |
| Anti-tau | 5E2 | Mouse monoclonal | Fetal heat stable MAPS | 1 : 2000 | Millipore |
| Anti-actin | Actin | Mouse IgG1kappa | β-actin | 1 : 10 000 | Chemicon International |
GSK, glycogen synthase kinase; PKA, protein kinase A.
Protein phosphatase 2A activity assay
Protein phosphatase 2A activity was assayed using a kit from Upstate (Billerica, MA, USA) with minor modification. Briefly, brains were homogenized (20 mM imidazole–HCl, 2 mM EDTA, 2 mM EGTA, pH 7.0, 1 μg/mL leupeptin, aprotinin, 1 mM benzamidine, and 1 mM PMSF), and centrifuged at 11 742 g for 10 min at 4°C. Then 100 μg of the supernatant were used for immunoprecipitation. Twenty microliters of protein A and 4 μg of anti-PP2A antibody were added to homogenate and shaked at 4°C for 2 h. Then precipitated PP2A was washed three times with phosphate-buffered saline followed by one wash with assay buffer (50 mM Tris–HCl, pH 7.0, 100 μM CaCl2). The beads were suspended in 80 μL assay buffer with 750 μM threonine phosphopeptide (K-R-pT-I-R-R) as the substrate. The reaction was performed for 10 min at various temperatures (4, 10, 20, 30, and 37°C) for hibernation AGS and summer-active AGS. Following a brief centrifugation 25 μL of supernatant was used to detect the release of phosphate.
Protein kinases activity assay
Arctic ground squirrel brains were first homogenized with five volume of lysis buffer (50 mM Tris–HCl, pH 7.4, 1% Nonidet P-40, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 1 mM Na3VO4, 1 μg/mL each of leupeptin, aprotinin, and pepstatin, 1 mM PMSF, 1 μM okadaic acid). The homogenate was centrifuged at 11 742 g for 10 min at 4°C, and the supernatant was used for immunoprecipitation. GSK-3β or Cdk5 was precipitated from 250 μg of homogenate using a specific antibody (GSK-3β, BD, Franklin Lakes, NJ, USA; Cdk5, Abcam, Cambridge, MA, USA). Precipitated GSK-3β or Cdk5 were added to 20 μL of kinases assay buffer (10 mM Tris–HCl, pH 7.0, 2 mM mercaptoethanol, 1 mM magnesium acetate, 2 mM EGTA, 1 mM NaF, 1 mM Na3VO4, 1 μM okadaic acid, 1 μg/mL of each leupeptin, aprotinin, pepstatin, 1 mM PMSF, 200 μM ATP. To more accurately reflect the kinase activity toward tau protein, we chose to use 100 μg/mL recombinant human tau protein as the substrate rather than optimal substrate for each kinase) and reacted for 15 min or 2 h (for Cdk5 activity assay) at 4, 10, 20, 30, and 37°C for both hibernator and summer-active AGS. The reaction was stopped by adding 20 μL of 2 × sample buffer (0.5 M Tris, 20% glycerol, 4.4% SDS, 0.1% bromophenol blue, 4% 2-mercaptoethanol) and boiling for 5 min. Phosphorylation level of tau protein at serine 404 was detected as the kinases activities toward tau protein.
Protein kinase A activity assay was conducted following manufacture’s instruction (StressGen, Ann Arbor, MI, USA) except that the temperatures were changed to 4, 10, 20, 30, and 37°C.
Data analysis
Data are expressed as group means ± SD, n ≥3. The criterion for statistical significance was p < 0.05.
Result
Tau protein phosphorylation during hibernation
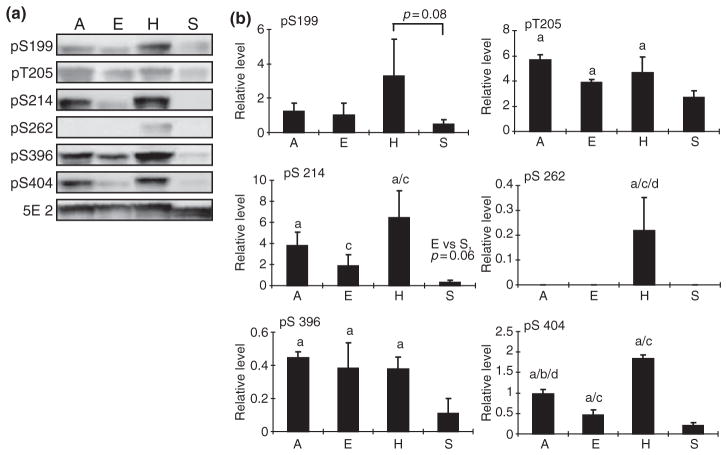
It was previously demonstrated that tau protein is reversibly phosphorylated during hibernation in European ground squirrels (Spermophilus citellus) (Arendt et al. 2003) which makes it an ideal model to determine the potential involvement of various kinases and phosphatases under physiological conditions. To pursue this end, in our AGS model, we first determined phosphorylation status of several specific sites on tau including both proline-directed and non-proline-directed sites that are known to be important in regulating tau function and are also hyperphosphorylated in AD. To control for a seasonal effect, we included one additional summer euthermic group of animals in addition to the three winter groups: (i) winter euthermic AGS, (ii) hibernating (or torpid) AGS, and (iii) aroused AGS. Six antibodies specific for tau phosphorylated at each of six phosphorylation sites were used. As shown in Fig. 1, in general, phosphorylation at each of these six sites is much lower in summer euthermic group compared with the three winter groups, suggesting an apparent seasonal effect on tau phosphorylation in this hibernating species.
