Abstract
Background
Although sorafenib is accepted as the standard of care in advanced hepatocellular carcinoma (HCC), its therapeutic benefit is marginal. Here, we aimed to compare the efficacy and safety of sorafenib monotherapy (S-M) and sorafenib-based loco-regional treatments (S-LRTs) in advanced HCC.
Methods
From 2007 to 2012, 290 patients with advanced HCC (Barcelona Clinic Liver Cancer stage C) with S-M (n = 226) or S-LRTs (n = 64) were reviewed retrospectively. Survival outcomes and treatment-related toxicities between two groups were analyzed.
Results
Variables related to tumor burden and liver function were similar between the groups (all P > 0.05). Within the entire population, the S-LRTs group had both longer median overall survival (OS) (8.5 vs 5.5 months, P = 0.001) and progression-free survival (PFS) (5.3 vs 3.0 months, P = 0.002) than the S-M group. Furthermore, the S-LRTs group had longer Os than the S-M group in a subgroup with neither extrahepatic spread (EHS) nor regional nodal involvement (RNI) (18.0 vs 7.8 months, P = 0.019) and in a subgroup with EHS and/or RNI (8.3 vs 4.8 months, P = 0.028). In addition, the S-LRTs group had longer PFS than the S-M group in the subgroup with neither EHS nor RNI (9.6 vs 3.2 months, P = 0.027).
Treatment
Related toxicity was similar between two groups.
Conclusion
Combined use of sorafenib and LRTs may provide better treatment outcomes without significantly increasing treatment-related toxicities, even in patients with EHS and/or RNI. Therefore, addition of active LRTs might be considered, if feasible.
Introduction
Hepatocellular carcinoma (HCC) is currently ranked as the fifth most common cancer and the third leading cause of cancer death worldwide [1]. Surgical resection or local ablative treatments such as radiofrequency ablation and percutaneous ethanol injection achieve the best outcomes, with a 5-year survival rate of 60–70% in patients treated during early stages. Unfortunately, this is not feasible for the majority of patients with HCC, because they present with advanced disease, consisting of extensive tumor burden with portal vein thrombosis, intra/extra tumor spread, or poor liver function [2,3].
To date, several palliative therapeutic options have been used to treat advanced HCC. These include trans-arterial chemoembolization (TACE), hepatic artery infusional chemotherapy (HAIC), external/internal irradiation, and molecular targeted agents [4-6]. Of these, sorafenib, a small-molecule multi-targeted tyrosine kinase inhibitor, is the first targeted agent approved for the systemic treatment of advanced HCC. Sorafenib inhibits vascular endothelial growth factor receptor, platelet-derived growth factor receptor, B-Raf, Fms-related tyrosine kinase, and c-kit at nanomolar concentrations [7,8]. Further, sorafenib demonstrates survival benefits when compared to the best supportive care [5,6] and has thus become the principal therapy for patients with advanced HCC or Barcelona Clinic Liver Cancer (BCLC) stage C. It is also currently being used in the control arm of ongoing clinical trials for new targeted agents [9-12]. Although sorafenib is the standard treatment for advanced HCC, most patients treated with sorafenib achieve only stable disease as the best radiologic response, with a median time to progression of from 2.2 to, at most, 5.5 months. More importantly, its therapeutic benefit might be substantially attenuated for patients with portal vein invasion and/or extrahepatic spread. Thus, these patients will have a much poorer overall survival compared to those without these two factors [13,14].
Therefore, additional treatment strategies need to be identified and optimized to improve the therapeutic response. Furthermore, given that more than two-thirds of patients with advanced HCC die of liver failure due to intrahepatic tumor progression rather than from progression of extrahepatic metastatic disease, loco-regional treatments (LRTs) targeting the primary tumor should be reappraised from the standpoint of therapeutic benefit for patients with advanced HCC. Indeed, before sorafenib treatment was widely accepted for advanced HCC, several researchers had investigated this issue and reported some benefits in patients with major vascular invasion or extrahepatic metastasis where LRTs were used to delay intra-hepatic tumor progression instead of the best supportive care [9,10,15,16]. More recently, several studies with small population have suggested that LRTs when combined with sorafenib may lead to better clinical outcomes than the traditional sorafenib-monotherapy (S-M) [17-20].
Here, we aimed to assess the efficacy and safety of the combined use of sorafenib and LRTs in advanced HCC and compare these outcomes to those achieved with S-M.
Materials and Methods
Patient Eligibility
Between December 2007 and February 2012 a total of 318 patients with unresectable HCC who were eligible for sorafenib treatment with or without LRTs were reviewed retrospectively. Among patients who underwent S-LRT or SM, 28 patients were excluded according to the following exclusion criteria; short duration of sorafenib administration (<2 week, n = 21), BCLC stage A/B (n = 5), Child-Pugh class C (n = 2), and Eastern Cooperative Oncology Group (ECOG) performance status ≥3 (n = 0). Finally, 290 patients with BCLC C and Child-Pugh class A or B were analyzed.
This study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from each participant or responsible family member after possible complications of the diagnostic procedures and anti-cancer treatments had been fully explained. This study was approved by the independent institutional review board of Severance Hospital.
Diagnosis of HCC
A diagnosis of HCC was based on histological examination or clinico-radiologic criteria according to guidelines proposed by The Korea Liver Cancer Study Group [21] as follows; a patient is considered positive for HCC if they have one or more risk factors (hepatitis B or C virus infection, cirrhosis) and one of the following: 1) serum α-fetoprotein (AFP) ≥ 400 ng/mL and a positive finding on at least one of three typical imaging studies [dynamic computed tomography (CT), dynamic magnetic resonance imaging (MRI), or hepatic angiography], or 2) serum AFP <400 ng/mL and positive findings on at least two of the three imaging studies. A positive finding for typical HCC on dynamic CT or MRI was defined as increased arterial enhancement followed by decreased enhancement compared with the liver (washout) in the portal or equilibrium phase.
