Abstract
Cytomegalovirus (CMV) has been suggested as a contributing force behind the impaired immune responsiveness in the elderly, with decreased numbers of naïve T-cells and an increased proportion of effector T-cells. Immunological impairment is also implicated as a part of the pathogenesis in Alzheimer’s disease (AD). The aim of this study was to investigate whether AD patients present with a different CMV-specific CD8 immune profile compared to non-demented controls. Blood samples from 50 AD patients and 50 age-matched controls were analysed for HLA-type, CMV serostatus and systemic inflammatory biomarkers. Using multi-colour flow cytometry, lymphocytes from peripheral blood mononuclear cells were analysed for CMV-specific CD8 immunity with MHC-I tetramers A01, A02, A24, B07, B08 and B35 and further classified using CD27, CD28, CD45RA and CCR7 antibodies. Among CMV seropositive subjects, patients with AD had significantly lower proportions of CMV-specific CD8 T-cells compared to controls, 1.16 % vs. 4.13 % (p=0.0057). Regardless of dementia status, CMV seropositive subjects presented with a lower proportion of naïve CD8 cells and a higher proportion of effector CD8 cells compared to seronegative subjects. Interestingly, patients with AD showed a decreased proportion of CMV-specific CD8 cells but no difference in general CD8 differentiation.
Introduction
Alzheimer’s disease (AD) is the most common form of dementing disorder and is characterised by a deterioration of cognitive and functional capacity. Due to ongoing demographic changes and the current lack of effective therapy, the socioeconomic burden of AD is estimated to increase globally in the years ahead [1].
Neuropathologically, the AD brain displays a progressive synaptic and neuronal loss together with extracellular plaques, mainly consisting of amyloid-β (Aβ), and intracellular neurofibrillary tangles of the microtubule-associated protein tau. According to the amyloid hypothesis, the pathogenesis is initiated by an increased production of Aβ followed by cytoskeletal changes and neuronal loss. Evidence for the primary role of Aβ has mainly been provided by the findings of disease-causing mutations in genes related to the generation of Aβ.
The pathology typically starts in the entorhinal cortex and other structures of the medial temporal lobe. However, with increased disease duration the pathology is extended in a hierarchical fashion to other cortical areas [2,3]. In addition to the main pathological changes, other features of the affected brain often include vascular alterations with deposition of Aβ in the vessel walls, especially in carriers of the Apolipoprotein (APOE ) ε4 allele [4], as well as various inflammatory reactions [5]. It is not completely understood how amyloid plaques, neurofibrillary degeneration, vascular alterations, inflammation and immune responses are related to each other and whether any infectious agents can influence the disease process.
The possible influence of viral infections on AD development has been investigated. For example, one study found that previous exposure to herpes simplex virus type 1 increased the risk of AD in carriers of the APOE ε4 allele [6] whereas later studies have failed to find such a correlation [7].
Whereas exposure to human cytomegalovirus (CMV) could influence the disease risk has not been extensively studied. CMV is a member of the betaherpesvirus group, causing a chronically persistent infection that in the immunocompetent adult rarely escapes immune surveillance, but can cause severe disease in patients with suppressed immune function [8,9]. Infection can occur in all stages of life, with a reported seroprevalence ranging from approximately 30 to 90% depending on age and ethnicity [10,11]. Recently, CMV has been shown to inflict a deep imprint in the host T-cell compartment that is characterised by an age-related oligoclonal expansion of differentiated CD8 (CD27-CD28-) cells and a corresponding decrease in proportion of naïve cells [12-15]. Also, the degree of differentiation in the CD4 compartments has been shown to correlate with levels of CMV IgG [16].
Alterations in systemic immunity have been shown to occur in the elderly, and the term immunosenescence is used to describe the age-related decline in capacity and regulatory balance of both innate and adaptive immune responses [17,18]. Dysregulation of immunoactive cells could also explain the progression of baseline systemic inflammation called inflammaging, which is considered a risk factor for several age-related diseases and where the role of CMV has been investigated [19]. Examples of clinically important immunosenescence include an impaired vaccine response that is especially pronounced in CMV seropositive patients [20,21] and an increased incidence of severe bacterial and viral infections in the elderly. In Swedish octogenarian and nonagenarian cohorts [22,23] a defined immune risk profile (IRP) consisting of a shift in CD4/CD8 ratio was associated with increased morbidity and mortality, but later studies conducted in different epidemiological settings have shown somewhat conflicting results [24].
