Abstract
Hibernation is a natural model of neuroprotection and adult synaptic plasticity. NMDA receptors (NMDAR), which play key roles in excitotoxicity and synaptic plasticity, have not been characterized in a hibernating species. Tolerance to excitotoxicity and cognitive enhancement in Arctic ground squirrels (AGS, Spermophilus parryii) suggests that NMDAR expression may decrease in hibernation and increase upon arousal. NMDAR consist of at least one NMDAR1 (NR1) subunit, which is required for receptor function. Localization of NR1 reflects localization of the majority, if not all, NMDAR complexes. The purpose of this study, therefore, was to characterize the distribution of NR1 subunits in AGS central nervous system using immunohistochemistry. In addition, we compare NR1 expression in hippocampus of hibernating AGS (hAGS) and inter-bout euthermic AGS (ibeAGS) and assess changes in cell somata size using NR1 stained sections in three hippocampal sub-regions (CA1, CA3, and dentate gyrus). For the first time, we report that immunoreactivity of anti-NR1 is widely distributed throughout the central nervous system in AGS and is similar to other species. No differences exist in the expression and distribution of NR1 in hAGS and ibeAGS. However, we report a significant decrease in size of hippocampal CA1 and dentate gyrus NR1-expressing neuronal somata during hibernation torpor.
Keywords: NMDAR1, Immunohistochemistry, Hibernation, Neuronal somata size, Hippocampus
1. Introduction
Heterothermic mammals, i.e., mammals that hibernate, provide extreme examples of neuroprotection in both the hibernating and euthermic (non-hibernating) state (Frerichs and Hallenbeck, 1998; Zhou et al., 2001; Ma et al., 2005; Ross et al., 2006; Dave et al., 2006). These animals also show pronounced synaptic plasticity (Popov et al., 1992; Popov and Bocharova, 1992) and evidence of adult cognitive enhancement following arousal from hibernation (Mihailovic et al., 1968; Weltzin et al., 2006). N-Methyl-D-aspartate receptors (NMDAR) play essential roles in excitotoxicity, synaptic plasticity, and learning and memory, yet no studies have begun to characterize NMDAR in heterothermic species.
NMDAR have three major subunits: NMDAR1 (NR1), NMDAR2A-D (NR2A-D), and NMDAR3 (NR3). All functional NMDAR consist of at least one NR1 subunit in various combinations with two or more NR2A-D subunits, and sometimes include an NR3 subunit. NR1 is expressed throughout the brain and throughout all stages of development. Localization of NR1 reflects localization of the majority, if not all, NMDAR complexes (Forrest et al., 1994; Sakimura et al., 1995; Nakanishi, 1992). The purpose of this study was, therefore, to characterize the distribution of NR1 subunits in the central nervous system of Arctic ground squirrels (AGS, Spermophilus parryii) using a NR1 antibody, which has been well characterized in rats and other species such as mouse, monkey, and human (Johnson et al., 1996; Bilak et al., 1995; Siegel et al., 1994; Huntley et al., 1994).
Hippocampal neurons are most vulnerable to ischemia and hippocampus shows structural (Popov et al., 1992; Popov and Bocharova, 1992) and functional changes (Weltzin et al., 2006) after arousal from hibernation. Therefore, the second aim of this study was to compare NR1 expression in hippocampus between hibernating AGS (hAGS) and inter-bout euthermic AGS (ibeAGS). In addition, Popov et al. (1992) reported a decrease in volume of CA3 somata in hibernating ground squirrels (Spermophilus undalatus), an Arctic species closely related to AGS. In their study, the volume of neuronal cell bodies was measured in gallocyanin-stained paraffin sections. Gallocyanin is a nuclear stain, and, therefore, decreased volume could only be attributed to the nucleus and not the entire cell body (Schulte et al., 1991). Previous studies show that changes of nuclear size in taste receptors occur during hibernation, but not cell bodies (Popov et al., 1999). Because the majority of NMDAR are found in the membrane fraction of AGS (Zhao et al., 2006) and are known to be distributed on the plasma membrane of neurons in other species (Monaghan and Cotman, 1985; Kharazia et al., 1996), NR1 stained neurons provide an opportunity to measure cell body size. Here, for the first time we report the distribution of NR1 in AGS using immunohistochemistry and show significant changes in the size of hippocampal CA1 and dentate gyrus neuronal somata during hibernation.
2. Materials and methods
2.1. Antibodies
Mouse anti-NR1 monoclonal antibody (Cat# MAb363, Chemicon, Temecula, CA) was used for immunohistochemistry and subsequent semi-quantitative Western blot analysis. This antibody was raised against a recombinant fusion protein containing glutathione transferase (GST) and NMDAR1 aa. 660–811 of rat NMDAR1 (Moriyoshi et al., 1991; Siegel et al., 1994), which is located on the extracellular loop between the third and fourth transmembrane regions. The specificity of mouse anti-NR1 monoclonal antibody in AGS was confirmed by western blot analysis and a single band of approximately 116 kDa was observed (Fig. 5). Mouse anti-β-actin monoclonal antibody (Cat# 5316, Sigma–Aldrich Corp. St. Louis, MO) was used as a loading control for immunoblotting. This antibody recognizes an epitope located on the N-terminal end of the β-isoform of actin (42 kDa) (Liao et al., 2000).
Fig. 5.
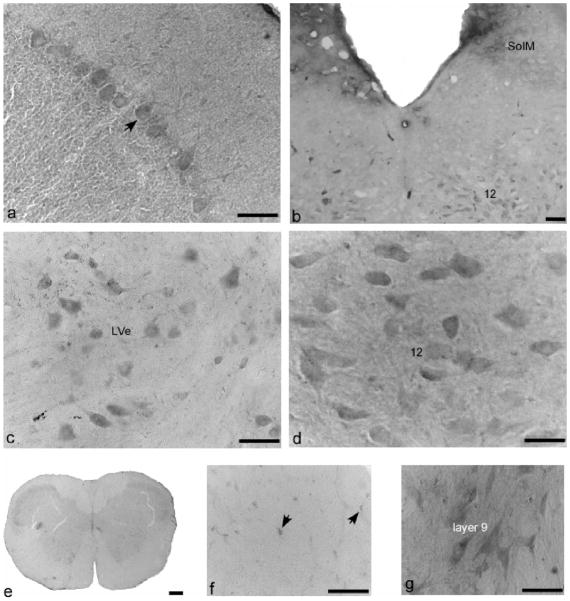
Immunostaining of NR1 in cerebellum, brain stem, and cervical spinal cord of AGS brain. (a) Cerebellar cortex, arrow indicates a Purkinje cell with two dendritic branches. (b) Brain stem, SolM, nucleus of solitary tract, medial part; 12, hypoglossal nucleus, also seen in (d). (c) Lateral vestibular nucleus, LVe; (e) spinal cord in lower power; (f) glia cell in the white matter of spinal cord (C2); (g) layer 9 of cervical spinal cord. (b), (c) and (e–g) are from ibeAGS, (a) and (d) are from hAGS. Scale bar in (e) is 200 μm, in others, 50 μm.