Study Design
Subjects were divided into two groups; 1) patients who were treated with sorafenib monotherapy (referred to as “S-M group”), and 2) patients who were treated by other LRTs in addition to sorafenib (referred to as “S-LRTs group”). Patients in the S-M group received 400 mg oral sorafenib (Nexavar Bayer Health Care AG, Leverkusen, Germany) twice daily on a continuous dosing schedule. In addition to the twice daily 400 mg oral sorafenib, patients in the S-LRTs group also received LRTs that included intra-arterial chemotherapy (either TACE or HAIC), external beam radiotherapy, or both to delay progression of intra-hepatic tumors. The detailed protocols of LRTs performed at our institution, such as TACE using doxorubicin, lipiodol, and gelatin sponge particles, HAIC via implantable port system using 5-fluorouracil and cisplatin, and three-dimensional conformal external beam radiotherapy were described previously [22-25]. S-LRTs were performed in advanced HCC with the tolerable liver function for anti-tumor therapy in Severance. However, S-M has been applied for patients who couldn’t afford S-LRTs because national insurance system didn’t guarantee only one method among chemotherapy and LRTs.
The time interval between LRTs and initiation of sorafenib is less than 14 days (median 4 days, range 0–14 days). In both groups, dose reduction and drug interruption were allowed in the case of significant drug-related adverse effects or poor general condition. Sorafenib was also administered until disease progression, the development of intolerable toxicities, or patient refusal. However, for patients in the S-LRTs group, when intrahepatic tumor progression was observed, an alternative LRTs was allowed to delay intrahepatic disease progression, according to physician discretion and patient eligibility.
Evaluation of Treatment Response and Safety Profile
Clinical examination and laboratory assessments were performed upon the initiation of sorafenib treatment. The response evaluation was carried out with a dynamic CT scan or MRI, if appropriate, every 8 weeks. We adopted the modified Response Evaluation Criteria in Solid Tumors as follows: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Objective response was defined as CR or PR, and disease control as CR, PR, or SD. Response was analyzed by intention-to-treat analysis. Toxicity grade was assessed before each treatment cycle using the National Cancer Institute Common Toxicity Criteria version 3.0
Statistical Analyses
Student’s t test or Mann-Whitney U tests, if appropriate, were used to compare continuous variables, and Chi-square or Fisher’s exact tests were used for categorical variables. The primary endpoint was overall survival (OS), whereas the secondary endpoints were progression-free survival (PFS) and treatment-related toxicity. OS was calculated as the time interval from the initiation date of either sorafenib or LRTs (provided that LRTs preceded the administration of sorafenib in the S-LRTs group) until the date of death or final follow-up. Similarly, PFS was calculated as the time interval from the initiation date of sorafenib or LRTs until the date of first progression or death. Survival time was estimated by the Kaplan-Meier method, and the survival difference between groups was assessed by the log-rank test. The Cox proportional hazards model was used for a multivariate analysis of survival. All variables found significant in the univariate analysis were included in the multivariate model. Statistical analyses were performed using SAS version 9.1.3 (SAS, Cary, NC). A two-sided P value < 0.05 was considered statistically significant.
Results
Baseline Characteristics
Table 1 shows the baseline characteristics of the entire population. The median age was 56.7 years and 243 (83.8%) patients were male. Most patients (n = 208, 71.7 %) showed preserved liver function of Child-Pugh class A. The median tumor size was 4.0 cm. Extrahepatic spreading (EHS) was identified in 182 (62.8%) patients, whereas regional lymph nodal involvement (RNI) was noted in 89 (36.0%) patients. The most common site for EHS was lung (n = 132, 45.5%). The median AFP and protein induced by vitamin K absence or antagonist (PIVKA) levels were 110 ng/mL and 112 AU/L, respectively. In S-LRTs group, sorafenib was combined with LRTs as follows; TACE (n = 24), HAIC (n = 5), HAIC with radiotherapy (n = 13), and radiotherapy alone (n = 22). The proportion of patients with a previous history of treatment for HCC and non-viral etiology was higher in the S-M group than in the S-LRTs group (79.6% vs. 67.2%, P = 0.037 and 15.9% vs. 6.2%, P = 0.047, respectively). There was no statistically significant difference in clinical variables between the two groups (Table 1).
Table 1. Baseline characters of patients in entire cohort.