Alzheimer’s disease has previously been associated with shifts in non CMV-specific CD4 as well as CD8 T-cell subsets [25-27]. Whether the CMV-specific CD8 immunity, shown to be of clinical importance for CMV disease in settings of more prominent immune deficiency [28], is affected in dementia patients has to our knowledge not previously been studied. Here, we have investigated if levels of CD8 T-cell CMV specificity and general CD8 differentiation differ in AD patients compared to non-demented (ND) controls.
Methods
Subjects
In the AD study group, a total of 51 patients were recruited from the Memory Clinic at the Department of Geriatrics in Uppsala University Hospital. All of them had recently undergone an extensive diagnostic work-up and received a clinical AD diagnosis in accordance with the NINCDS-ADRDA criteria [29] and DSM-IV criteria. Thus, all patients described a clinical picture of AD as well as a CT or MRI scan consistent with the diagnosis, i.e. with the absence of significant vascular abnormalities. Mini-mental State Examination (MMSE) scores were available from all but one and ranged from 10 to 27. In the ND control group, 52 age-matched subjects were recruited from a database of listed volunteers from the same geographical area as the AD group. Subjects in the ND control group had been recruited via local advertising and did not have any subjective cognitive impairment. Due to a change in diagnosis from AD to frontotemporal dementia, one participant in the AD group was later excluded and in the ND group two patients were excluded due to technical problems with sample handling prior to analysis, rendering a total of 50 AD patients and 50 ND controls. Dementia status was coded, allowing blinding during all laboratory work.
Informed consent was obtained in writing from all study participants, together with written consent from a close relative if there was any uncertainty on whether the subject was capable of providing informed consent him- or herself. The study was approved by the Regional Ethical Review Board in Uppsala, Sweden.
Sampling and routine analyses
Blood samples were acquired by venipuncture, performed in accordance with standard clinical protocol. Routine analyses, including clinical chemistry and apolipoprotein E (APOE) genotyping, were made at the Department of Clinical Chemistry and Pharmacology, whereas HLA genotyping (PCR-SSO) was performed at the Department of Clinical Immunology and Transfusion Medicine, both accredited laboratories at Uppsala University Hospital.
PBMCs were isolated from blood samples collected in BD Vacutainer CPT™ Cell Preparation Tubes with sodium citrate. The tubes were kept at room temperature, before centrifugation and washing steps in accordance with the manufacturer’s protocol. A total of between 12-76 x 106 cells were acquired per patient and frozen in batches of 5 x 106 cells in medium consisting of 15% dimethyl sulfoxide (DMSO) and 85% foetal calf serum (FCS). All samples were stored in liquid nitrogen until analysis. The excess plasma from PBMC isolation was separately frozen in -20°C until analysed for CMV IgG (SIEMENS Enzygnost anti-CMV/IgG) at the Department of Clinical Microbiology at Uppsala University Hospital, and study participants were classified as either CMV seropositive or seronegative.
Flow cytometry
For each study participant, one batch of 5 x 106 cells were quickly thawed in a 37°C water bath and diluted in 40 ml cold wash buffer consisting of phosphate buffered saline (PBS) with 2.5% foetal bovine serum (FBS, Invitrogen) and 0.1% sodium azide. The suspension was centrifuged at 250 x g for 5 min and the supernatant was discarded. Next, the pellet was resuspended in 400 µl wash buffer and divided into four aliquots. In accordance with HLA typing results, titrated amounts of PE-labelled CMV-specific iTAg™Class 1 MHC tetramers (Beckman Coulter) were added to separate aliquots, if alleles matched one to four of the following: HLA-A*0101, HLA-A*0201, HLA-A*2402, HLA-B*0702, HLA- B*0801 or HLA-B*3501. If no tetramers matched, one sample was still analysed without any tetramers, rendering a total of 197 samples. Each sample was also concomitantly stained with titrated amounts of fluorochrome labelled antibodies targeting CD3 APC-H7, CD19 Alexa Fluor 700, CD4 BD Horizon V500, CD8 BD Horizon V450, CD27 PerCP-Cy5.5, CD28 APC, CCR7 PE-Cy7 and CD45RA FITC (all from BD Biosciences) and was incubated for 60 minutes in a light-protected environment at 2°C.