2.2. Animals
The Institutional Animal Care and Use Committee of the University of Alaska Fairbanks approved all animal procedures. AGS were trapped on the northern slope of the Brooks Range, Alaska in July under permit from the Alaska Department of Fish and Game. Upon arrival at the university, AGS were quarantined for at least 14 days. All AGS were housed individually in cages at an ambient temperature (Ta) of approximately 18 °C, fed approximately 40 g of Mazuri Rodent Chow per day, and kept on natural lighting for 64° latitude where the light:dark cycle changed from 20 h:4 h to 16 h:8 h before AGS were moved to “winter” conditions. In mid-August, AGS were fed 10–15 sunflower seeds each day for 2 weeks and were then moved to environmental chambers to facilitate hibernation. Chambers were set to a Ta of 2 °C and 4 h:20 h light:dark cycle. While housed in environmental chambers, all AGS were fed rodent chow ad libitum although animals do not eat when hibernating.
Groups of AGS (n = 4 animals per group, hAGS and ibeAGS) were matched for age, sex, and body weight. At the time of tissue collection, hAGS had experienced at least three bouts of torpor and were at least 3 days into their current bout. Respiratory rate was less than 5 breaths per minute, and wood shavings placed on an animal’s back 24 h previously remained undisturbed. ibeAGS had experienced at least two bouts of torpor. For the immunohistochemistry experiment, ibeAGS aroused naturally in the cold chamber (Ta, 2 °C). For the Western blot analysis, ibeAGS were aroused by transferring to a warm room (Ta, 18 °C). Tissues for both experiments were collected approximately 24 h later. Respiration rate for ibeAGS was more than 80 breaths per minute, body temperature was more than 35.5 °C, and animals responded quickly to touch.
2.3. Immunohistochemistry
Animals were anaesthetized with 5% halothane and maintained at 3% halothane mixed with 100% oxygen, delivered at a flow rate of 1.5 l/min. Prior to perfusion, the descending aorta was clamped to achieve more efficient perfusion of the brain. AGS were perfused transcardially with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were quickly removed and post-fixed in 4% paraformaldehyde at 4 °C overnight, and then cryoprotected in 30% sucrose until brains sank. Brains were rapidly frozen in isopentane at −30 to −40 °C, which was pre-cooled with dry ice or liquid N2. Coronal sections were cut with a cryostat (Leica CM 1800) at 50 μm and stored in cyroprotectant (1% (w/v) polyvinylpyrrolidone, 30% sucrose, and 30% ethylene glycol in 0.1 M PBS) at −20 °C until use.
Sections containing complete hippocampal regions from hAGS and ibeAGS were stained in parallel for comparison of NR1 staining intensity and hippocampal neuronal size. For remaining brain areas, approximately 20 sections per animal were processed for immunohistochemistry. The best quality sections from each region were selected for further examination and illustration. All incubations were carried out with gentle agitation at room temperature unless otherwise stated. Immunostaining was carried out according to standard procedure (Petralia et al., 1994; Huntley et al., 1994). Briefly, free-floating sections were labeled with anti-NR1 (1:1000) overnight followed by incubating with secondary antibody (biotinylated anti-mouse IgG, 1:200, Vector Laboratory, Burlingame, CA). After incubation in avidin-biotin-peroxidase (vectastain ABC kit, Vector Laboratory, Burlingame, CA), sections were treated with 0.03% of 3′,3-diaminobenzidine tetrahydrochloride (DAB, Sigma–Aldrich Corp. St. Louis, MO). After washing, the sections were mounted on slides and coversliped with Permount (Fisher, Pittsburgh, PA). PBS controls, in which primary antibody was omitted and replaced by PBS, were run in every experiment under the same conditions.
Images of immunostained brain slices were taken with a Zeiss Axioplan 2 imaging microscope (Zeiss, Germany). Level of staining was described using a relative scale from 0 to 4, where 0 indicates the level of staining seen in corresponding control sections and 4 indicates the level of densest staining.
The cell body area of pyramidal neurons in hippocampal CA1 and CA3 regions and granular cells in hippocampal dentate gyrus was compared in both hAGS and ibeAGS. For each region of interest, 30 or more neurons within the dorsal hippocampus, corresponding to Figs. 33–35 of the rat Atlas (Paxinos and Watson, 1998), were measured by manually tracing around the perimeter of each cell body using Metamorph software 6.2 (Meridian Instrument Co., Kent, WA). Areas of 30 or more neurons were averaged for each animal such that sample size equaled the number of animals per group (n = 4 AGS per group). Since no statistically significant difference in cell size was observed in CA3, we further quantified NR1 staining intensity in the CA3 region between hAGS and ibeAGS. Average staining intensity in CA3 was calculated by subtracting the background value from integrated intensity of objects using ImageQuant 5.2 software (Amersham Biosciences, Piscataway, NJ).
2.4. Western blot analysis
Because a difference in the total number of positive numbers or density of NR1-immunoreactivity could be influenced by cell size, we used Western blotting to semi-quantitatively compare NR1 expression between hAGS and ibeAGS. Animals were lightly anesthetized and brains were removed quickly after decapitation. Hippocampi were dissected, quickly frozen in liquid N2, and stored at −80 °C.
Total protein lysate preparation and western blotting were performed according to a previously published procedure (Zhao et al., 2006). Briefly, total protein lysate was prepared from approximately 20–30 mg of hippocampal tissue in 200–300 μl (10× volumes) of ice cold 1% NP-40 lysis buffer (50 mM Tris–HCl (pH 7.6), 0.02% sodium azide, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP-40, 150 mM NaCl with protease/phosphatase inhibitors 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 10 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 μg/ml antipain) with a motor-driven polytron homogenizer for 30–40 s. Homogenates were left on ice for 40 min and then centrifuged at maximal speed for 10 min using a microcentrifuge. The supernatant was collected and termed total protein lysate. Protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). Twenty micrograms of protein was separated on 8% polyacrylamide gels using SDS-PAGE and then transferred to nitrocellulose membranes. The membranes were incubated with anti-NR1 (1:1000) and in TBST (TBS, 0.1% Tween 20) with 1% bovine serum albumin overnight at 4 °C with gentle agitation. The membranes were washed with TBST and incubated with horseradish peroxidase-conjugated secondary antibody (anti-mouse IgG, 1:2000, Bio-Rad, Hercules, CA) for 1 h. Immunoreactive bands were visualized using enhanced chemiluminescence (ECL, Perkin-Elmer, Boston, MA) and determined by SDS-PAGE standard markers (BIO-RAD, Hercules, CA). Then membranes were stripped by incubation with 10 mM Tris–HCl (pH 2), 150 mM NaCl for 30 min. Equal loading was confirmed by reprobing with anti-actin (1:5000) diluted in TBST with 1% bovine serum albumin. Scans of ECL exposures were analyzed using ImageQuant 5.2 software (Amersham Biosciences, Piscataway, NJ).