| Variables | Entire population (n = 290) | S-M group (n= 226, 77.9%) | S-LRTs group (n = 64, 22.1%) | P | |
|---|---|---|---|---|---|
| Age (years) | 56.7 (27.0–85.0) | 57.0 (27.0–85.0) | 56.0 (28.0–79.0) | NS | |
| Male gender | 243 (83.8) | 188 (83.2) | 55 (85.9) | NS | |
| Etiology, viral/ non-viral | 250 (86.2)/40 (13.8) | 190 (84.1)/36 (15.9) | 60 (93.8)/4 (6.2) | 0.047 | |
| ECOG, 0/1~2 | 84 (29.0)/206 (71.0) | 63 (27.9)/163 (72.1) | 21 (32.8)/43 (67.2) | NS | |
| Child-Pugh class, A/B | 208(71.7)/82 (28.3) | 161 (71.2)/65 (28.8) | 47 (73.4)/17 (26.6) | NS | |
| Tumor size (cm) | 4.0 (0.7–20.0) | 4.0 (1.0–20.0) | 4.7 (0.7–16.0) | NS | |
| Presence of EHS | 182 (62.8) | 147 (65.0) | 35 (54.7) | NS | |
| Presence of lung metastasis | 132 (45.5) | 109 (48.2) | 23 (35.9) | 0.081 | |
| Presence of bone metastasis | 44 (15.2) | 39 (17.3) | 5 (7.8) | 0.063 | |
| Presence of distant LN metastasis | 26 (9.0) | 17 (7.5) | 9 (14.1) | NS | |
| Presence of RNI | 89 (30.7) | 66 (29.2) | 23 (35.9) | NS | |
| Prior history of HCC treatment¶ | 223 (76.9) | 180 (79.6) | 43 (67.2) | 0.037 | |
| Surgery | 17 | 13 | 4 | ||
| RFA | 2 | 2 | 0 | ||
| PEIT | 0 | 0 | 0 | ||
| TACE | 97 | 71 | 26 | ||
| TACE with RFA | 4 | 1 | 3 | ||
| TACE with radiotherapy | 9 | 9 | 0 | ||
| HAIC with radiotherapy | 86 | 78 | 8 | ||
| HAIC | 5 | 4 | 1 | ||
| Radiotherapy | 3 | 2 | 1 | ||
| AFP, ng/mL | 110 (0.9–120,000.0) | 231.3 (1.1–120,000.0) | 160.5 (0.9–83,000.0) | NS | |
| PIVKA, AU/L | 112 (6.2–75,000.0) | 733.0 (6.2–75,000.0) | 329.5 (10.0–2,000.0) | 0.098 | |
| Ln total dosage (mg | 10.7 (5.9–13.4) | 10.7 (5.9–13.4) | 10.9 (8.5–12.8) | NS |
Abbreviations: S-M, sorafenib monotherapy; S-LRTs, sorafenib combined with loco-regional treatments; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; EHS, extrahepatic spread; LN, lymph node; RNI, regional nodal involvement; RFA, radiofrequency ablation; PEIT, percutaneous ethanol injection therapy; TACE, trans-arterial chemoembolization; HAIC, hepatic arterial infusional chemotherapy; AFP, α-fetoprotein; PIVKA, protein induced by vitamin K absence; Ln, natural logarithm; NS, not significant.
Values are expressed as median (range) or no. (%).
¶ If different anti-cancer treatment modalities were performed repeatedly before enrollment, the latest treatment modality was recorded as the “prior history of HCC treatment”.
Treatment Outcomes and Variables Affecting OS
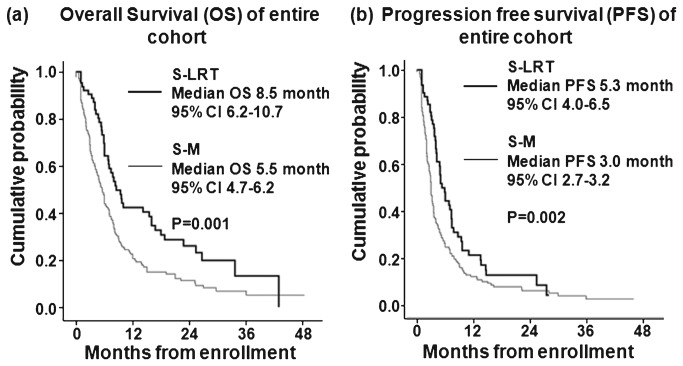
The median OS in the entire population was 6 months [95% confidence interval (CI) 5.2–6.7]. Subjects in the S-LRTs group had a significantly longer median OS than those in the S-M group [8.5 months (95% CI 6.2–10.7 months) vs. 5.5 months (95% CI 4.7–6.2 months); P = 0.001] (Figure 1). In addition, Child-Pugh class, tumor size, EHS and/or RNI, AFP level ≥400 ng/mL, PIVKA level ≥1,000 AU/L, and the cumulative dose of sorafenib (transformed by natural logarithm) significantly predicted OS in univariate analysis (all P < 0.05) (Table 2). Subsequent multivariate analysis revealed that combined LRTs modality with sorafenib remained as the independent predictor for the better OS [adjusted hazard ratio (HR) 0.5, 95% CI 0.3–0.8, P = 0.002], together with Child-Pugh class (adjusted HR 1.8, 95% CI 1.2–2.5, P < 0.001), tumor size (adjusted HR 1.5, 95% CI 1.1–2.3, P = 0.030), EHS and/or RNI (adjusted HR 1.7, 95% CI 1.2–2.4, P = 0.001), AFP level (adjusted HR 1.6, 95% CI 1.1–2.1, P = 0.002), and the cumulative dose of sorafenib (transformed by natural logarithm) (adjusted HR 0.5, 95% CI 0.4–0.6, P < 0.001 ) (Table 2).
Figure 1. Kaplan-Meier analysis of overall survival (A) and progression-free survival (B) in patients.
Data were stratified by the treatment modalities treated with sorafenib monotherapy (S-M) and sorafenib-based loco-regional treatments (S-LRTs). The thick line indicates S-LRTs and the thin line S-M. Median OS and PFS are significantly longer in S-LRTs than S-M.