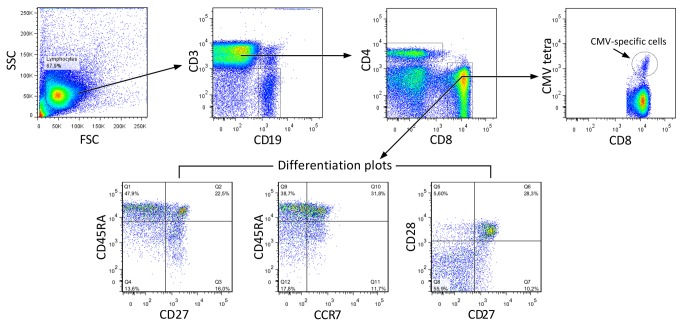
All samples were analysed using a BD LSR II Special Order System, controlled by the BD FACSDiva 6.0 software (BD Biosciences). Compensation for spectral overlap was calculated based on data from unstained and single-colour stained BD CompBeads, using the antibody-fluorochrome conjugates specified above. A preliminary forward scatter (FSC) vs. side scatter (SSC) gate was used to identify lymphocytes and, depending on sample size, a total of up to 100 000 in-gate events were recorded. All datasets were migrated to FlowJo 7.6.5 (Treestar Inc.) for further gating and analysis. Gating was performed as specified in Figure 1 and only visually distinct tetramer-positive populations were counted.
Figure 1. Gating flow-chart.
The CD3+CD8+ subset was identified from the FSC/SSC lymphocyte window, and further analysed for CMV tetramer staining and CD27/CD28/CD45RA/CCR differentiation.
Statistical analysis
R version 2.15.1 (The R Foundation for Statistical Computing) was used for statistical analysis. All comparisons were made using the non-parametric Mann-Whitney U-test. The primary outcome variable was the proportion of CMV-specific cells of total CD8, and ad hoc statistical analyses comparing AD and ND groups were performed without any correction for multiplicity. All other variables were considered secondary and significance tests were adjusted with the Bonferroni correction method.
Post hoc, to correct for possible influence by age and gender distribution differences between groups, a linear model was constructed correlating the rank of the primary outcome variable with dementia status, age and gender [30]. Box plots were defined with boxes containing quartiles 2 and 3 and whiskers displaying quartiles 1 and 4, excluding any outliers outside 1.5 times the interquartile range.
Results
Background data
The epidemiological and laboratory background data of the AD and ND subjects is described in Table 1. As a small number of analytic results were returned blank or erroneous, the mean values have been calculated on the available data.
Table 1. Summary of baseline characteristics in patients with Alzheimer’s disease (AD) and non-demented controls (ND).
| Continuous data reported as mean (Standard Deviation) | AD (N=50) | ND (N=50) |
|---|---|---|
| Age, years | 77.5 (6.9) | 74.0 (8.0) |
| Gender, male/female | 28/22 | 22/28 |
| Mini-mental State Examination score | 19.9 (4.8) | NA |
| Haemoglobin, g/L | 139.1 (10.6) | 137.9 (10.4) |
| Leukocytes, 109/L | 6.75 (1.78) | 6.28 (1.67) |
| Lymphocytes, 109/L | 1.95 (0.89) | 1.88 (0.61) |
| C-reactive protein, mg/L | 3.59 (7.05) | 3.18 (4.84) |
| Interleukin-6, ng/L | 2.24 (2.94) | 2.23 (3.08) |
| APOE ε4 allele carriers, hetero-/homozygote | 28/4 | 16/2 |
| CMV IgG positive | 84% (n=42) | 78% (n=39) |
| HLA-A02 positive | 60% (n=30) | 70% (n=36) |
| HLA-A02 and CMV IgG positive | 52% (n=26) | 60% (n=30) |
| HLA-A01/A02/A24/B07/B08/B35 positive | 94% (n=47) | 90% (n=45) |
| HLA-A01/A02/A24/B07/B08/B35 positive and CMV IgG positive | 78% (n=39) | 72% (n=36) |
NA = Not Available
The AD and ND groups were similar regarding total leukocyte count, total lymphocyte count, C-reactive protein and interleukin-6. The standard deviation of some parameters exceeded the mean in magnitude, suggesting skewed data distribution. CMV serostatus and HLA allele distribution were also comparable between groups, but the AD group contained a larger proportion of APOE ε4 allele carriers. Due to incomplete HLA coverage of available tetramers and CMV serostatus, the total number of participants analysable for CMV MHC-1 specificity was 39 in the AD and 36 in the ND group.