All data were analyzed using one-way ANOVA (Sigmastat Ver3.0, SYSSTAT Software Inc., Chicago, IL). Data were expressed as group means ± S.E.M. The criterion for statistical significance was p < 0.05.
3. Results
3.1. Controls
Staining of sections throughout the brains produced a pattern of NR1 immunoreactivity that was similar to previous studies (Huntley et al., 1994; Kharazia et al., 1996; Petralia et al., 1994). NR1 positive cells throughout the brain including pyramidal neurons in hippocampus and cortex showed classic morphology and distribution pattern (Petralia et al., 1994; Siegel et al., 1994). Specificity of NR1 antibody was confirmed using western blot analysis of NR1. A single band was observed and is shown in Fig. 6. Specificity of the secondary antibody was confirmed by omitting the primary antibody and replacing it with PBS. Specific labeling in these sections was completely abolished (Fig. 1a).
Fig. 6.
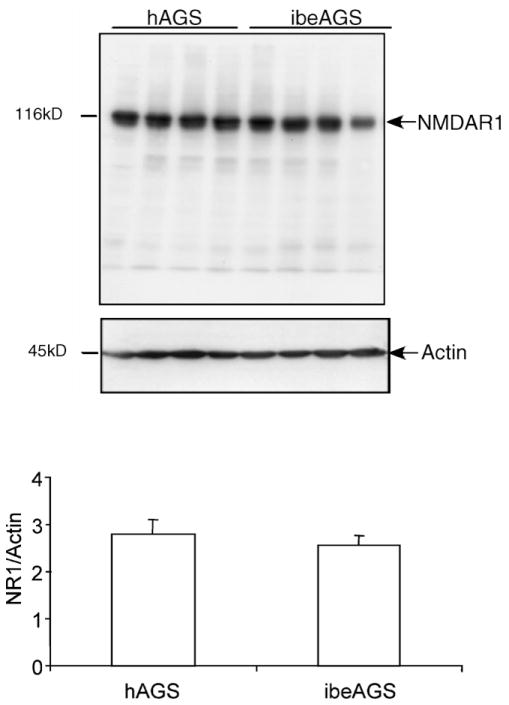
NR1 abundance is similar in total protein lysates prepared from hAGS and ibeAGS hippocampi. Immunoblots (top) show representative results of a membrane labeled with anti-NR1 antibody and reprobed with actin as a loading control. Densitometric analysis of NR1 immunoreactivity relative to loading control (bottom graph) shows no difference in NR1 abundance between hAGS and ibeAGS (p > 0.05, n = 4 AGS per group).
Fig. 1.
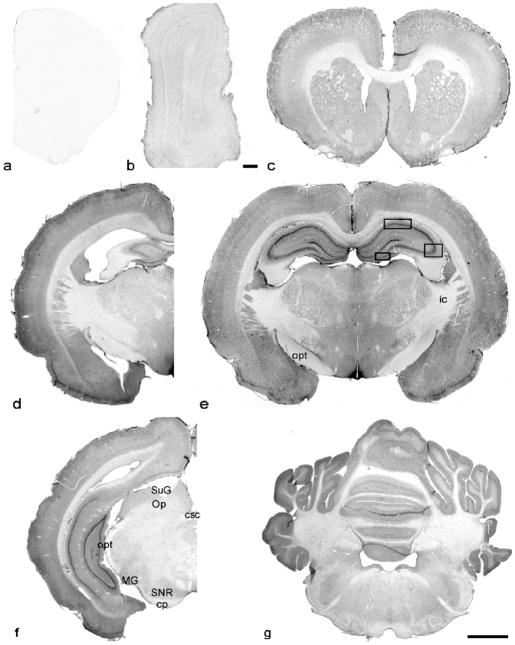
Immunolabeling of NR1 in coronal sections of AGS brain from forebrain to cerebellum: (a) control section at the same level as the NR1 stained section shown in (c); (b–f) olfactory bulb to cerebellum; (a–c), (d) and (f) are from ibeAGS; (e) and (g) are from hAGS. opt, optic tract; ic, internal capsule; csc, commissural of the superior colliculus; SuG, superficial gray layer of the superior colliculus; Op, optic nerve layer of the superior colliculus; MG, medial geniculate nucleus; SNR, substantia nigra, reticular part; cp, cerebral peduncle, basal part. Scale bar in (b), 100 μm, in others, 2.5 mm. Frames in (e) indicate the areas where neuronal soma size was measured.
3.2. Overall NR1 distribution
Immunoreactivity of anti-NR1 was widely distributed throughout the central nervous system in both ibeAGS and hAGS (Table 1). In hippocampus where NR1 distribution in hAGS and ibeAGS were run in parallel for comparison, no qualitative differences were noted. In other regions, the following description of NR1 staining pattern includes observations from hAGS or ibeAGS.
Table 1.