Fig. 1.
Phosphorylation levels of tau at different sites in the brain homogenates of arctic ground squirrels (AGS) from different groups (E: euthermic; H: hibernating; A: aroused; and S: summer active) (n = 5/ group) were analyzed by western blot (a). Six phosphorylation-dependent and site-specific antibodies (i.e. pS199, pT205, pS214, pS262, pS396, and pS404) were used to detect tau phosphorylation levels at these sites, respectively. Phosphorylation independent tau antibody 5E2 was used to measure the total tau level. (b) The immunoreactivity in western blot was quantitated, normalized to total tau levels, and expressed as mean ± SD (‘a’ indicates significant difference when compared with summer euthermic with p < 0.05; ‘b’ indicates significant difference between aroused and cold euthermic squirrels with p < 0.05; ‘c’ indicates significant difference between euthermic and hibernating squirrels with p < 0.05, and ‘d’ indicates significant difference between aroused and hibernating squirrels with p < 0.05).
In the three winter groups, phosphorylation is site and group specific. Within these three groups, the winter euthermic group typically displays the lowest degree of tau phosphorylation and the hibernating group typically shows the highest degree of tau phosphorylation. Specifically, levels of tau phosphorylated at T205, S214, S262, and S404 are significantly higher in hibernating group compared with the winter euthermic group, and levels of tau phosphorylated at T205 and S404 are significantly higher in aroused animals compared with winter euthermic animals. There is no significant difference in levels of tau phosphorylated at S199 and S396 among the three winter groups, demonstrating increased tau phosphorylation only at selected sites during hibernation. Significantly lower levels of tau phosphorylated at S262 and S404 in aroused animals compared with hibernating (torpid) animals was also noted, confirming the reversible tau phosphorylation at these sites. Notably, there is no detectable phosphorylation at S262 in any group other than the torpid, hibernating group, suggesting that phosphorylation at this site may play a critical role. The total tau level indicated by 5E2, which recognizes all the tau proteins, was not changed among all four groups, but the mobility of tau protein in summer euthermic squirrels appears faster compared with the other three groups, suggesting a lower molecular weight, consistent with the notion that tau is less phosphorylated in this group compared with the other three winter groups of AGS.
Kinases activity during hibernation
The level of tau phosphorylation depends upon the balance of kinases and phosphatases, of which GSK-3β and Cdk5 are the two most implicated tau kinases (Iqbal and Grundke-Iqbal 2006). To determine the cause of increased tau phosphorylation at selective sites during hibernation, we first examined the level and/or activity of these kinases in the different groups of AGS among different groups.
Glycogen synthase kinase-3β activity is inhibited by phosphorylation at serine 9 and stimulated by phosphorylation at tyrosine 216 (Dajani et al. 2001; Frame et al. 2001; Grimes and Jope 2001). Therefore, to measure GSK-3β activity, GSK-3β phosphorylation at both serine 9 and tyrosine 216 were measured by immunoblot with phospho-specific antibodies. As shown in Fig. 2a–c, there was no change in GSK-3β level among all four groups of squirrels. Interestingly, the inactive form of phospho-GSK-3β (Ser9) was significantly higher in hibernating and aroused squirrels compared with winter euthermic and summer-active groups (Fig. 2a and b), and consistent with changes in levels of the inactive form, the stimulatory form of phospho-GSK-3β (Tyr216) was much lower in hibernating and aroused AGS compared with summer active groups (Fig. 2a and c), seemingly contradictory to a role of GSK-3β in the increased tau phosphorylation in these two groups. To further clarify the potential involvement of GSK-3β in tau phosphorylation during hibernation, we conducted a GSK-3β activity assay using recombinant tau as a substrate. Because of the sample limitation, only two groups with the largest differences in tau phosphorylation (i.e. hibernating group and summer active group) were used for this activity assay. Indeed, consistent with the immunoblot results, the GSK-3β kinase activity assay confirmed that GSK-3β activity is decreased significantly in hibernating squirrels compared with summer-active squirrels to a level approximately 25% of that of summer active AGS. As the decrease or increase in body temperature during hibernation is a gradual process, we reasoned that it is important to perform kinetic analysis of GSK-3β kinase activity at different temperatures (i.e. 4, 10, 20, 30, and 37°C) to clarify its involvement of tau phosphorylation during hibernation. Interestingly, kinetic study of GSK-3β kinase activity of brain homogenates from hibernating ground squirrels and summer-active ground squirrels revealed an interesting phenomenon (Fig. 2d): during the decrease of temperature from 37 to 4°C, GSK-3β peaked at 20°C, and then gradually decreased suggesting that activity peaks and then declines as brain temperature passes through 20°C during immergence and emergence from torpor.
Fig. 2.