Table 2. Univariate and multivariate analysis for variables affecting overall and progression-free survival.
| Variables |
Overall survival
|
Progression-free survival
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate analysis
|
Univariate |
Multivariate analysis
|
||||||
| P | Adjusted HR (95% CI) | P | P | Adjusted HR (95% CI) | P | ||||
| S-LRTs (vs. S-M) | <0.001 | 0.5 (0.3–0.8) | 0.002 | 0.002 | 0.6 (0.4–0.9) | 0.025 | |||
| Age ≥65 (vs. < 65 years) | 0.819 | - | - | 0.116 | - | - | |||
| Male (vs. female) | 0.809 | - | - | 0.221 | - | - | |||
| Viral etiology (vs. non-viral) | 0.428 | - | - | 0.75 | - | - | |||
| ECOG 0 (vs. 1/2) | 0.944 | - | - | 0.297 | - | - | |||
| Child-Pugh class B (vs. A) | <0.001 | 1.8 (1.2–2.5) | <0.001 | 0.003 | 1.4 (1.1–1.9) | 0.029 | |||
| Tumor size≥10 cm (vs. <10 cm) | <0.001 | 1.5 (1.1–2.3) | 0.03 | 0.006 | 1.6 (1.1–2.4) | 0.012 | |||
| Presence of EHS and/or RNI (vs. no) | <0.001 | 1.7 (1.2–2.4) | 0.001 | <0.001 | 1.7 (1.2–2.4) | <0.001 | |||
| Prior history of HCC treatment (vs. no) | 0.949 | - | - | 0.992 | - | - | |||
| AFP ≥400 ng/mL (vs. <400 ng/mL) | <0.001 | 1.6 (1.1–2.1) | 0.002 | <0.001 | 1.9 (1.4–2.5) | <0.001 | |||
| PIVKA ≥1,000 AU/L (vs. <1,000 AU/L) | <0.001 | 1.2 (0.9–1.6) | 0.161 | <0.001 | 1.0 (0.7–1.3) | 0.883 | |||
| Ln total dosage (mg) | <0.001 | 0.5 (0.4–0.6) | <0.001 | <0.001 | 0.6 (0.5–0.7) | <0.001 | |||
Abbreviations: HR, hazard ratio; CI, confidence interval; S-M, sorafenib monotherapy; S-LRTs, sorafenib combined with loco-regional treatments; ECOG, Eastern Cooperative Oncology Group; EHS, extrahepatic spread; RNI, regional nodal involvement; HCC, hepatocellular carcinoma; AFP, α-fetoprotein; PIVKA, protein induced by vitamin K absence; Ln, natural logarithm.
Because lung and/or bone metastasis significantly predicted OS in univariate analysis (P = 0.033), it was entered into multivariate analysis, adjusting other significant covariates such as S-LRTs, Child-Pugh class, tumor size, AFP level ≥400 ng/mL, PIVKA level ≥1,000 AU/L, and the cumulative dose of sorafenib. However, presence of EHS and/or RNI was not incorporated into this analysis since presence of EHS and/or RNI partly included lung and/or bone metastasis. Finally, lung and/or bone metastasis was also selected as one of the independent prognostic factor for OS (adjusted HR 1.2, 95% CI 1.1-1.8, P = 0.031) (Table S1).
Treatment Outcomes and Variables Affecting PFS
The median PFS for the entire population was 3.4 months (95% CI 3.0–3.7). Subjects in the S-LRTs group had a significantly longer median PFS than those in the S-M group [5.3 months (95% CI 4.0–6.5 months) vs. 3.0 months (95% CI 2.7–3.2 months); P = 0.002; Figure 1]. In addition, Child-Pugh class, tumor size, EHS and/or RNI, AFP level ≥400 ng/mL, PIVKA level ≥1,000 AU/L, and the cumulative dose of sorafenib (transformed by natural logarithm) significantly predicted PFS in univariate analysis (all P < 0.05; Table 2). Subsequent multivariate analysis revealed that combined LRTs modality with sorafenib remained as the independent predictor for the better PFS (adjusted HR 0.6, 95% CI 0.4–0.9, P = 0.025), together with Child-Pugh class (adjusted HR 1.4, 95% CI 1.1–1.9, P = 0.029), tumor size (adjusted HR 1.6, 95% CI 1.1–2.4, P = 0.012), EHS and/or RNI (adjusted HR 1.7, 95% CI 1.2–2.4, P < 0.001 ), AFP level (adjusted HR 1.9, 95% CI 1.4–2.5, P < 0.001), and the cumulative dose of sorafenib (transformed by natural logarithm) (adjusted HR 0.6, 95% CI 0.5–0.7, P < 0.001) (Table 2).
Because lung and/or bone metastasis significantly predicted PFS in univariate analysis (P = 0.001), it was entered into multivariate analysis, adjusting other significant covariates such as S-LRTs, Child-Pugh class, tumor size, AFP level ≥400 ng/mL, PIVKA level ≥1,000 AU/L, and the cumulative dose of sorafenib. However, presence of EHS and/or RNI was not incorporated into this analysis since presence of EHS and/or RNI partly included lung and/or bone metastasis. Finally, lung and/or bone metastasis was also selected as one of the independent prognostic factor for PFS (adjusted HR 1.5, 95% CI 1.1-2.1, P = 0.005) (Table S1).
Subgroup Analysis According to EHS or Regional Node Metastasis and AFP
The OS and PFS of subgroups according to the presence of EHS and/or RNI were compared (Table 3). In each subgroup, patients treated with S-LRTs had longer OS compared to those treated with S-M (18.0 months vs. 7.8 months in a subgroup with neither EHS nor RNI and 8.3 months vs. 4.8 months in a subgroup with EHS and/or RNI; all P < 0.05). In addition, patients treated with S-LRTs had longer PFS compared to those treated with S-LRTs in a subgroup with neither EHS nor RNI (9.6 months vs. 3.2 months, P = 0.027). However, the therapeutic benefit of LRTs was only marginal in a subgroup with EHS and/or RNI (4.9 months vs. 2.9 months, P = 0.069).