NR1 expression in hibernating or inter-bout euthermic AGS braina
| Brain regions | Level of staining |
|---|---|
| Olfactory region | |
| Anterior olfactory nucleus (AO) | 2 |
| Main olfactory bulb | |
| Glomerular layer of olfactory bulb (Gl) | 1 |
| Periglomerular cell (Pg) | 1 |
| External plexiform layer olfactory bulb (EPl) | 1.5 |
| Mitral cell layer of olfactory bulb (Mi) | 2 |
| Internal plexiform layer olfactory bulb (Ipl) | 1 |
| Granular cell layer of olfactory bulb (Gro) | 1–1.5 |
| Cortex | |
| Piriform cortex (pir) | 3.5 |
| Frontal association cortex (FrA) and prelimbic cortex (PrL) | |
| Layer 1 | 0–0.5 |
| Layers 2–3 | 2.5 |
| Layer 4–6 | 2 |
| Parietal association cortex (PtA) and primary motor cortex (M1) | |
| Layer 1 | 0–0.5 |
| Layers 2–3 | 3.5 |
| Layer 4 | 2–2.5 |
| Layer 5 | 3–3.5 |
| Layer 6 | 2.5–3 |
| Hippocampus | |
| CA1 | |
| Stratum oriens (Or) | 1–1.5 |
| Pyramidal layer (Py) | 3.5–4 |
| Stratum radiatum (Ra) | 2.5 |
| Stratum lacunosum-moleculare (LM) | 1.5 |
| CA3 | |
| Stratum oriens (Or) | 1–1.5 |
| Pyramidal layer (Py) | 3–3.5 |
| Stratum lucidum (Lu) | 3 |
| Dentate gyrus | |
| Molecular layer (Mo) | 1.5 |
| Granular layer (Gr) | 3.5–4 |
| Polymorph layer (hilus) | 3–3.5 |
| Amygdala | |
| Posterolateral cortical amygdale nucleus (PLCo) | 2.5 |
| Posteromeidal cortical amygdale nucleus (PMCo) | 2.5 |
| Dorsal endopiriform nucleus (Den) | 1.5 |
| Lateral amygdaloid nucleus (La) | 2.5–3 |
| Basolateral amygaloid nucleus (BL) | 3 |
| Septum | |
| Medial septal nucleus (MS) | 2 |
| Lateal septal nucleus, dorsal (LSD) | 1 |
| Lateal septal nucleus, intermediate (LSI) | 1 |
| Nucleus of horizontal limb diagonal band (HDB) | 2 |
| Nucleus of vertical limb diagonal band (VDB) | 2 |
| Basal ganglia | |
| Caudate nucleus | 2–2.5 |
| Putamen | 3 |
| Nucleus accumbens core (Acbc) | 2 |
| Lateral global pallidum (LGP) | 2 |
| Ventral pallidum (VP) | 2 |
| Thalamus | |
| Lateral habenular nucleus (LHb) | 2 |
| Medial habenular nucleus (MHb) | 3.5 |
| Stria medullairs of thalamus (sm) | 0–0.5 |
| Paraventricular thalamic nucleus, posterior (PVP) | 3–3.5 |
| Intermediodorsal thalamic nucleus (IMD) | 2.5–3 |
| Centeral medial thalamic nucleus (CM) | 3 |
| Reuniens thalamic nucleus (Re) | 3 |
| Laterodorsal thalamic nucleus (LDV) | 2.5–3 |
| Ventral thalamic nuclear group (Vl, VM) | 2.5–3 |
| Ventral posterior thalamic neulear group (VPM, VPL) | 2.5–3 |
| Posterior thalamic nuclear group (Po) | 2.5–3 |
| Hypothalamus | |
| Lateral hypothalamic area (LH) | 1.5 |
| Magnocellular nucleus of lateral hypothamus | 2.5 |
| Dorsomedial nucleus, dorsal (DMD) | 2 |
| Perifornical nucleus (PeF) | 2 |
| Periventrical hypothalamic nucleus (Pe), neuropilar | 1.5 |
| Ventromed nucleus (VMH) | |
| Dorsomedial part | 2.5–3 |
| Ventrolateral part | 2 |
| Arcuate nucleus, medial part (ArcM) | 3.5 |
| Medial eminence (ME) | 3.5 |
| Suprachiasmatic nucleus (SCN) | 1 |
| Lateral preoptic area (LPO) | 0.5–1 |
| Supraopic nucleus (SO) | 1 |
| Midbrain | |
| Superficial gray layer of superior colliculus (SuG) | 2 |
| Optic nuclear layer of superior colliculus (OP) | 1 |
| Optic tract | 0 |
| Medial genic nucleus (MG) | 1.5 |
| Substantia nigra (SNR) | 1.5 |
| Commissural of superior colliculus (CSC) | 0.5 |
| Cerebral peduncle (CP) | 0.5 |
| Cerebellum | |
| Cerebellar cortex | |
| Molecular layer | 2 |
| Stellate cells | 1.5 |
| Granular layer | 2 |
| Purkinje cell body | 2.5 |
| Purkinje cell dendrities | 2–2.5 |
| Golgi cell | 1.5–2 |
| Brain stem | |
| Nucleus of the solitary trat, parvicellular part (SolPC) | 3–3.5 |
| Nucleus of the solitary tract, medial part (SolM) | 2 |
| Subpostrema dorsal (SubPD) | 3.5 |
| Hypoglossal (12) | 2.5–3 |
| Lateral reticular nucleus (LRt) | 2 |
| Lateral recticular nucleus, parvicell (LRtPC) | 1.5–2 |
| Superior paraolivary nucleus (SPO) | 1.5 |
| Medial vestibular nuclei, magnocellular part (MVeMC) | 2.5 |
| Facial nucleus (7) | 2 |
| Inferior olive (IO) | 2 |
| Dorsal motor nucleus of vagus (10) | 2 |
| Spinal trigeminal tract (sp5) | 0.5–1 |
| Spinal 5 nucleus (Sp5C, Sp5O) | 1–1.5 |
| Cervical spinal cord | |
| Lateral cervical nucleus (Latc) | 1.5 |
| Central cervical nucleus (CeCv) | 2 |
| Intermediomedial cell column (IMM) | 1.5 |
| Laminae I–III | 2 |
| Laminae IV–VIII | 1.5 |
| Motor neurons of laminae IX | 2.5 |
| Laminae X | 1.5 |
Level of staining is described using a relative scale from 0 to 4, in which 0 indicates the level seen in corresponding control sections and 4 indicates the densest staining. NR1 expression in hibernating and euthermic brain was compared systematically in hippocampus. Observations from other regions were made in brain sections from hAGS or ibeAGS.
The densest staining with anti-NR1 antibody was found throughout the hippocampus. Staining was also dense in layers 2–3 and layer 5 of the cerebral cortex. Staining appeared to be moderate in the putamen and thalamus, and light to moderate in the hypothalamus. Immunostaining in other regions such as the olfactory bulb, cerebellum, and the brain stem was light, but some neurons and nuclei were moderately stained (Fig. 1).
Immunostaining was described as neuronal staining and neuropilar staining. Neuronal staining indicates the staining of cell bodies without staining of the nucleus and major dendrites. Neuropilar staining includes the processes and unresolvable matrix between cells without tracing to specific cell bodies.