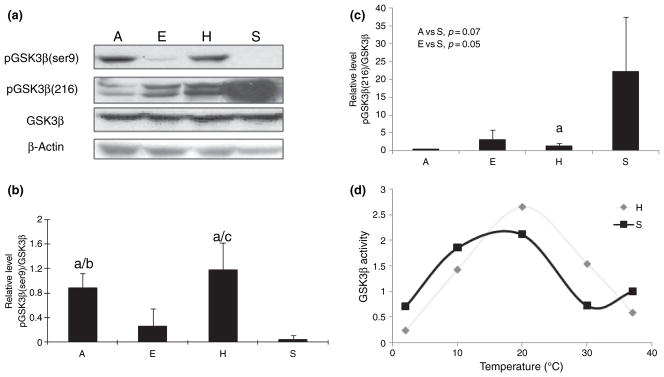
(a) Level of total glycogen synthase kinase (GSK)-3β, GSK-3β phosphorylated at Ser9 (i.e. inactive form) and GSK3β phosphorylated at Tyr216 (i.e. active form) in the brain homogenates of arctic ground squirrels (AGS) from different groups (E: euthermic; H: hibernating; A: aroused; and S: summer active) (n = 5/group) were analyzed by western blot. The immunoreactivity of pGSK-3β(Ser9) (b) and pGSK3(Y216) (c) in western blot was quantitated, normalized to total GSK-3β levels, and expressed as mean ± SD (‘a’ indicates significant difference when compared with summer euthermic with p < 0.05; ‘b’ indicates significant difference between aroused and cold euthermic squirrels with p < 0.05; and ‘c’ indicates significant difference between euthermic and hibernating squirrels with p < 0.05). (d) The kinetic of GSK-3β activity at different temperature of brain homogenates of AGS from torpid and summer-active groups was measured.
Like GSK-3β, Cdk5 is also highly expressed in the brain and can phosphorylate tau at a number of sites. Cdk5 activity is regulated by its binding to p35 (Uchida et al. 1994). Our data (Fig. 3a and b) showed that Cdk5 expression in summer euthermic squirrels is higher, but not significantly different from the three winter groups of squirrels. There were almost no differences in its activator p35 in different groups of squirrels (Fig. 3a and c). We also performed the kinase activity of Cdk5 toward tau in brain homogenates from hibernating AGS and summer active AGS. The in vitro Cdk5 activity toward tau proteins was much less significant compared with GSK-3β as no tau phosphorylation was detected in Cdk5 assay after 15 min incubation in brain homogenates from either hibernating or summer-active AGS while significant tau phosphorylation can be detected in the GSK-3β assay under the same conditions in both groups. Tau phosphorylation by Cdk5 only became detectable after a 2-h incubation, and it appeared that Cdk5 activity is higher in summer-active AGS than that in hibernating AGS at 37°C but remained undetectable even after longtime incubation at 4°C in both groups (Fig. 3d). This result suggests a less important role for Cdk5 in tau phosphorylation during hibernation in AGS.
Fig. 3.
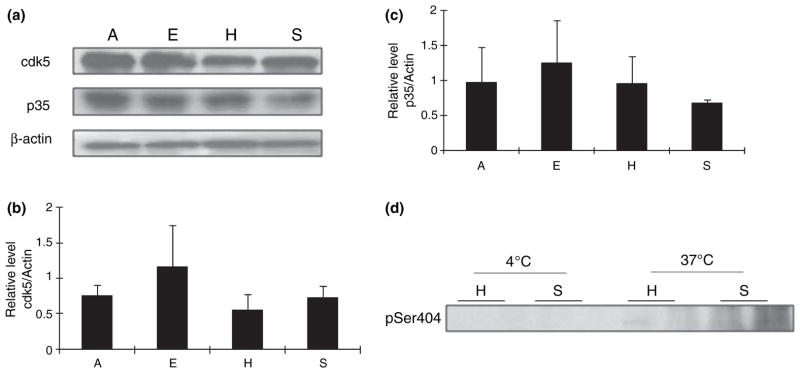
(a) Levels of cdk5 and p35 in the brain homogenates of arctic ground squirrels (AGS) from different groups (E: euthermic; H: hibernating; A: aroused; and S: summer active) (n = 5/group) were analyzed by western blot. The immunoreactivity of Cdk5 (b) and p35 (c) was quantitated, normalized to the levels of actin, and expressed as mean ± SD; no significant difference was found between any groups. (d) cdk5 activity at 4 and 37°C of brain homogenates of AGS from torpid and summer-active groups was measured.
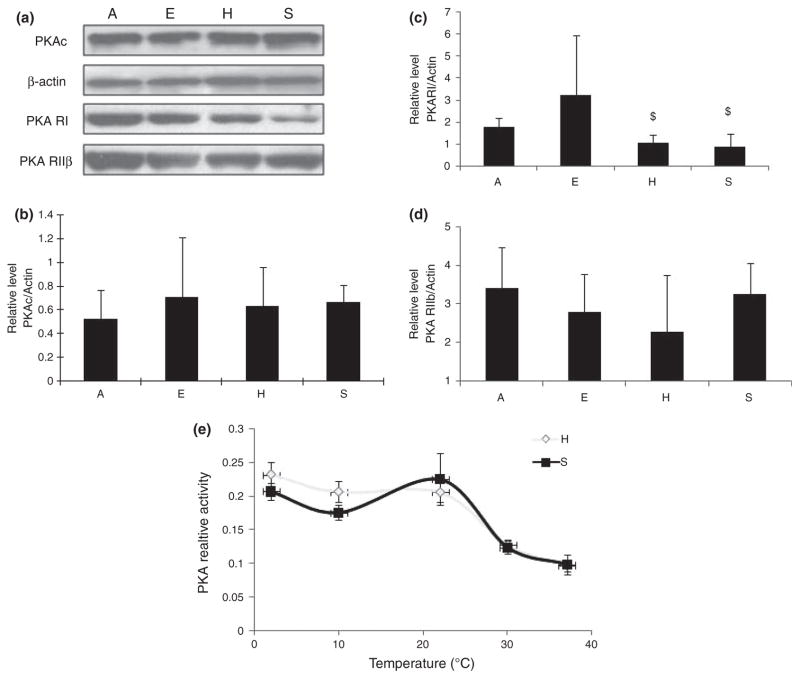
It has been reported that tau phosphorylated by other kinases such as PKA becomes a better substrate for GSK-3β (i.e. priming effect) (Liu et al. 2004), such that even basal levels of GSK-3β activity may result in tau hyperphosphorylation. Additionally, PKA can also phosphorylate tau at several sites (i.e. Ser214, Thr217, Ser262, Ser396/404, and Ser416). To this end, we also measured PKA during hibernation. PKA is composed of catalytic subunits (C) and regulatory subunits (R, including RI and RII), and its activity is regulated by association and disassociation of C subunit from R subunit (Sandberg et al. 1990; Skalhegg et al. 1992). Immunoblot and quantification analysis of the expression level of PKA catalytic subunit (i.e. PKAc) (Fig. 4a and b) or PKA RIIβ (Fig. 4a and d) revealed no difference between the four groups examined but demonstrated significantly lower levels of PKA RI subunit in hibernating and summer-active AGS than in aroused AGS (Fig. 4a and c). The kinetic study of PKA activity at different temperatures, however, revealed an interesting finding: During the decrease in temperature from 37 to 4°C, PKA activity gradually increased in both hibernating and summer-active AGS such that PKA activity at 4°C increased to about 2.4-fold of that of 37°C (Fig. 4e). In fact, PKA activity at 4°C tended to be higher in the hibernating group compared with the summer-active group.