Table 3. Overall and progression-free survival according to tumor factors in subgroups.
| Population |
Median OS (95% CI) (months)
|
Hazard Ratio (95% CI) for S-LRTs (vs. S-M) | P | ||
|---|---|---|---|---|---|
| S-M | S-LRTs | ||||
| Entire cohort | 5.5 (4.7–6.2) | 8.5 (6.2–10.7) | 0.5 (0.4–0.7) | 0.001 | |
| Subgroup with neither EHS nor RNI | 7.8 (6.2–9.3) | 18.0 (3.7–33.2) | 0.4 (0.2–0.9) | 0.019 | |
| Subgroup with EHS and/or RNI | 4.8 (3.7–5.8) | 8.3 (5.0–11.5) | 0.6 (0.4–0.9) | 0.028 | |
| Subgroup with AFP <400 ng/mL | 7.8 (6.6–8.9) | 8.3 (5.2–11.3) | 0.8 (0.5–1.3) | 0.449 | |
| Subgroup with AFP ≥400 ng/mL | 3.4 (2.5–4.2) | 8.0 (0.0–19.0) | 0.3 (0.1–0.6) | 0.001 | |
| Population |
Median PFS (95% CI) (months)
|
Hazard Ratio (95% CI) for S-LRTs (vs. S-M) | P | ||
| S-M | S-LRTs | ||||
| Entire cohort | 3.0 (2.7–3.2) | 5.3 (4.0–6.5) | 0.6 (0.4–0.8) | 0.002 | |
| Subgroup with neither EHS nor RNI | 3.2 (1.8–4.5) | 9.6 (3.1–16.0) | 0.5 (0.2–0.9) | 0.027 | |
| Subgroup with EHS and/or RNI | 2.9 (2.5–3.2) | 4.9 (4.0–5.7) | 0.7 (0.4–1.0) | 0.069 | |
| Subgroup with AFP <400 ng/mL | 4.0 (2.6–5.3) | 6.8 (4.1–9.4) | 0.7 (0.4–1.1) | 0.225 | |
| Subgroup with AFP ≥400 ng/mL | 2.0 (1.6–2.3) | 4.2 (2.6–5.7) | 0.5 (0.3–0.8) | 0.012 | |
Abbreviations: OS, overall survival; PFS, progression-free survival; CI, confidence interval; S-M, sorafenib monotherapy; S-LRTs, sorafenib combined with loco-regional treatments; EHS, extrahepatic spread; RNI, regional nodal involvement.
The OS and PFS of subgroups were also affected by AFP level (<400 ng/mL vs. ≥400 ng/mL). In the subgroup with low AFP level (<400 ng/mL), the therapeutic benefit of LRTs to prolong OS did not reach the statistical significance (P = 0.449). However, in the subgroup with high AFP level (≥400 ng/mL), the LRTs was effective to improve the OS (3.4 months vs. 8.0 months, P = 0.001). Regarding PFS, similar results were obtained. Although the S-LRTs could not increase PFS in the subgroup of low AFP level (<400 ng/mL) (P = 0.225), it extended PFS significantly in the subgroup of high AFP level (≥400 ng/mL) (2.0 months vs. 4.2 months, P = 0.012).
Treatment-Related Toxicity
The treatment-related adverse effects of grade 2 or more are described in Table 4. The most common toxicity related to sorafenib was hand foot-skin reaction (23.0% in S-M group vs. 23.4% in S-LRTs group), followed by diarrhea (16.4% in S-M group vs. 15.6% in S-LRTs group). Grade 3 or 4 toxicities were relatively uncommon and hand foot-skin reaction of grade 3 or more was found in 8 (4.8%) patients in the S-M group and 2 (3.7%) patients in the S-LRTs group. Except anorexia, the addition of LRTs to sorafenib did not increase the occurrence of significant toxicities (all P > 0.05). All adverse events were manageable and there was no significance difference of the sorafenib discontinuation due to adverse effects between two groups.
Table 4. Treatment-related adverse effects.
| S-M | S-LRTs | P | ||
|---|---|---|---|---|
| Major adverse effects of grade 2 or more | ||||
| HFSR | 52 (23.0) | 15 (23.4) | NS | |
| Diarrhea | 37 (16.4) | 10 (15.6) | NS | |
| Skin eruption | 17 (7.5) | 7 (10.9) | NS | |
| Anorexia | 10 (4.4) | 8 (12.5) | 0.034 | |
| Abdominal pain | 19 (8.4) | 8 (12.5) | NS | |
| Sorafenib discontinuation due to adverse effects | 64 (28.3) | 19 (29.7) | NS |
Abbreviations: S-M, sorafenib monotherapy; S-LRTs, sorafenib combined with loco-regional treatments; HFSR, hand foot-skin reaction; NS, not significant.
Discussion
For advanced-stage disease, the multikinase inhibitor sorafenib is the new standard treatment with a proven survival benefit [5,6]. However, since sorafenib predominantly results in delayed tumor progression by inhibiting tumor cell proliferation rather than shrinking tumors, most patients treated with sorafenib alone achieve only stable disease as the best radiologic response, with a median time to progression of from 2.2 to at most 5.5 months. Thus, new methods beyond sorafenib treatment are urgently required for HCC. In contrast, active LRTs including intra-arterial chemotherapy (TACE or HAIC) and/or external beam radiotherapy induce complete or partial radiologic response of approximately 30%, even in advanced HCC [22,24,26,27]. Considering that more than two-thirds of patients with advanced HCC die of liver failure due to intrahepatic tumor progression rather than from progression of extrahepatic metastatic disease, addition of such radical LRTs targeting the primary tumor might be a reasonable and effective approach for treating advanced-stage HCC, even in the presence of MVI, regional lymph node metastasis or EHS. To obtain pilot data, we investigated the benefits of providing active LRTs with sorafenib to patients with advanced-stage HCC.