Flow cytometry
The FSC/SSC gate generated an average number of 103 315 events for the AD group and 96 815 for the ND group, respectively. When comparing proportions of the total sum of CMV-specific CD8 cells for all HLA types per CMV positive subject (Figure 2a), there was a significant difference (p=0.0057) between AD (1.16%) and ND (4.13%) groups. This difference was still significant (p=0.0012) when correcting for age and gender. When comparing only HLA-A02 tetramer data from HLA-A02 positive CMV positive subjects (Figure 2b) there was a trend (p=0.066) indicating a similar numerical difference between AD (1.26%) and ND (3.07%) groups, also unaffected (p=0.058) by correction for age and gender. The overall CD4/CD8-ratio did not differ between groups (Figure 2c).
Figure 2a-c. Comparison of proportions of CMV-specific CD8 cells and overall CD4/CD8 ratio in seropositive subjects with Alzheimer´s disease (AD) and non-demented controls (ND).
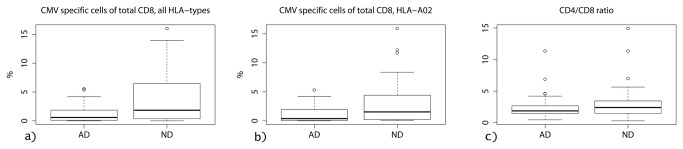
a) Comparing total cell count for all HLA-types, there is a clear difference with significantly lower proportions of CMV-specific CD8 cells in AD compared to ND group; 1.16 % versus 4.13 % (p=0.0057) b) Comparing only subjects with HLA-A02 tetramer data, there is a trend towards lower proportions of CD8 cells in the AD compared to the ND group; 1.26 % versus 3.07% (p=0.066) c) The overall CD4/CD8-ratio did not differ between groups.
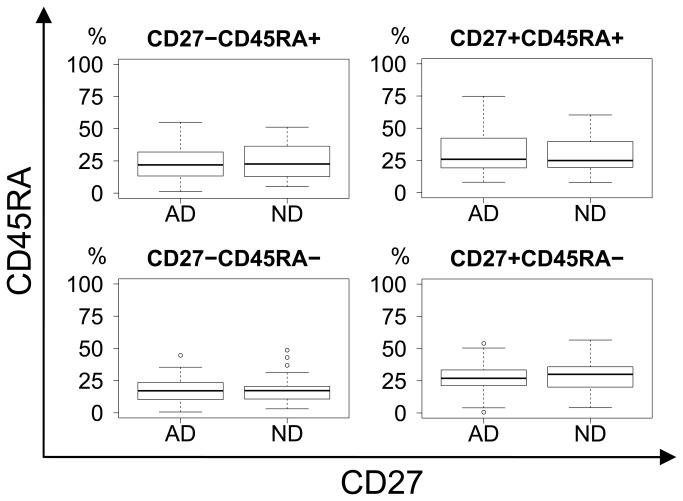
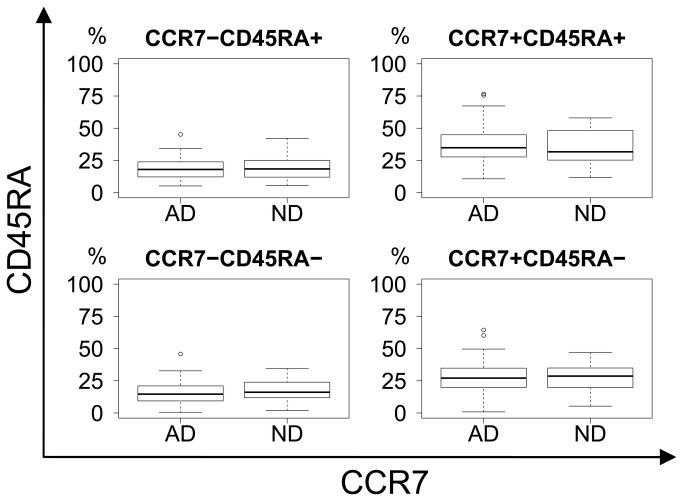
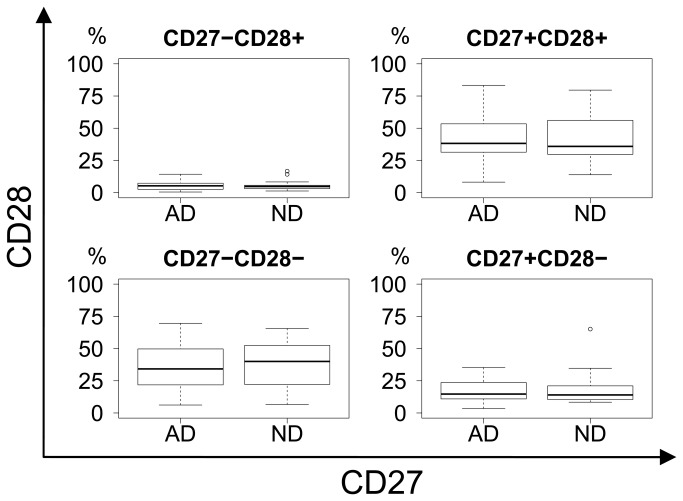
General CD8 differentiation was illustrated by CD27/CD45RA, CCR7/CD45RA and CD27/CD28-projections of the four-dimensional CD27/CD28/CCR7/CD45RA phenotypic space. No significant differences in differentiation were seen when comparing AD and ND groups (Figures 3-5).