Fig. 4.
(a) Levels of protein kinase A (PKA) catalytic subunit (PKAc), PKA regulatory subunits RI and RIIβ in the brain homogenates of arctic ground squirrels (AGS) from different groups (E: euthermic; H: hibernating; A: aroused; and S: summer active) (n = 5/group) were analyzed by immunoblot. The immunoreactivity of PKAc (b), PKA RI (c), and PKA RIIβ (d) was quantitated, normalized to the levels of actin, and expressed as mean ± SD. ‘$’ indicates significant differences compared with aroused squirrels with p ≤ 0.05. (e) The kinetic of PKA activity at different temperature of brain homogenates of AGS from torpid and summer-active groups was measured.
Protein phosphatase 2A activity during hibernation
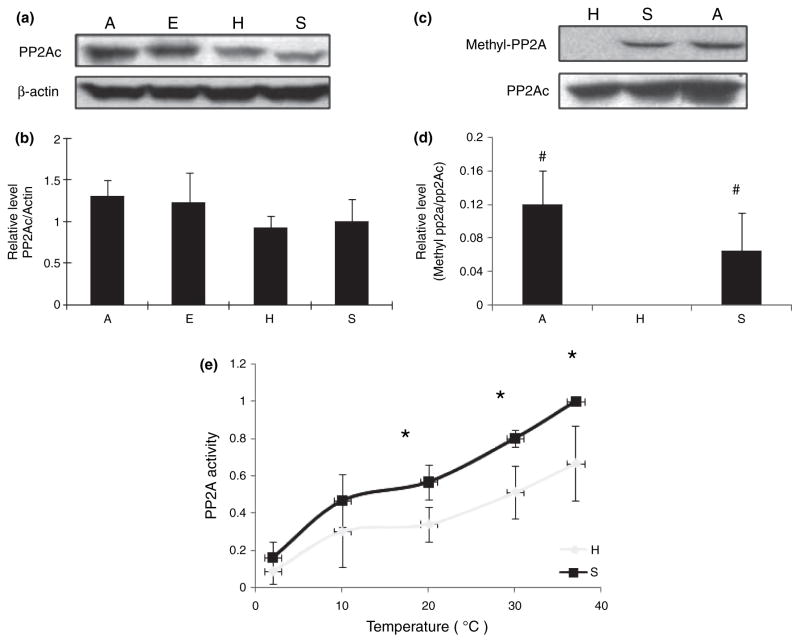
Among all the potential tau phosphatases, it is suggested that the inhibition of PP2A probably plays the most critical role in hyperphosphorylation of tau in AD (Liu et al. 2005). We also examined the expression level and phosphatase activity of PP2A among the four groups in AGS. Immunoblot analysis of PP2Ac revealed no significant difference in the expression levels of PP2Ac among four groups (Fig. 5a and b). PP2A is considered to be activated by methylation of its catalytic subunit and down-regulation of PP2A carboxyl methylation and methyltransferase was reported in AD (Sontag et al. 2004, 2007). We therefore also explored the changes in PP2A methylation levels in AGS during hibernation. As shown in Fig. 5c and d, PP2A methylation was significantly reduced in hibernating AGS but restored to a more comparable level in aroused AGS when compared with summer-active AGS. The kinetic analysis of phosphatase activity of PP2A in brain homogenates from hibernating and summer-active AGS at different temperatures revealed that as temperature decreased from 37 to 4°C, PP2A activity gradually and significantly decreased such that only about 20% of activity remained at 4°C compare with that of 37°C (Fig. 5e). Interestingly, PP2A activity is higher in brain extracts from summer-active AGS compared with hibernating AGS at all temperatures tested and the difference is significant at higher temperatures (Fig. 5e).
Fig. 5.