The OS of the S-M group in our study was relatively shorter than that of Asia-Pacific trial (5.5 months vs. 6.5 months) [6]. This can be explained in part by the higher proportion of patients with older age (median 56 years vs. 51 years), poorer liver function (Child-Pugh class B, 28.3% vs. 2.7%), and more advanced stage of HCC (BCLC C, 100% vs. 95.3%) in our study population. Nevertheless, in our study S-LRTs prolonged the median OS by up to 3 months (8.5 months, P = 0.001), compared to that of the S-M group (5.5 months). This benefit of combined treatment was also confirmed through multivariate analysis after adjusting for other predictors such as Child-Pugh class, tumor size, EHS and/or RNI, alpha-fetoprotein level, and cumulative dosage of sorafenib. The beneficial effects of S-LRTs on OS were similarly observed in subgroups of patients with neither EHS nor RNI and those with EHS and/or RNI. Likewise, regarding PFS, S-LRTs prolonged the median PFS by up to 2.3 months (P = 0.002). Furthermore, when lung and/or bone metastasis were incorporated into multivariate analysis instead of EHS and/or RNI, the independent prognostic values of lung and/or bone metastasis and S-LRT were also similarly maintained for both OS and PFS.
Interestingly, when tumor burden was high, reflected by high AFP level (≥400 ng/mL), the effectiveness of S-LRTs became more prominent in improving OS and PFS (Table 3). These results support again the rationale that LRTs should be considered for advanced HCC with high tumor burden. Taken together, S-LRTs might delay intra-hepatic tumor progression and by extension, result in preserving the remnant liver function and ultimately prolonging the OS. The mechanism of action remains to be further investigated.
Although the promising results of active LRTs warrant further validation in larger prospective trials, our study had several strengths. First, the sample size in this study was larger and the follow-up period was longer than any previous studies [18-20]. Second, we have suggested how to improve the treatment responses of sorafenib treatment on the assumption that active LRTs might delay the hepatic failure due to the intrahepatic tumor progression. Thus, this study might be expected to provide a basic reference for further research on the addition of LRTs with sorafenib administration. Third, we performed subgroup analysis according to tumor status in order to identify who are more likely to benefit from additional LRTs. The therapeutic benefit for both OS and PFS was greater in the subgroup with neither EHS nor RNI than in the subgroup with EHS and/or RNI. However, since additional LRTs are aimed primarily at controlling the disease progression in the liver, the therapeutic effect for prolonging PFS is only marginal in a certain subgroup with EHS and/or RNI where the progression of EHS or RNI is beyond the effect of additional LRTs. Strikingly, even in such a subgroup, additional LRTs were helpful in reducing the events of hepatic failure due to intrahepatic tumor progression, ultimately leading to significantly prolonged OS. Therefore, the concurrent use of active LRTs might be a reasonable approach for treating advanced HCC.
Notably, among other predictors, the cumulative dosage of sorafenib, which depends on the daily administration dosage and duration of treatment, proved to be an independent prognostic predictor for both OS and PFS. This observation indicates that sorafenib should be administered as long as patients tolerate the treatment and further confirms the importance of sorafenib at the core of HCC therapy. In the current study the incidence of adverse effects due to sorafenib treatment were similar to those reported in other investigations [28-30]. More importantly, the addition of active LRTs did not increase the overall incidence of adverse events owing to sorafenib administration. However, physicians should always exercise caution when combining modalities.
There are several possible explanations for the beneficial effects of combining sorafenib with other LRTs. Although TACE induces tumor hypoxia, angiogenic factors such as VEGF temporarily increase after TACE. Therefore, the enhanced expression of circulating or tissue VEGF after TACE treatment could adversely affect the outcome of HCC patients, through revascularization, tumor progression, and distant metastasis [31]. From this view point, combining an anti-angiogenic agent with TACE may provide complementary inhibition of neovascularization and tumor growth [32,33]. Similarly, radiation exposure may act as a stressing event, inducing the compensatory activation of multiple intracellular signaling pathway mediators, such as phosphoinositide 3-kinase, mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase, and nuclear factor-kappa B [34,35]. In particular, VEGF levels increased in a time- and dose-dependent manner after sublethal irradiation of HCC cells, which translated to enhanced intratumor angiogenesis in vivo [36]. Therefore, sorafenib-mediated blockage of the Raf/MAPK and vascular endothelial growth factor receptor pathways might enhance the efficacy of radiation [37,38].
This study has a few limitations. The first drawback is the retrospective nature of the work, which could lead to selection bias in determining the treatment modalities. However, we consecutively enrolled subjects during the study period and the baseline characteristics between the two groups were very similar, differing only in the proportion of HCCs with viral etiologies and previous history of treatment for HCC. As a matter of fact, the etiologies for HCC and prior treatment history did not affect the clinical outcomes. Second, active LRTs used in this study include heterogeneous modalities such as TACE, HAIC, external-beam radiotherapy, and their combinations. Although such LRTs demonstrate comparable outcomes [15,22,34,39], prospective trials are required to solve this issue. In addition, treatment regimen decisions were based not only on medical issues, but also non-medical and/or economic considerations.
In conclusion, the addition of active LRTs to sorafenib treatment achieved promising results in unresectable HCC without significantly increasing treatment-related toxicities. Further, the therapeutic benefits for OS were maintained regardless of the presence of EHS. These results now require validation in another population through prospective trials.