Figure 3. CD27 vs. CD45RA differentiation plot.
No difference in differentiation between AD and ND groups in terms of CD27 and CD45RA expression. Upper right: Naïve. Lower right: Memory. Upper left: Effector.
Figure 4. CCR7 vs. CD45RA differentiation plot.
No difference in differentiation between AD and ND groups in terms of CCR7 and CD45RA expression. Upper right: Naïve. Lower right: Central memory. Upper left: Effector. Lower left: Effector-memory.
Figure 5. CD27 vs. CD28 differentiation plot.
No difference in differentiation between AD and ND groups in terms of CD27 and CD28 expression. Upper right: Early memory and naïve. Lower right: Intermediate memory. Lower left: Late memory.
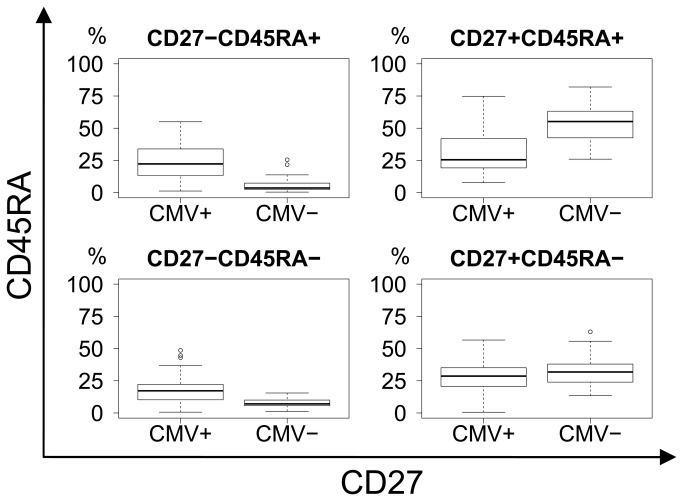
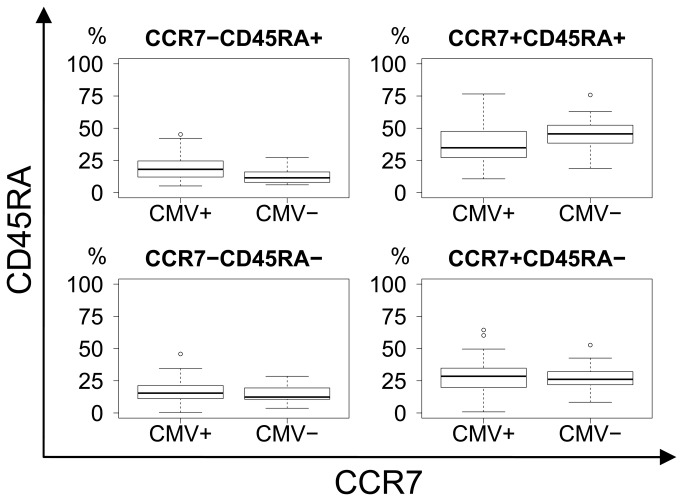
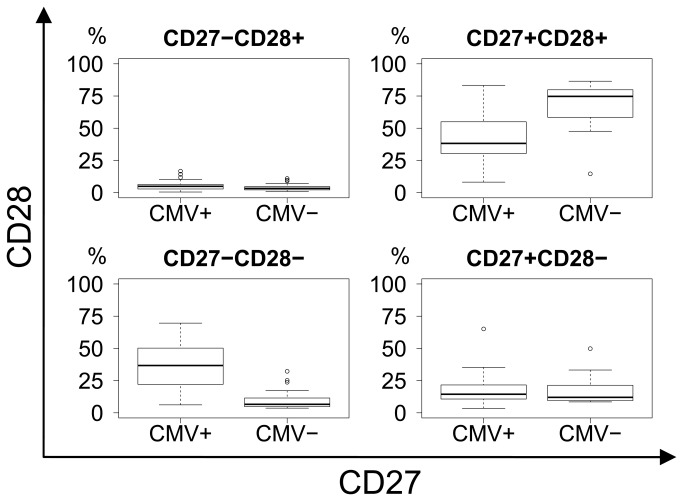
When comparing CMV seropositive and CMV seronegative subjects, regardless of dementia status, there was a clear difference in CD8 differentiation as CMV seropositive subjects presented with substantial shifts in phenotype, from naïve and early memory towards late memory and effector differentiation (Figures 6-8). All p−values were calculated using Bonferroni correction for a multiplicity of 27.