(a) Levels of protein phosphatase 2A (PP2A) catalytic unit (PP2Ac) in the brain homogenates of arctic ground squirrels (AGS) from different groups (E: euthermic; H: hibernating; A: aroused; and S: summer active) (n = 5/group) were analyzed by western blot. (b) The immunoreactivity of PP2Ac was quantitated, normalized to the levels of actin, and expressed as mean ± SD. No significant difference was found between any groups. (c) Methylation levels of PP2A catalytic unit in the brain homogenates of AGS from three different groups (n = 4/group) were analyzed by western blot. (d) The immunoreactivity of PP2Ac methylation was quantified, normalized to the level of PP2Ac, and expressed as mean ± SD. ‘#’ indicates significant difference as compared with hibernating AGS with p ≤ 0.05. (e) The kinetics of PP2A activity at different temperature of brain homogenates of AGS from torpid and summer-active groups was measured. Data was presented as mean ± SD. ‘*’ indicates significant difference when compared with summer euthermic with p < 0.05.
Discussion
In this study, we demonstrated that tau protein was differentially phosphorylated at six sites among different groups of AGS examined in this study: during the long torpor status of hibernation (i.e. hibernating group, body temperature went down to only about 4°C) tau protein was highly phosphorylated at all six sites we checked; three of these sites (i.e. S199, S262, and S404) became dephosphorylated while the other three sites remained phosphorylated in the aroused AGS, demonstrating a reversible phosphorylation at selective sites during hibernation; phosphorylation level at two sites (i.e. T205 and S404) remained higher in aroused AGS comparing with euthermic AGS. Summer-active AGS demonstrated the lowest tau phosphorylation levels of all the sites examined. Given the potential involvement of tau phosphorylation in AD pathogenesis, it is of paramount importance to understand the mechanisms underlying reversible tau phosphorylation at selected sites during hibernation without causing obvious neurological consequence. The measurement of expression levels and enzyme activity of various potential tau kinases and phosphatases during hibernation revealed that increased PKA activity and decreased PP2A activity, as well as contribution from GSK-3β, likely play a key role in increased tau phosphorylation during hibernation in AGS.
It was previously demonstrated that reversible tau phosphorylation parallels the gradual regression/reappearance of the mossy fiber system during hibernation (Arendt et al. 2003), implicating that tau phosphorylation underlies neuronal plasticity in hibernating animals. One most interesting aspect of tau phosphorylation during hibernation in our studies is the reversible tau phosphorylation at several sites (i.e. S199, S262, and S404) but not other sites (i.e. T205, S214, and S396). Although the possibility that phosphorylation at the latter sites (i.e. T205, S214, and S396) may become reversible if examined at later time points during arousal cannot be entirely ruled out, in our opinion, this is less likely. As revealed by kinetic assays, the most important factor in tau phosphorylation during hibernation is temperature. The group of aroused animals used in this study all reached a peak body temperature during arousal period (i.e. above 34°C) and no difference in core body temperature was noted between groups of aroused AGS and euthermic or summer-active AGS. Additionally, the entire arousal period (body temperature increase, stabilize, and decrease to ambient temperature) lasts < 24 h, therefore, it is less likely that there will be further dephosphorylation even if tested at a later time point. The difference in reversibility in tau phosphorylation at different sites suggests potentially different roles of these sites in the regulation of tau function during hibernation. It is suggested that S262 plays an important role in binding and stabilizing tau interaction with microtubules (Sengupta et al. 1998) and the phosphorylation of which has a strong disruptive effect on microtubules. Consistent with previous reports, S262 becomes completely dephosphorylated in aroused AGS comparing with hibernating AGS and it remains unphosphorylated in winter euthermic and summer-active AGS, suggesting a crucial role for phosphorylation at this site in determining tau function during hibernation. Along this line of reasoning, it appears that S199 and S404 likely play a similar role in tau function during hibernation. Indeed, recent evidence suggested a potentially synergistic effect between S404 phosphorylation and phosphorylation of S199 and S262 which diminished the ability of tau to protect against microtubule depolymerization (Ding et al. 2006). Given the phosphorylation/dephosphorylation of tau at these sites temporally parallels the synaptic regression and subsequent reinnervation during hibernation, one possibility is that phosphorylation at these sites increases a disrupted microtubule structure to enhance its dynamics which facilitates the structural changes of mossy fiber system during hibernation. However, phosphorylation at T205, S214, and S396 in aroused AGS is not reversed in aroused AGS and remains at a similarly high level comparing with hibernating AGS, suggesting phosphorylation at these sites is not crucial for tau function associated with the regression and reestablishment of mossy fiber system during hibernation. As hyperphosphorylation might confer tau resistance to protease degradation (Litersky and Johnson 1992), it is possible that phosphorylation at these sites may serve as a mechanism to stabilize/preserve tau proteins during the rapid changes which occur during hibernation. It must be noted that although phosphorylation at S396 was suggested to be a critical event during neurofibrillary tangle formation (Wang et al. 1996; Alonso Adel et al. 2004) and phosphorylation at T205 and S214 are found in PHF-tau in AD, no fibril was found during hibernation despite the incomplete reversibility of tau phosphorylation at these sites (Arendt et al. 2003).
Another interesting aspect of our tau phosphorylation data is that, despite reversible tau phosphorylation only at selective sites during hibernation, phosphorylation at all these sites eventually become reversed in summer-active animals as evidenced by the lowest phosphorylation levels in summer-active AGS of all six sites examined in this study. This likely indicates that lower phosphorylation levels of tau are essential for AGS to maintain normal brain function and an active life during summer. Additionally, with the inclusion of a group of summer-active AGS, we were able to demonstrate that tau phosphorylation at multiple sites is increased in winter animals compared with summer-active AGS. Although hypothermia or cold water stress induces tau phosphorylation in mice (Korneyev et al. 1995; Okawa et al. 2003; Planel et al. 2004), our observation is likely because of a seasonal effect rather than the difference in body temperatures as winter euthermic AGS keep their body temperature, similar to that of summer-active AGS, constant despite the low ambient temperature. It is tempting to speculate that such a seasonal effect may serve as a molecular signal for the animal to prepare for hibernation during winter time.