3.3. Olfactory bulb
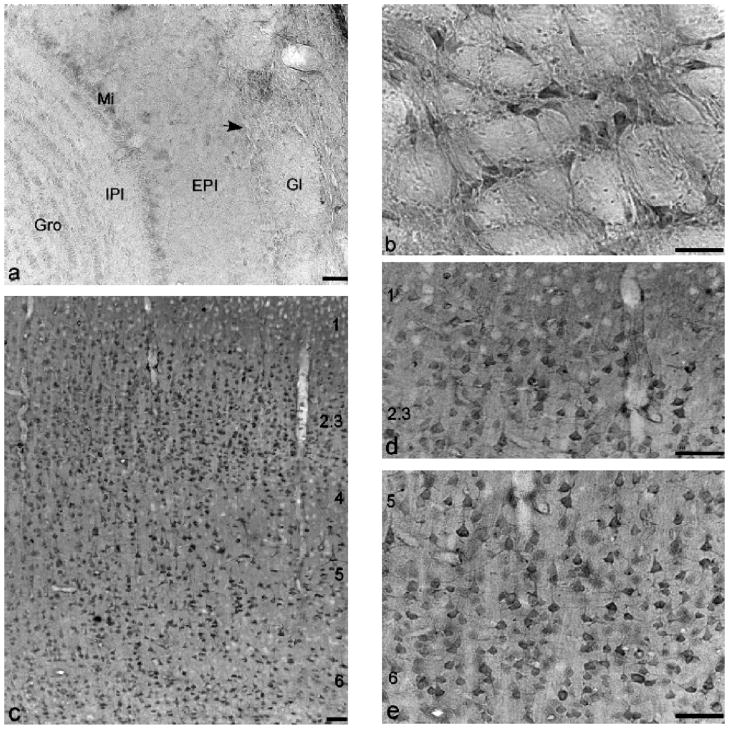
Immunostaining of the main olfactory bulb (Figs. 1b and 2a) was very light in the glomerular layer (Gl) and internal plexiform layer (Ipl), and light in the external plexiform layer (EPl) and granular cell layer (Gro). Immunostaining of mitral cells (Mi) was denser than other areas. Some periglomerular cells (Pg) were stained very lightly in the edge between the glomerular layer and external plexiform layer of the olfactory bulb. Neuropilar staining of the olfactory bulb was light to moderate compared with other brain regions.
Fig. 2.
Immunolabeling of NR1 in olfactory bulb, putamen, and cerebral cortex of AGS brain: (a) main olfactory bulb; (b) putamen; (c) low power of cortex; (d) cortex layers 1–3; (e) cortex layers 5–6. (a), (c–e) are from ibeAGS, and (b) is from hAGS. Gl, glomerular layer of olfactory bulb; EPl, external plexiform layer olfactory bulb; Mi, mitral cell layer of olfactory bulb; IPl, internal plexiform layer olfactory bulb; Gro, granular cell layer of olfactory bulb. Arrow indicates a periglomerular cell, numbers in (c–e) indicate the layers of cortex. Scale bar, 50 μm.
3.4. Cortex
Immunostaining of NR1 in the frontal association cortex (FrA), primary motor cortex (M1), and parietal association cortex (PtA) (Fig. 2c–e) was surveyed. The overall laminar pattern of NR1 immunoreactivity was similar in most of cerebral cortexes. Immunostaining of NR1 was present in most layers. Neuropilar staining was light to moderate and homogeneous throughout the cortical layers. Neuronal staining patterns in layers varied slightly with regions. Little or no staining was seen in the molecular layer (layer 1). The densest neuronal staining was found in the external granular layer (layer 2) and external pyramidal layer (layer 3). Since most immunostained small and middle size pyramidal neurons in layers 2 and 3 were highly packed, the staining of layers 2 and 3 appeared to be noticeably denser than the other layers. In the frontal association cortex, neurons in layers 4–6 were stained lightly to moderately. The staining patterns of layers 4–6 in primary motor cortex and parietal association cortex were similar. Neuronal staining in layer 4 was light. Large pyramidal neurons as well as their long vertically oriented dendrites in the layer 5 were densely stained. Neuronal staining of the multiform layer (layer 6) was light to moderate.
3.5. Hippocampus
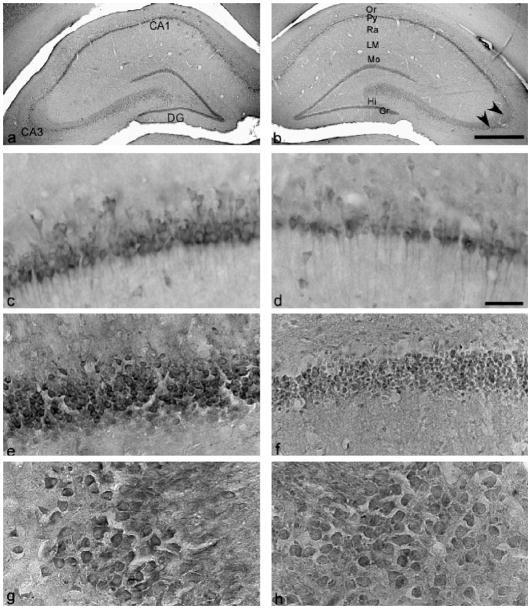
Immunoreactivity of anti-NR1 was found in most of major fields of the hippocampus (Figs. 1e and f and 3). Immunostaining of pyramidal neurons in CA1 and CA3 areas and granular neurons in dentate gryus was the densest. Staining of hilar polymorphic cells in the hilus of the dentate gyrus were moderate to dense. In the hippocampus, neuronal staining was well defined on the cell membrane. The dendrites of pyramidal neurons in CA1 and CA3 were clearly traced and intensely labeled. Neuropilar staining was light to moderate in dentate gyrus and CA1 including the stratum oriens (Or), stratum radiatum (Ra), stratum lacunosum-moleculare (LM), and stratum moleculare (Mo). Neuropilar staining was remarkably dense in the stratum lucidum of CA3, an area in which apical dendrites of the CA3 pyramidal neurons are located.
Fig. 3.
Immunostaining of NR1 in hippocampus in hAGS and ibeAGS brain. Left panel is from ibeAGS and right panel is from hAGS: (a and b) low power of hippocampus; (c and d) CA1; (e and f) dentate gyrus and hilus; (g and h) CA3. Or, stratum oriens; Py, stratum pyramidale; Ra, stratum radiatum; LM, stratum lacunosum-moleculare; Mo, stratum moleculare; Gr, stratum granulare. Arrowhead indicates the stratum lucidum of CA3 region. Hi, hilus of dentate gyrus. Scale bar in (a) and (b), 500 μm; and in (c–h), 50 μm.
3.6. Basal ganglia
The structure of the basal ganglia was slightly different in AGS compared with rats. In rats, the caudate nucleus and putamen are not separated by the internal capsule. In contrast, the internal capsule clearly delineates the caudate nucleus and putamen in AGS. The overall neuropilar staining in the basal ganglia was light to moderate. Neuronal staining in putamen was moderate and slightly denser than in the caudate nucleus (Figs. 1c and 2b).