Figure 6. CD27 vs. CD45RA differentiation plot.
Significant shift from the CD27+CD45RA+ naïve (p=8.13E-05) to CD27-CD45RA+ effector (p=1.76E-06) and CD27-CD45RA- (p=1.17E-04) subsets with CMV status. Upper right: Naïve. Lower right: Memory. Upper left: Effector.
Figure 7. CCR7 vs. CD45RA differentiation plot.
Non-significant trend in shift from the CCR7+CD45RA+ naïve (p=0.062) to the CCR7-CD45RA+ effector (p=0.13) subset with CMV status. Upper right: Naïve. Lower right: Central memory. Upper left: Effector. Lower left: Effector-memory.
Figure 8. CD27 vs. CD28 differentiation plot.
Significant shift from the CD27+CD28+ early memory and naïve (p=3.19E-05) to the CD27-CD28- late memory (p=2.73E-07) subset with CMV status. Upper right: Early memory and naïve. Lower right: Intermediate memory. Lower left: Late memory.
Discussion
To our knowledge, this is the first study investigating CMV-specific immunity in AD. Studying an age-related disease, we had expected AD patients to present with a phenotype of premature immunosenescence and expanded clones of CMV-specific CD8 cells. Interestingly, this group instead presented with a significantly lower proportion of CMV-specific cells compared to ND controls. There were no differences in denominators such as CD4/CD8 ratio or total lymphocyte count that could explain the difference in CMV specificity, nor any obvious signs of more advanced immunosenescence in the AD group in terms of CD27/CD28/CD45RA/CCR7 differentiation which confirms results from Pellicanò et al [25]. Also, the groups were similar in levels of system inflammatory biomarkers such as C-reactive protein and interleukin-6, providing no evidence that inflammaging is a cofactor for AD development. CMV serostatus and HLA allele distribution were comparable between groups, but as expected [31] the AD group contained a larger proportion of APOE ε4 allele carriers.
When comparing CMV seronegative with seropositive subjects regardless of dementia status, significant shifts in the CD8 subsets became obvious. This confirms the results of previous studies that CMV infection itself induces T-cell differentiation towards late effector phenotypes [12,32], but does not infer a link to the risk of developing AD.
If the lower proportion of CMV-specific CD8 cells in AD patients reflects a partially impaired cellular immunity, present before or in early stages of the AD pathophysiological process, CMV reactivation in brain macrophages or vascular endothelial cells could be contributing to local inflammation and disease progression. Another theory would be that CMV immunity, which in normal individuals engages a rather large proportion of the CD8 compartment, is suppressed by AD specific immunological processes, i.e. immunity directed towards amyloid beta or other components related to AD development.
We believe that our data is relevant, as it is obtained from a fairly large number of AD patients, diagnosed by current clinical protocols and compared to matched controls. We are not yet able to directly link our findings to the AD pathophysiological process but think that they can contribute to new angles of approach to future research.
There are some inherent methodological weaknesses to this study. Firstly, when working with frozen PBMC samples, there is a risk of losing a proportion of more sensitive cell types, which could possibly skew the results. Efforts to avoid biasing effects include standardised, simultaneous and blinded handling of study and control samples, which should distribute errors evenly between study groups. Secondly, the tetramer staining technique carries limitations, mainly in terms of limited HLA coverage and immune dominance, but as our results are similar when comparing data from multiple HLA types with the subgroup of only HLA-A02 AD/ND subjects, they should be generalisable to other populations. However, the comparison between HLA-A02 AD/ND subjects did not quite reach statistical significance (p=0.058 after correction) which could be due to the HLA-A02 groups being of insufficient size.