Supporting Information
Univariate and multivariate analysis for variables including lung and/or bone metastasis to affect overall and progression-free survival.
(DOC)
Funding Statement
This study was supported in part by a grant of the Korea Healthcare technology R & D project, Ministry of Health and Welfare, Republic of Korea (number A102065). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74-108. doi: 10.3322/canjclin.55.2.74. PubMed: 15761078. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362: 1907-1917. doi: 10.1016/S0140-6736(03)14964-1. PubMed: 14667750. [DOI] [PubMed] [Google Scholar]
- 3. Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42: 1208-1236. doi: 10.1002/hep.20933. PubMed: 16250051. [DOI] [PubMed] [Google Scholar]
- 4. Lencioni R, Crocetti L (2012) Local-regional treatment of hepatocellular carcinoma. Radiology 262: 43-58. doi: 10.1148/radiol.11110144. PubMed: 22190656. [DOI] [PubMed] [Google Scholar]
- 5. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378-390. doi: 10.1056/NEJMoa0708857. PubMed: 18650514. [DOI] [PubMed] [Google Scholar]
- 6. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S et al. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25-34. doi: 10.1016/S1470-2045(08)70285-7. PubMed: 19095497. [DOI] [PubMed] [Google Scholar]
- 7. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A et al. (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64: 7099-7109. doi: 10.1158/0008-5472.CAN-04-1443. PubMed: 15466206. [DOI] [PubMed] [Google Scholar]
- 8. Chang YS, Adnane J, Trail PA, Levy J, Henderson A et al. (2007) Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol 59: 561-574. doi: 10.1007/s00280-006-0393-4. PubMed: 17160391. [DOI] [PubMed] [Google Scholar]
- 9. Kim BK, Kang WJ, Kim JK, Seong J, Park JY et al. (2011) (18) F-fluorodeoxyglucose uptake on positron emission tomography as a prognostic predictor in locally advanced hepatocellular carcinoma. Cancer 117: 4779-4787. doi: 10.1002/cncr.26099. PubMed: 21469082. [DOI] [PubMed] [Google Scholar]
- 10. Kim HY, Park JW, Nam BH, Kim HK, Choi JI et al. (2011) Survival of patients with advanced hepatocellular carcinoma: sorafenib versus other treatments. J Gastroenterol Hepatol 26: 1612-1618. doi: 10.1111/j.1440-1746.2011.06751.x. PubMed: 21517968. [DOI] [PubMed] [Google Scholar]
- 11. Qu XD, Chen CS, Wang JH, Yan ZP, Chen JM et al. (2012) The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer 12: 263. doi: 10.1186/1471-2407-12-263. PubMed: 22721173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM et al. (2012) Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys 82: 2004-2011. doi: 10.1016/j.ijrobp.2011.03.019. PubMed: 21621346. [DOI] [PubMed] [Google Scholar]
- 13. Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R et al. (2008) Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol 103: 914-921. doi: 10.1111/j.1572-0241.2007.01712.x. PubMed: 18177453. [DOI] [PubMed] [Google Scholar]
- 14. Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ et al. (2008) Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol 49: 523-529. doi: 10.1080/02841850801958890. PubMed: 18568538. [DOI] [PubMed] [Google Scholar]
- 15. Yoo DJ, Kim KM, Jin YJ, Shim JH, Ko GY et al. (2011) Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol 26: 145-154. doi: 10.1111/j.1440-1746.2010.06341.x. PubMed: 21175808. [DOI] [PubMed] [Google Scholar]
- 16. Lee JM, Han KH (2010) Positioning and indication of sorafenib in the treatment algorithm and real practice setting: Western and eastern approach--Asian perspective. Oncology 78: 167-171. doi: 10.1159/000315246. PubMed: 20616600. [DOI] [PubMed] [Google Scholar]
- 17. Cabrera R, Pannu DS, Caridi J, Firpi RJ, Soldevila-Pico C et al. (2011) The combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinoma. Aliment Pharmacol Ther 34: 205-213. doi: 10.1111/j.1365-2036.2011.04697.x. PubMed: 21605146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park JW, Koh YH, Kim HB, Kim HY, An S et al. (2012) Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol 56: 1336-1342. doi: 10.1016/j.jhep.2012.01.006. PubMed: 22314421. [DOI] [PubMed] [Google Scholar]
- 19. Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N et al. (2011) Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol 29: 3960-3967. doi: 10.1200/JCO.2011.37.1021. PubMed: 21911714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY et al. (2011) Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 47: 2117-2127. doi: 10.1016/j.ejca.2011.05.007. PubMed: 21664811. [DOI] [PubMed] [Google Scholar]
- 21. Liver Korean Cancer Study Group and National Cancer Center (2009) Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol 15: 391-423. doi: 10.3350/kjhep.2009.15.3.391. PubMed: 19783891. [DOI] [PubMed] [Google Scholar]
- 22. Han KH, Seong J, Kim JK, Ahn SH, Lee do Y et al. (2008) Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer 113: 995-1003. doi: 10.1002/cncr.23684. PubMed: 18615601. [DOI] [PubMed] [Google Scholar]