3.7. Thalamus and hypothalamus
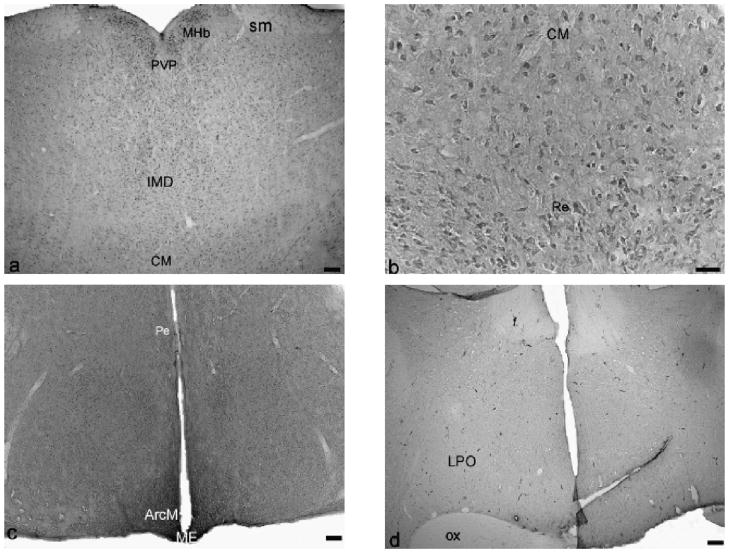
Neuronal staining in the thalamus and hypothalamus varied with nuclei(Fig. 1e). In the midline region of the thalamus(Fig. 4a and b), neuronal staining in the medial habenular nucleus (MHb) and posterior periventricular nucleus of the thalamus (PVP) was moderate to dense. The central medialthalamic nucleus(CM)and reuniens thalamic nucleus (Re) were moderately stained and surrounded with lightly stained neuropil. Both neuronal and neuropilar staining in the lateral habenular nucleus was light. Neuronal staining in the medial habenular nucleus (MHb) was moderate to dense. The ventral nuclear group (VP) and the posterior thalamus nuclear group (Po) were stained lightly to moderately. The most remarkable staining in the hypothalamus (Figs. 1e and 4c and d) was found in the arcuate nucleus (Arc) and median eminence (ME) where neuropilar staining was moderate to dense. Neuropilar staining in other areas of the hypothalamus was moderate but greater than in thalamus (Fig. 1e). Light to moderate staining was found in the lateral hypothalamus (LH) and periventrical hypothalamic nucleus (Pe). At the base of the hypothalamus, the size of the optic tract (opt, Fig. 1f) and optic chiasm (ox, Fig. 4d) was considerably larger in AGS than in rats consistent with a well developed visual system in ground squirrels (Reme and Young, 1977).
Fig. 4.
Immunostaining of NR1 in thalamus, and hypothalamus of hAGS brain: (a and b) thalamus, (c and d) hypothalamus. MHb, medial habenular nucleus; sm, stria medullaris of the thalamus; PVP, paraventricular thalamus nucleus, posterior part; IMD, intermediodorsal thalamic area; CM, centeral medial thalamus nucleus; Re, reuniens thalamus nucleus; Pe, periventricular hypothalamus nucleus; ArcM, medial part of arcuate nucleus; ME, median eminence; LPO, lateral preoptic area; f, fornix; ox, optic chiasm. Scale bar, 50 μm.
3.8. Cerebellum
Overall, cerebellar cortex (Fig. 5a) staining was light to moderate. Neuropilar staining in the molecular layer and granular layer was light. Some small stellate cells in the molecular layer were lightly stained, granular cells were uniformly stained and the plasma membrane was not well defined. Purkinje cell dendritic branches were labeled lightly to moderately and could be traced in some cells. Staining of Purkinje cell bodies varied from light to moderate. Glial cells in white matter were lightly stained.
3.9. Brain stem and cervical spinal cord
Neuropilar staining in brain stem was light. Neuronal staining varied with nuclei. Large motor neurons in the hypoglossal nucleus (12) (Fig. 5b and d) and neurons in the lateral vestibular nuclei (LVe, Fig. 5c) were stained moderately. Light staining was found in the superior paraolivary nucleus (SPO), facial nucleus (7), lateral reticular nucleus (LRt), spinal trigeminal tract (sp5), and interpolar part of spinal 5 nucleus (Sp5I). In cervical spinal cord (Fig. 5e), neuropilar staining was very light, and glia cells in the white matter (Fig. 5f) were lightly stained. Staining of gray matter was relatively denser than that of white matter. Staining in laminae 1–3 and lamina 10 was light; staining in laminae 4–8 was very light to light. The staining of large motor neurons in lamina 9 was moderate (Fig. 5g).
3.10. Smaller neurons in hAGS hippocampus
Distinct staining of the plasma membrane allowed for accurate tracing of cell bodies. The area of neurons in hippocampal sub-regions was measured and results are shown in Table 2. Neurons in CA1 and in the dentate gyrus were 28% and 31% smaller in hAGS (72 ± 4% and 69 ± 0.7%) relative to ibeAGS (100 ± 5% and 100 ± 3%) (p < 0.05, n = 4 AGS per group). In contrast, the size of CA3 neurons was not different between these two groups. Further studies using densitometry found that NR1 staining intensity of CA3 neurons was similar between the two groups (NR1 staining intensity in hAGS was 76 ± 14% of that in ibeAGS (100 ± 14%), p > 0.05, n = 4 AGS per group).
Table 2.
Comparison of neuronal soma area in AGS hippocampal subfields
| Subfields | Area of neurons (μm2)
|
|
|---|---|---|
| hAGS | ibeAGS | |
| CA1 | 120.7 ± 7.6 | 167.4 ± 7.9* |
| CA3 | 198.2 ± 8.7 | 204.6 ± 7.6 |
| Dentate gyrus | 48.9 ± 0.5 | 70.5 ± 2.4** |
p < 0.05,
p < 0.01, n = 4 AGS per group.
3.11. NR1 expression in hippocampus
Densitometric analysis of NR1 staining was not done in CA1 or DG where difference in cell size could confound interpretation. Rather, NR1 expression in whole hippocampus was quantified using Western blots. Expression of NR1 in the hippocampus evaluated semi-quantitatively using Western blotting was similar between hAGS and ibeAGS (p > 0.05, n = 4 AGS per group, Fig. 6).
4. Discussion
In the current study, we report that the cellular pattern of NR1 expression in AGS is widely distributed throughout the central nervous system. Hippocampus was chosen for quantitative analysis of cell size and NR1 expression because of hippocampal neuroprotection and neuron plasticity observed in AGS (Ross et al., 2006; Weltzin et al., 2006). hAGS have similar NR1 expression patterns to ibeAGS in hippocampus and the amount of NR1 protein in hippocampus does not differ between the two groups of animals. No difference in NR1 expression suggests that NMDAR function may be altered through other mechanisms in hAGS, such as phosphorylation (Zhao et al., 2006). Moreover, neuronal soma size decreases in CA1 and dentate gyrus during hibernation, but not in CA3. Description of NR1 expression and distribution as well as changes in neuronal size is a necessary first step toward investigating the role of NMDAR in neuroprotection and plasticity in AGS (Zhou et al., 2001; Ross et al., 2006; Weltzin et al., 2006).