Tau phosphorylation is regulated by a delicate balance of kinases and phosphatases. Many kinases have been suggested as candidate tau kinases in vivo, among which GSK-3β and Cdk5 are the most implicated as both kinases phosphorylate tau at a large number of sites in vitro, are highly expressed in the brain, and have been shown to be associated with all stages of neurofibrillary pathology in AD (Pei et al. 1997, 1998, 1999). Indeed, activation of GSK-3β and Cdk5 could result in tau hyperphosphorylation in mouse models (Ahlijanian et al. 2000; Lucas et al. 2001; Noble et al. 2003). Therefore, we evaluated the potential contribution of these two kinases to reversible phosphorylation of tau during hibernation. Interestingly, although both immunoblot analysis [i.e. phospho-GSK-3β (Ser9) or phospho-GSK-3β (Tyr216)] and kinase activity assay suggested that GSK-3β is inactivated in hibernating and aroused AGS, the kinetic study of GSK-3β kinase activity at different temperatures revealed that, during the decrease of temperature from 37 to 4°C, GSK-3β activity initially increased and peaked at 20°C, and then gradually decreased. These data suggest that GSK-3β may not play an important role in maintaining the high steady-state level of tau phosphorylation in hibernating AGS, but might play a very important role in increased tau phosphorylation during the process when body temperature of AGS drops during hibernation. More importantly, we found that during the decrease of temperature from 37 to 4°C, PKA activity gradually increased in both hibernating and summer-active AGS such that PKA activity at 4°C increased to about 2.4-fold of that of 37°C. The implications of these data in relation to tau phosphorylation during hibernation are twofold: (i) PKA may directly phosphorylate tau at specific sites during the process of body temperature decrease and maintain the steady-state tau phosphorylation during long torpor in hibernating AGS; and (ii) as tau phosphorylated by PKA becomes a better substrate for GSK-3β, increased PKA activity likely enhances tau phosphorylation by GSK-3β both during body temperature change and during long torpor. In this regard, it is of interest to note that total PKA activity in the brain was the highest among six major organs including brown adipose tissue where thermogenesis is required for AGS to arouse from torpor during hibernation, emphasizing the importance of this kinase in the brain during hibernation (MacDonald and Storey 1998). Importantly, our observation of increased PKA activity at lower temperature is consistent with prior studies with purified PKAc from Richardson’s ground squirrels (i.e. a hibernating species) and rabbit (i.e. a non-hibernating species) which demonstrated a species-specific and temperature-dependent change in PKAc kinetics in a manner that would facilitate a rapid and large activation of PKA at low temperature (MacDonald and Storey 1998). Our finding that levels of PKA RI subunit were reduced in hibernating AGS comparing with aroused AGS suggested that the levels of PKA RI may be an additional factor that contributes to the increased activation of PKA in hibernating AGS. Given the fact that levels of PKA RI subunit were also reduced in summer-active AGS, it is likely that levels of RI, in addition to temperature, are also affected by seasonal effect. Comparing with GSK-3β, it appears that Cdk5 plays a minor role in tau phosphorylation during hibernation as its activity toward tau is many folds lower than GSK-3β at all conditions measured, consistent with previous studies (Plattner et al. 2006). MAPKs (i.e. extracellular signal-regulated kinase, c-Jun N-terminal kinase and p38) are also implicated in tau phosphorylation (Zhu et al. 2001a,b, 2002). Given that MAPKs demonstrated lower activity in hibernating groups (Zhu et al. 2005), it is likely that MAPKs do not play a major role in tau phosphorylation during hibernation either.
In AD, activity of protein phosphatases including PP2A, PP1, and PP5 was decreased, paralleling the increase in tau phosphorylation. It is believed that among all these protein phosphatases, PP2A likely plays the most important role in mediating tau hyperphosphorylation in AD neurons (Liu et al. 2005). Studies have shown that starved mice, reduced glucose metabolism, and anesthesia could lead to tau phosphorylation by inhibition of PP2A, indicating the importance of PP2A in regulation of tau phosphorylation (Planel et al. 2001, 2004, 2007). In our studies, there was no change in the expression level of PP2A catalytic unit between all four groups of AGS. However, the levels of PP2A methylation were significantly reduced in hibernating AGS but were restored in aroused AGS when compared with summer-active AGS. It is known that PP2A methylation enhances its activity through alteration of PP2A substrate specificity (Tolstykh et al. 2000; Yu et al. 2001). In the specific case of tau dephosphorylation, methylation is required for efficient assembly of Bα-containing heterotrimers, which is the major PP2A enzyme that binds to and dephosphorylates tau (Tolstykh et al. 2000; Yu et al. 2001). Our finding of reduced PP2A methylation in hibernating AGS suggested a lower PP2A activity in this group of AGS. Indeed, the activity assay indicated that PP2A activity decreased significantly in hibernating AGS compared with summer-active AGS. As increased tau phosphorylation was found in hibernating AGS, these findings suggest that reduced PP2A activity contributes to increased tau phosphorylation during hibernation and reduced PP2A methylation is likely one of the mechanisms responsible for reduced PP2A activity during hibernation. Importantly, PP2A activity dramatically decreased with temperature decline and only around 20% of activity remained at 4°C compared with 37°C, suggesting that decreased PP2A activity not only plays an important role in maintaining high steady-state tau phosphorylation in hibernating AGS, but also may contribute to changes in tau phosphorylation during the process of gradual decline in body temperature when an animal enters hibernation. Notably, the fact that PP2A activity is higher in brain extracts from summer-active AGS compared with hibernating AGS at all temperatures tested suggests an intrinsic difference in PP2A enzyme or its regulation between these two groups of AGS in which the difference in methylation may be a factor.