Based on the results of this study we conclude that CMV CD8 T-cell frequency is significantly lower in AD than in non-demented controls, possibly affecting CMV immunity. Any causality between CMV and AD remains to be shown, but we believe that this has brought new insight into how immunity in AD differs from the normal ageing process. Should future studies prove a causative role of CMV in the AD pathophysiology this could increase the benefit of a future CMV vaccine, given that this does not trigger the same pathology as the infection itself. However, we find it more likely that the altered CMV immunity in AD patients reflects more profound changes in systemic immunity and that other forms of immunotherapy might be considered. Further studies in AD, comparing CMV specific CD4 immunity, cellular reactivity on CMV antigen challenge and specific immunity against other chronically persistent viruses, could put this into better context.
Acknowledgments
We are very grateful for the invaluable practical and intellectual assistance from Susanne Lindblom, Johan Brännström, Jan Grawé, Käthe Ström, Rose-Marie Brundin and Frida Ekholm Pettersson.
Funding Statement
This work was funded by grants from The Family Ohlinder-Nielsen’s Foundation, Uppsala University, Landstinget i Uppsala län, Swedish Research Council (2006-2822, 2006-6326 and 2006-3464), Swedish Brain Foundation, Swedish Alzheimer Foundation and Swedish Society of Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L et al. (2005) Global prevalence of dementia: a Delphi consensus study. Lancet 366: 2112-2117. PubMed: 16360788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL (1984) Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science 225: 1168-1170. doi: 10.1126/science.6474172. PubMed: 6474172. [DOI] [PubMed] [Google Scholar]
- 3. Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: 239-259. doi: 10.1007/BF00308809. PubMed: 1759558. [DOI] [PubMed] [Google Scholar]
- 4. Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN (1996) Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer's disease. Am J Pathol 148: 2083-2095. PubMed: 8669492. [PMC free article] [PubMed] [Google Scholar]
- 5. Nilsson L, Rogers J, Potter H (1998) The essential role of inflammation and induced gene expression in the pathogenic pathway of Alzheimer's disease. Front Biosci 3: d436-d446. PubMed: 9545438. [DOI] [PubMed] [Google Scholar]
- 6. Itzhaki RF, Lin W-R, Shang D, Wilcock GK, Faragher B et al. (1997) Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet 349: 241-244. doi: 10.1016/S0140-6736(96)10149-5. PubMed: 9014911. [DOI] [PubMed] [Google Scholar]
- 7. Marques AR, Straus SE, Fahle G, Weir S, Csako G et al. (2001) Lack of association between HSV-1 DNA in the brain, Alzheimer's disease and apolipoprotein E4. J Neurovirol 7: 82-83. doi: 10.1080/135502801300069773. PubMed: 11519487. [DOI] [PubMed] [Google Scholar]
- 8. Boeckh M, Nichols WG (2004) The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 103: 2003-2008. doi: 10.1182/blood-2003-10-3616. PubMed: 14644993. [DOI] [PubMed] [Google Scholar]
- 9. Sund F, Lidehäll AK, Claesson K, Foss A, Tötterman TH et al. (2010) CMV-specific T-cell immunity, viral load, and clinical outcome in seropositive renal transplant recipients: a pilot study. Clin Transplant 24: 401-409. PubMed: 19222507. [DOI] [PubMed] [Google Scholar]
- 10. Ahlfors K (1984) IgG antibodies to cytomegalovirus in a normal urban Swedish population. Scand J Infect Dis 16: 335-337. doi: 10.3109/00365548409073957. PubMed: 6098960. [DOI] [PubMed] [Google Scholar]
- 11. Bate SL, Dollard SC, Cannon MJ (2010) Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis 50: 1439-1447. doi: 10.1086/652438. PubMed: 20426575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA et al. (2002) Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol 169: 1984-1992. PubMed: 12165524. [DOI] [PubMed] [Google Scholar]
- 13. Vescovini R, Biasini C, Fagnoni FF, Telera AR, Zanlari L et al. (2007) Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J Immunol 179: 4283-4291. PubMed: 17785869. [DOI] [PubMed] [Google Scholar]
- 14. Vescovini R, Telera A, Fagnoni FF, Biasini C, Medici MC et al. (2004) Different contribution of EBV and CMV infections in very long-term carriers to age-related alterations of CD8+ T cells. Exp Gerontol 39: 1233-1243. doi: 10.1016/j.exger.2004.04.004. PubMed: 15288697. [DOI] [PubMed] [Google Scholar]