Most brain regions including the olfactory bulb, cerebral cortex, hippocampus, and the putamen showed immunostaining similar to NR1 distribution in rats (Monaghan and Cotman, 1985; Petralia et al., 1994; Huntley et al., 1994; Bilak et al., 1995; Trombley and Westbrook, 1990). The densest staining was found in the hippocampus and cerebral cortex of AGS, consistent with RNA blot analysis, in situ hybridization, autoradiography, and immunohistochemistry in rats (Moriyoshi et al., 1991; Monaghan and Cotman, 1985; Petralia et al., 1994). Slight differences in the relative level of staining in some brain regions between AGS and rats were observed. For example, we found equally dense staining in CA1, CA3, dentate gyrus, and hilus regions in AGS hippocampus while, in rats, others have found denser staining in CA1 and CA3 (Petralia et al., 1994) or in CA1 and the hilus region of the dentate gyrus (Johnson et al., 1996). These disparities may be due to strain or species differences or to a difference in antibodies used. Different antibodies produce different NR1 staining patterns (Johnson et al., 1996). In rats, Petralia et al. (1994) found the densest staining in CA1 and CA3 and moderate staining in DG using an NR1 antibody raised in rabbit that recognizes a peptide corresponding to the C terminal residue 909–938. When Johnson and his colleagues used anti-rabbit NR1-C1 that recognizes polypeptide sequence 864–900, another epitope on the C terminus of the NR1 subunit, they reported the densest labeling in CA1 and the hilus of the dentate gyrus. Therefore, differences in antibodies used might explain differences in expression patterns among these studies and our results. Staining of glial cells was absent in control sections and is consistent with other reports of NR1 expression in astrocytes (Petralia et al., 1994).
In heterothermic animals, brains in the hibernating state show many changes in structure (Jacobs, 1996; Reme and Young, 1977; Azzam et al., 2000) and these structural changes may be related to energy conservation and or hypofunction. For instance, Purkinje cell nucleoli of the cerebellum are smaller in hibernating hedgehogs compared to euthermic hedgehogs (Giacometti et al., 1989). Cone cells in the retina of hibernating 13-lined ground squirrels undergo many changes including reduction in the diameters of the membranous discs as well as the size and number of mitochondria (Reme and Young, 1977).
Other cells outside of the central nervous system also show different expression during hibernation. Malatesta et al. (2002) found that the total cell and cytoplasm area of hepatocytes from hibernating dormice (Muscardinus avellanarius) was significantly reduced compared with those from euthermic dormice. High energy phosphates are maintained in hibernation; decreases in actin-ATP hydrolysis and turnover may contribute to energy conservation and changes in cell size (Lust et al., 1989; Storey, 1997; Bernstein and Bamburg, 2003). Malatesta et al. (2002) suggested that the change in cell structure was related to marked reduction in hepatocyte function found in the hibernating dormouse. Whether similar mechanisms are involved in neuronal cell size changes in AGS remains to be elucidated.
Our results show that the area of CA3 neuronal cell bodies in hAGS is similar to that in ibeAGS. This is in contrast to Popov et al.’s results (1992a). They reported that the soma volume of CA3 pyramidal neurons was smaller in hibernating ground squirrels (Spermophilus undalatus) than those in euthermic ground squirrels using a nuclear stain. Here we measured soma size using NR1 as a plasma membrane marker. Thus, discrepancies between findings reported here and by Popov et al., may be due to differential effects of hibernation torpor on soma and nuclei. While Popov et al., found that CA3 nuclei decrease in size during hibernation torpor we see no change in CA3 soma size. In contrast, we find a decrease in soma size in CA1 and DG that were not studied previously. Region specific differences in soma and nuclear size may be due to differential modulation of the cytoskeleton (Schoenenberger et al., 2005) that maintains the structure of the nucleus and the plasma membrane. Alternatively, species differences or the level of the hippocampus that was evaluated may account for the discrepant results.
Interestingly, tau protein in CA3 region of European ground squirrels undergoes a reversible paired helical filament (PHF)-like hyperphosphorylation during hibernation. Immunoreactivity of phophorylated tau (AT8) is particularly high in CA3 pyramidal neurons compared with neurons in CA1 and DG (Arendt et al., 2003). Hyperphosphorylation of tau may increase the stability of cytoskeleton and therefore, maintain the cell structure in CA3 neurons. Further studies are warranted to address the possible association between hyperphosphorylation and neuronal size in hippocampus between hAGS and ibeAGS.
In summary, we report expression and distribution of NR1 in AGS brain. In addition, CA1 and dentate gyrus neuronal cell bodies are significantly smaller in hAGS, and NR1 expression in hippocampus does not change in the hibernating state suggesting that NMDAR function may be altered by other means or non-NMDAR mechanisms are involved in neuroprotection (Zhao et al., 2006; Ross et al., 2006) and neuroplasticity (Weltzin et al., 2006) in AGS.
Acknowledgments
This work was supported by Alaska SNRP (NIH U54-NS 41069 funded by NINDS, NIMH, NCRR, and NCMHD) and Alaska EPSCoR (ESP-0092040). The authors thank Dr. Lique Coolen for advice and comments on a prior version of the manuscript.
Footnotes
Note added to proof
von der Ohe et al. (2006) report temperature dependent decreases in somasize in cortical spiny stellate neurons, ventral posterior thalamic relayneurons and hippocampal CA3 neurons in torpid, hibernating Golden-manteledground squirrels. These recent results suggest that sustained size of CA3 neurons in AGS during torpor may be unique to AGS.