In conclusion, our studies confirmed tau phosphorylation at multiple sites in hibernating AGS. Importantly, tau phosphorylation was reversible in aroused AGS at some sites but not at other sites suggesting differential roles of these sites in determining tau function during hibernation. Furthermore, GSK-3β and PKA, along with decreased PP2A activity, may play a significant role in increased tau phosphorylation during hibernation.
Acknowledgments
This work was supported by the Alzheimer’s Association (NIRG-05-13985), US Army Medical Research and Material Command Grant No. 05178001, USAMRMC No. 05178001 and NS041069-06 (National Institute of Neurological Disorders and Stroke, National Institute of Mental Health).
Abbreviations used
- AD
Alzheimer disease
- AGS
arctic ground squirrels
- C
catalytic subunit
- Cdk5
cyclin dependent kinase 5
- GSK
glycogen synthase kinase
- MAP
microtubule associated protein
- NFTs
neurofibrillary tangles
- PHF
paired helical filaments
- PKA
protein kinase A
- PKAc
PKA catalytic subunit
- PMSF
phenylmethylsulfonyl fluoride
- PP2A
protein phosphatase 2A
- R
regulator subunit
- SDS
sodium dodecyl sulfate
References
- Ahlijanian MK, Barrezueta NX, Williams RD, et al. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA. 2000;97:2910–2915. doi: 10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso Adel C, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by fronto-temporal dementia tau mutations. J Biol Chem. 2004;279:34873–34881. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- Arendt T, Stieler J, Strijkstra AM, Hut RA, Rudiger J, Van der Zee EA, Harkany T, Holzer M, Hartig W. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci. 2003;23:6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Morphological criteria for the recognition of Alzheimer’s disease and the distribution pattern of cortical changes related to this disorder. Neurobiol Aging. 1994;15:355–356. doi: 10.1016/0197-4580(94)90032-9. discussion 379–380. [DOI] [PubMed] [Google Scholar]
- Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, Pearl LH. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–732. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Ding H, Matthews TA, Johnson GV. Site-specific phosphorylation and caspase cleavage differentially impact tau-microtubule interactions and tau aggregation. J Biol Chem. 2006;281:19107–19114. doi: 10.1074/jbc.M511697200. [DOI] [PubMed] [Google Scholar]
- Drew KL, Rice ME, Kuhn TB, Smith MA. Neuro-protective adaptations in hibernation: therapeutic implications for ischemia-reperfusion, traumatic brain injury and neurodegenerative diseases. Free Radic Biol Med. 2001;31:563–573. doi: 10.1016/s0891-5849(01)00628-1. [DOI] [PubMed] [Google Scholar]
- Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- Gomez-Ramos A, Smith MA, Perry G, Avila J. Tau phosphorylation and assembly. Acta Neurobiol Exp (Wars) 2004;64:33–39. doi: 10.55782/ane-2004-1489. [DOI] [PubMed] [Google Scholar]
- Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Dysregulation of protein phosphorylation/dephosphorylation in Alzheimer’s disease: a therapeutic target. J Biomed Biotechnol. 2006:31825. doi: 10.1155/JBB/2006/31825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3 beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I. Discoveries of tau, abnormally hyperphosphorylated tau and others of neurofibrillary degeneration: a personal historical perspective. J Alzheimers Dis. 2006;9:219–242. doi: 10.3233/jad-2006-9s325. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I. Developing pharmacological therapies for Alzheimer disease. Cell Mol Life Sci. 2007;64:2234–2244. doi: 10.1007/s00018-007-7221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenagy GJ, Sharbaugh SM, Nagy KA. Annual cycle of energy and time expenditure in a golden-mantled ground squirrel population. Oecologia. 1989;78:269–282. doi: 10.1007/BF00377166. [DOI] [PubMed] [Google Scholar]
- Korneyev A, Binder L, Bernardis J. Rapid reversible phosphorylation of rat brain tau proteins in response to cold water stress. Neurosci Lett. 1995;191:19–22. doi: 10.1016/0304-3940(95)11546-3. [DOI] [PubMed] [Google Scholar]
- Litersky JM, Johnson GV. Phosphorylation by cAMP-dependent protein kinase inhibits the degradation of tau by calpain. J Biol Chem. 1992;267:1563–1568. [PubMed] [Google Scholar]
- Liu SJ, Zhang JY, Li HL, et al. Tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated by PKA in rat brain. J Biol Chem. 2004;279:50078–50088. doi: 10.1074/jbc.M406109200. [DOI] [PubMed] [Google Scholar]
- Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3β conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JA, Storey KB. cAMP-dependent protein kinase from brown adipose tissue: temperature effects on kinetic properties and enzyme role in hibernating ground squirrels. J Comp Physiol. 1998;168:513–525. doi: 10.1007/s003600050172. [DOI] [PubMed] [Google Scholar]