- 15. Derhovanessian E, Maier AB, Hähnel K, Beck R, de Craen AJ et al. (2011) Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol 92: 2746-2756. doi: 10.1099/vir.0.036004-0. PubMed: 21813708. [DOI] [PubMed] [Google Scholar]
- 16. Alonso Arias R, Moro-García MA, Echeverría A, Solano-Jaurrieta JJ, Suárez-García FM et al. (2013) Intensity of the humoral response to cytomegalovirus is associated with the phenotypic and functional status of the immune system. J Virol 87: 4486-4495. doi: 10.1128/JVI.02425-12. PubMed: 23388717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solana R, Tarazona R, Aiello AE, Akbar AN, Appay V et al. (2012) CMV and Immunosenescence: from basics to clinics. Immun Ageing 9: 23. doi: 10.1186/1742-4933-9-23. PubMed: 23114110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herndler-Brandstetter D, Landgraf K, Tzankov A, Jenewein B, Brunauer R et al. (2012) The impact of aging on memory T cell phenotype and function in the human bone marrow. J Leukoc Biol 91: 197-205. doi: 10.1189/jlb.0611299. PubMed: 22013229. [DOI] [PubMed] [Google Scholar]
- 19. Bartlett DB, Firth CM, Phillips AC, Moss P, Baylis D et al. (2012) The age-related increase in low-grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging Cell 11: 912-915. doi: 10.1111/j.1474-9726.2012.00849.x. PubMed: 22708923. [DOI] [PubMed] [Google Scholar]
- 20. Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ et al. (1994) The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA 272: 1661-1665. doi: 10.1001/jama.1994.03520210045030. PubMed: 7966893. [DOI] [PubMed] [Google Scholar]
- 21. Trzonkowski P, Myśliwska J, Szmit E, Wieckiewicz J, Lukaszuk K et al. (2003) Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—an impact of immunosenescence. Vaccine 21: 3826-3836. doi: 10.1016/S0264-410X(03)00309-8. PubMed: 12922116. [DOI] [PubMed] [Google Scholar]
- 22. Wikby A, Johansson B, Olsson J, Löfgren S, Nilsson BO et al. (2002) Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol 37: 445-453. doi: 10.1016/S0531-5565(01)00212-1. PubMed: 11772532. [DOI] [PubMed] [Google Scholar]
- 23. Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG (1998) Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev 102: 187-198. doi: 10.1016/S0047-6374(97)00151-6. PubMed: 9720651. [DOI] [PubMed] [Google Scholar]
- 24. Colonna-Romano G, Akbar AN, Aquino A, Bulati M, Candore G et al. (2007) Impact of CMV and EBV seropositivity on CD8 T lymphocytes in an old population from West-Sicily. Exp Gerontol 42: 995-1002. doi: 10.1016/j.exger.2007.05.006. PubMed: 17611062. [DOI] [PubMed] [Google Scholar]
- 25. Pellicanò M, Larbi A, Goldeck D, Colonna-Romano G, Buffa S et al. (2012) Immune profiling of Alzheimer patients. J Neuroimmunol 242: 52-59. doi: 10.1016/j.jneuroim.2011.11.005. PubMed: 22153977. [DOI] [PubMed] [Google Scholar]
- 26. Larbi A, Pawelec G, Witkowski JM, Schipper HM, Derhovanessian E et al. (2009) Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer's disease. J Alzheimers Dis 17: 91-103. PubMed: 19494434. [DOI] [PubMed] [Google Scholar]
- 27. Pirttilä T, Mattinen S, Frey H (1992) The decrease of CD8-positive lymphocytes in Alzheimer's disease. J Neurol Sci 107: 160-165. doi: 10.1016/0022-510X(92)90284-R. PubMed: 1564514. [DOI] [PubMed] [Google Scholar]
- 28. Gratama JW (2001) Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood 98: 1358-1364. doi: 10.1182/blood.V98.5.1358. PubMed: 11520783. [DOI] [PubMed] [Google Scholar]
- 29. McKhann G, Drachman D, Folstein M, Katzman R, Price D et al. (1984) Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34: 939-944. doi: 10.1212/WNL.34.7.939. PubMed: 6610841. [DOI] [PubMed] [Google Scholar]
- 30. Mansouri H (1995) A comparative study of some rank tests for interaction. Comput Statist Data Anal. [Google Scholar]
- 31. Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA et al. (1993) Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology 43: 1467-1472. doi: 10.1212/WNL.43.8.1467. PubMed: 8350998. [DOI] [PubMed] [Google Scholar]
- 32. Pita-Lopez ML, Gayoso I, DelaRosa O, Casado JG, Alonso C et al. (2009) Effect of ageing on CMV-specific CD8 T cells from CMV seropositive healthy donors. Immun Ageing 6: 11. doi: 10.1186/1742-4933-6-11. PubMed: 19715573. [DOI] [PMC free article] [PubMed] [Google Scholar]