- 23. Aaltonen T, Adelman J, Akimoto T, Albrow MG, González BA et al. (2008) Forward-backward asymmetry in top-quark production in pp[over] collisions at sqrt[s]=1.96 TeV. Phys Rev Lett 101: 202001. doi: 10.1103/PhysRevLett.101.202001. PubMed: 19113329. [DOI] [PubMed] [Google Scholar]
- 24. Park JY, Ahn SH, Yoon YJ, Kim JK, Lee HW et al. (2007) Repetitive short-course hepatic arterial infusion chemotherapy with high-dose 5-fluorouracil and cisplatin in patients with advanced hepatocellular carcinoma. Cancer 110: 129-137. doi: 10.1002/cncr.22759. PubMed: 17508408. [DOI] [PubMed] [Google Scholar]
- 25. Seong J, Lee IJ, Shim SJ, Lim do H, Kim TH et al. (2009) A multicenter retrospective cohort study of practice patterns and clinical outcome on radiotherapy for hepatocellular carcinoma in Korea. Liver Int 29: 147-152. doi: 10.1111/j.1478-3231.2008.01873.x. PubMed: 18795897. [DOI] [PubMed] [Google Scholar]
- 26. Kim JK, Han KH, Lee JT, Paik YH, Ahn SH et al. (2006) Long-term clinical outcome of phase IIb clinical trial of percutaneous injection with holmium-166/chitosan complex (Milican) for the treatment of small hepatocellular carcinoma. Clin Cancer Res 12: 543-548. doi: 10.1158/1078-0432.CCR-05-1730. PubMed: 16428498. [DOI] [PubMed] [Google Scholar]
- 27. Koom WS, Seong J, Han KH, Lee do Y, Lee JT (2010) Is local radiotherapy still valuable for patients with multiple intrahepatic hepatocellular carcinomas? Int J Radiat Oncol Biol Phys 77: 1433-1440. doi: 10.1016/j.ijrobp.2009.07.1676. PubMed: 19896779. [DOI] [PubMed] [Google Scholar]
- 28. Lipworth AD, Robert C, Zhu AX (2009) Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology 77: 257-271. doi: 10.1159/000258880. PubMed: 19923864. [DOI] [PubMed] [Google Scholar]
- 29. Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L et al. (2012) Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: Subanalyses of a phase III trial. J Hepatol 57: 821-829. doi: 10.1016/j.jhep.2012.06.014. PubMed: 22727733. [DOI] [PubMed] [Google Scholar]
- 30. Di Costanzo GG, Tortora R, Iodice L, Lanza AG, Lampasi F et al. (2012) Safety and effectiveness of sorafenib in patients with hepatocellular carcinoma in clinical practice. Dig Liver Dis 44: 788-792. doi: 10.1016/j.dld.2012.04.001. PubMed: 22579445. [DOI] [PubMed] [Google Scholar]
- 31. Li X, Feng GS, Zheng CS, Zhuo CK, Liu X (2004) Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol 10: 2878-2882. PubMed: 15334691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang H, Meng Q, Tan H, Pan S, Sun B et al. (2007) Antiangiogenic therapy enhances the efficacy of transcatheter arterial embolization for hepatocellular carcinomas. Int J Cancer 121: 416-424. doi: 10.1002/ijc.22655. PubMed: 17330237. [DOI] [PubMed] [Google Scholar]
- 33. Sieghart W, Pinter M, Reisegger M, Müller C, Ba-Ssalamah A et al. (2012) Conventional transarterial chemoembolisation in combination with sorafenib for patients with hepatocellular carcinoma: a pilot study. Eur Radiol 22: 1214-1223. doi: 10.1007/s00330-011-2368-z. PubMed: 22215073. [DOI] [PubMed] [Google Scholar]
- 34. Cheng JC, Chou CH, Kuo ML, Hsieh CY (2006) Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene 25: 7009-7018. doi: 10.1038/sj.onc.1209706. PubMed: 16732316. [DOI] [PubMed] [Google Scholar]
- 35. Chung YL, Jian JJ, Cheng SH, Tsai SY, Chuang VP et al. (2006) Sublethal irradiation induces vascular endothelial growth factor and promotes growth of hepatoma cells: implications for radiotherapy of hepatocellular carcinoma. Clin Cancer Res 12: 2706-2715. doi: 10.1158/1078-0432.CCR-05-2721. PubMed: 16675562. [DOI] [PubMed] [Google Scholar]
- 36. Ma BB, Bristow RG, Kim J, Siu LL (2003) Combined-modality treatment of solid tumors using radiotherapy and molecular targeted agents. J Clin Oncol 21: 2760-2776. doi: 10.1200/JCO.2003.10.044. PubMed: 12860956. [DOI] [PubMed] [Google Scholar]
- 37. Cao C, Albert JM, Geng L, Ivy PS, Sandler A et al. (2006) Vascular endothelial growth factor tyrosine kinase inhibitor AZD2171 and fractionated radiotherapy in mouse models of lung cancer. Cancer Res 66: 11409-11415. doi: 10.1158/0008-5472.CAN-06-2414. PubMed: 17145887. [DOI] [PubMed] [Google Scholar]
- 38. Kim BK, Park JY, Choi HJ, Kim do Y, Ahn SH et al. (2011) Long-term clinical outcomes of hepatic arterial infusion chemotherapy with cisplatin with or without 5-fluorouracil in locally advanced hepatocellular carcinoma. J Cancer Res Clin Oncol 137: 659-667. doi: 10.1007/s00432-010-0917-5. PubMed: 20552225. [DOI] [PubMed] [Google Scholar]
- 39. Kim BK, Ahn SH, Seong JS, Park JY, Kim do Y et al. (2011) Early alpha-fetoprotein response as a predictor for clinical outcome after localized concurrent chemoradiotherapy for advanced hepatocellular carcinoma. Liver Int 31: 369-376. doi: 10.1111/j.1478-3231.2010.02368.x. PubMed: 21083802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariate and multivariate analysis for variables including lung and/or bone metastasis to affect overall and progression-free survival.
(DOC)