- Matsuo ES, Shin RW, Billingsley ML, Van deVoorde A, O’Connor M, Trojanowski JQ, Lee VM. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer’s disease paired helical filament tau. Neuron. 1994;13:989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Noble W, Olm V, Takata K, et al. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- Okawa Y, Ishiguro K, Fujita SC. Stress-induced hyperphosphorylation of tau in the mouse brain. FEBS Lett. 2003;535:183–189. doi: 10.1016/s0014-5793(02)03883-8. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Tanaka T, Tung YC, Braak E, Iqbal K, Grundke-Iqbal I. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol. 1997;56:70–78. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Grundke-Iqbal I, Iqbal K, Bogdanovic N, Winblad B, Cowburn RF. Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer’s disease neurofibrillary degeneration. Brain Res. 1998;797:267–277. doi: 10.1016/s0006-8993(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Distribution of active glycogen synthase kinase 3 beta (GSK-3β) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Planel E, Yasutake K, Fujita SC, Ishiguro K. Inhibition of protein phosphatase 2A overrides tau protein kinase I/glycogen synthase kinase 3 beta and cyclin-dependent kinase 5 inhibition and results in tau hyperphosphorylation in the hippocampus of starved mouse. J Biol Chem. 2001;276:34298–34306. doi: 10.1074/jbc.M102780200. [DOI] [PubMed] [Google Scholar]
- Planel E, Miyasaka T, Launey T, et al. Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer’s disease. J Neurosci. 2004;24:2401–2411. doi: 10.1523/JNEUROSCI.5561-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E, Richter KE, Nolan CE, et al. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci. 2007;27:3090–3097. doi: 10.1523/JNEUROSCI.4854-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner F, Angelo M, Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem. 2006;281:25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- Roder HM. Prospect of therapeutic approaches to tauopathies. J Mol Neurosci. 2003;20:195–202. doi: 10.1385/jmn:20:2:195. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Skalhegg B, Jahnsen T. The two mRNA forms for the type I alpha regulatory subunit of cAMP-dependent protein kinase from human testis are due to the use of different polyadenylation site signals. Biochem Biophys Res Commun. 1990;167:323–330. doi: 10.1016/0006-291x(90)91768-n. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Kabat J, Novak M, Wu Q, Grundke-Iqbal I, Iqbal K. Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch Biochem Biophys. 1998;357:299–309. doi: 10.1006/abbi.1998.0813. [DOI] [PubMed] [Google Scholar]
- Skalhegg BS, Landmark B, Foss KB, Lohmann SM, Hansson V, Lea T, Jahnsen T. Identification, purification, and characterization of subunits of cAMP-dependent protein kinase in human testis. Reverse mobilities of human RII alpha and RII beta on sodium dodecyl sulfate–polyacrylamide gel electrophoresis compared with rat and bovine RIIs. J Biol Chem. 1992;267:5374–5379. [PubMed] [Google Scholar]
- Sontag E, Hladik C, Montgomery L, Luangpirom A, Mudrak I, Ogris E, White CL., III Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J Neuropathol Exp Neurol. 2004;63:1080–1091. doi: 10.1093/jnen/63.10.1080. [DOI] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Sontag JM, Diaz-Arrastia R, Ogris E, Dayal S, Lentz SR, Arning E, Bottiglieri T. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J Neurosci. 2007;27:2751–2759. doi: 10.1523/JNEUROSCI.3316-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Wang J. Role of serine/threonine protein phosphatase in Alzheimer’s disease. Neurosignals. 2002;11:262–269. doi: 10.1159/000067425. [DOI] [PubMed] [Google Scholar]
- Tolstykh T, Lee J, Vafai S, Stock JB. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Ishiguro K, Ohnuma J, Takamatsu M, Yonekura S, Imahori K. Precursor of cdk5 activator, the 23 kDa subunit of tau protein kinase II: its sequence and developmental change in brain. FEBS Lett. 1994;355:35–40. doi: 10.1016/0014-5793(94)01163-x. [DOI] [PubMed] [Google Scholar]
- Wang JZ, Grundke-Iqbal I, Iqbal K. Restoration of biological activity of Alzheimer abnormally phosphorylated tau by dephosphorylation with protein phosphatase-2A, -2B and -1. Brain Res Mol Brain Res. 1996;38:200–208. doi: 10.1016/0169-328x(95)00316-k. [DOI] [PubMed] [Google Scholar]
- Yu XX, Du X, Moreno CS, Green RE, Ogris E, Feng Q, Chou L, McQuoid MJ, Pallas DC. Methylation of the protein phosphatase 2A catalytic subunit is essential for association of Balpha regulatory subunit but not SG2NA, striatin, or polyoma-virus middle tumor antigen. Mol Biol Cell. 2001;12:185–199. doi: 10.1091/mbc.12.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Rottkamp CA, Boux H, Takeda A, Perry G, Smith MA. Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J Neuropathol Exp Neurol. 2000;59:880–888. doi: 10.1093/jnen/59.10.880. [DOI] [PubMed] [Google Scholar]
- Zhu X, Castellani RJ, Takeda A, Nunomura A, Atwood CS, Perry G, Smith MA. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the ‘two hit’ hypothesis. Mech Ageing Dev. 2001a;123:39–46. doi: 10.1016/s0047-6374(01)00342-6. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Boux H, Smith MA. Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J Neurochem. 2001b;76:435–441. doi: 10.1046/j.1471-4159.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee HG, Raina AK, Perry G, Smith MA. The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neurosignals. 2002;11:270–281. doi: 10.1159/000067426. [DOI] [PubMed] [Google Scholar]
- Zhu X, Smith MA, Perry G, Wang Y, Ross AP, Zhao HW, Lamanna JC, Drew KL. MAPKs are differentially modulated in arctic ground squirrels during hibernation. J Neurosci Res. 2005;80:862–868. doi: 10.1002/jnr.20526. [DOI] [PubMed] [Google Scholar]