References
- Arendt T, Stieler J, Strijkstra AM, Hut RA, Rudiger J, Van der Zee EA, Harkany T, Holzer M, Hartig W. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci. 2003;23 (18):6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam NA, Hallenbeck JM, Kachar B. Membrane changes during hibernation. Nature. 2000;407 (6802):317–318. doi: 10.1038/35030294. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Actin-ATP hydrolysis is a major energy drain for neurons. J Neurosci. 2003;23 (1):1–6. doi: 10.1523/JNEUROSCI.23-01-00002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilak SR, Bilak MM, Morest DK. NMDA receptor expression in the mouse cerebellar cortex. Synapse. 1995;20 (3):257–268. doi: 10.1002/syn.890200310. [DOI] [PubMed] [Google Scholar]
- Dave KR, Prado R, Raval AP, Drew KL, Perez-Pinzon MA. The Arctic Ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke. 2006 Mar 30; doi: 10.1161/01.STR.0000217409.60731.38. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13 (2):325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab. 1998;18 (2):168–175. doi: 10.1097/00004647-199802000-00007. [DOI] [PubMed] [Google Scholar]
- Giacometti S, Scherini E, Bernocchi G. Seasonal changes in the nucleoli of Purkinje cells of the hedgehog cerebellum. Brain Res. 1989;488 (1–2):365–368. doi: 10.1016/0006-8993(89)90732-4. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Vickers JC, Janssen W, Brose N, Heinemann SF, Morrison JH. Distribution and synaptic localization of immunocytochemically identified NMDA receptor subunit proteins in sensory-motor and visual cortices of monkey and human. J Neurosci. 1994;14 (6):3603–3619. doi: 10.1523/JNEUROSCI.14-06-03603.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LF. The economy of winter: phenotypic plasticity in behavior and brain structure. Biol Bull. 1996;191 (1):92–100. doi: 10.2307/1543068. [DOI] [PubMed] [Google Scholar]
- Johnson RR, Jiang X, Burkhalter A. Regional and laminar differences in synaptic localization of NMDA receptor subunit NR1 splice variants in rat visual cortex and hippocampus. J Comp Neurol. 1996;368 (3):335–355. doi: 10.1002/(SICI)1096-9861(19960506)368:3<335::AID-CNE2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kharazia VN, Phend KD, Rustioni A, Weinberg RJ. EM colocalization of AMPA and NMDA receptor subunits at synapses in rat cerebral cortex. Neurosci Lett. 1996;210 (1):37–40. doi: 10.1016/0304-3940(96)12658-6. [DOI] [PubMed] [Google Scholar]
- Liao J, Xu X, Wargovich MJ. Direct reprobing with anti-beta-actin antibody as an internal control for Western blotting analysis. Biotechniques. 2000;28 (2):216–218. doi: 10.2144/00282bm05. [DOI] [PubMed] [Google Scholar]
- Lust WD, Wheaton AB, Feussner G, Passonneau J. Metabolism in the hamster brain during hibernation and arousal. Brain Res. 1989;489 (1):12–20. doi: 10.1016/0006-8993(89)90003-6. [DOI] [PubMed] [Google Scholar]
- Ma YL, Zhu X, Rivera PM, Toien O, Barnes BM, LaManna JC, Smith MA, Drew KL. Absence of cellular stress in brain following hypoxia induced by arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2005;289 (5):R1297–R1306. doi: 10.1152/ajpregu.00260.2005. [DOI] [PubMed] [Google Scholar]
- Malatesta M, Zancanaro C, Baldelli B, Gazzanelli G. Quantitative ultrastructural changes of hepatocyte constituents in euthermic, hibernating and arousing dormice (Muscardinus avellanarius) Tissue Cell. 2002;34 (6):397–405. doi: 10.1016/s0040816602000745. [DOI] [PubMed] [Google Scholar]
- Mihailovic L, Petrovic-Minic B, Protic S, Divac I. Effects of hibernation on learning and retention. Nature. 1968;218 (137):191–192. doi: 10.1038/218191a0. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5 (11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354 (6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258 (5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academics; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994;14 (2):667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS. Hibernation-induced structural changes in synaptic contacts between mossy fibres and hippocampal pyramidal neurons. Neuroscience. 1992;48 (1):53–62. doi: 10.1016/0306-4522(92)90337-2. [DOI] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS, Bragin AG. Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience. 1992;48 (1):45–51. doi: 10.1016/0306-4522(92)90336-z. [DOI] [PubMed] [Google Scholar]
- Popov VI, Ignat’ev DA, Lindemann B. Ultrastructure of taste receptor cells in active and hibernating ground squirrels. J Electron Microsc (Tokyo) 1999;48 (6):957–969. doi: 10.1093/oxfordjournals.jmicro.a023770. [DOI] [PubMed] [Google Scholar]
- Reme CE, Young RW. The effects of hibernation on cone visual cells in the ground squirrel. Invest Ophthalmol Vis Sci. 1977;16 (9):815–840. [PubMed] [Google Scholar]
- Ross AP, Christian SL, Zhao HW, Drew KL. Persistent tolerance to oxygen and nutrient deprivation and N-methyl-D-aspartate in cultured hippocampal slices from hibernating Arctic ground squirrel. J Cereb Blood Flow Metab. 2006 Jan 4; doi: 10.1038/sj.jcbfm.9600271. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373 (6510):151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Schoenenberger CA, Buchmeier S, Boerries M, Sutterlin R, Aebi U, Jockusch BM. Conformation-specific antibodies reveal distinct actin structures in the nucleus and the cytoplasm. J Struct Biol. 2005;152 (3):157–168. doi: 10.1016/j.jsb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Schulte EK, Lyon H, Prento P. Gallocyanin chromalum as a nuclear stain in cytology. I A cytophotometric comparison of the Husain–Watts Gallocyanin chromalum staining protocol with the Feulgen procedure. Histochem J. 1991;23 (5):241–245. doi: 10.1007/BF01462247. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Brose N, Janssen WG, Gasic GP, Jahn R, Heinemann SF, Morrison JH. Regional, cellular, and ultrastructural distribution of N-methyl-D-aspartate receptor subunit 1 in monkey hippocampus. Proc Natl Acad Sci USA. 1994;91 (2):564–568. doi: 10.1073/pnas.91.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey KB. Metabolic regulation in mammalian hibernation: enzyme and protein adaptations. Comp Biochem Physiol A: Physiol. 1997;118 (4):1115–1124. doi: 10.1016/s0300-9629(97)00238-7. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Westbrook GL. Excitatory synaptic transmission in cultures of rat olfactory bulb. J Neurophysiol. 1990;64 (2):598–606. doi: 10.1152/jn.1990.64.2.598. [DOI] [PubMed] [Google Scholar]
- von der Ohe CG, Darian-Smith C, Garner CC, Heller HC. Ubiquitous and temperature-dependent neural plasticity in hibernators. J Neurosci. 2006;26 (41):10590–10598. doi: 10.1523/JNEUROSCI.2874-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzin MM, Zhao HW, Drew KL, Bucci DJ. Arousal from hibernation alters contextual learning and memory. Behav Brain Res. 2006;167 (1):128–133. doi: 10.1016/j.bbr.2005.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HW, Ross AP, Christian SL, Buchholz JN, Drew KL. Decreased NR1 phosphorylation and decreased NMDAR function in hibernating Arctic ground squirrels. J Neurosci Res. 2006;84 (2):291–298. doi: 10.1002/jnr.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Zhu X, Castellani RJ, Stimmelmayr R, Perry G, Smith MA, Drew KL. Hibernation, a model of neuroprotection. Am J Pathol. 2001;158 (6):2145–2151. doi: 10.1016/S0002-9440(10)64686-X. [DOI] [PMC free article] [PubMed] [Google Scholar]