Abstract
Objective: Information regarding the development of the enteric nervous system (ENS) is important for understanding the functional abnormalities of the gut. Because fertilized chicken eggs provide easy access to embryos, chicken models have been widely used to study embryonic development of myenteric plexus; however, no study has been focused on the postnatal period. The aim of this study was to perform a qualitative and quantitative analysis of the nitrergic neurons in the myenteric plexus of developing chickens in the postnatal period. Methods: Whole-mount preparations of the myenteric plexus were made in 7-d, 15-d, and 40-d old (adult) chickens of either sex (n=15). The myenteric plexus was studied after nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) histochemistry using light microscopy, digital photography, and Image-Pro Plus 6.0 software. The numbers of positively stained neurons and ganglia were counted in the duodenum, jejunum, ileum, caecum, and colon in the different age groups. Data were expressed as mean±standard deviation (SD), and statistical analysis was performed using a one-way analysis of variance (ANOVA) test. Results: The positively stained neurons showed various morphologies and staining intensities, and formed bead-shaped and U-shaped arrangements in the myenteric plexus. The densities of neurons and ganglia increased with age. However, the number of positive neurons per ganglion increased. The number of NADPH-d-positive neurons was highest in the colon, followed by the ileum, the jejunum, the duodenum, and the caeca in all age groups. Conclusions: Developmental changes in the myenteric plexus of chickens continue in the postnatal period, indicating that the maturation process of the gastrointestinal function is gradual. In addition, no significant difference is happening among different intestinal segments during postnatal development, suggesting that the function of different intestinal segments had been determined after birth.
Keywords: NADPH-d histochemistry, Enteric nervous system (ENS), Development, Myenteric plexus, Chicken
1. Introduction
The enteric nervous system (ENS), the largest and most complex division of the autonomic nervous system, regulates the motility of the intestine. Abnormalities of the ENS, especially deficiency of the inhibitory innervation, may lead to motility disorders, impaired peristalsis or functional bowel obstruction, such as congenital hypertrophic pyloric stenosis, Hirschsprung’s disease, hypoganglionosis, intestinal neuronal dysplasia, and internal anal sphincter achalasia (Vanderwinden et al., 1992; Hanani et al., 1995; Hirakawa et al., 1995). Studying the development of ENS is important for understanding functional abnormalities.
The enteric neurons can be classified by their neurotransmitters. The three largest groups are the cholinergic, catecholamninergic (or adrenergic), and nonadrenergic-noncholinergic (NANC) enteric neurons (Sri Paran et al., 2009). Identifying one of these neurotransmitter groups is a feasible technique to follow developmental changes in the myenteric plexus.
Bult et al. (1990) reported that nitric oxide (NO) is released on stimulation of NANC nerves. Since then, it has been postulated that NO acts as an NANC neurotransmitter and mediates relaxation of the smooth muscle of the gastrointestinal tract (Boeckxstaens et al., 1990; Burleigh, 1992; Stark et al., 1993). NO regulates smooth muscle cell tone, platelet aggregation and adhesion, cell growth, apoptosis, neurotransmission, and injury, as well as infection-induced immune reactions (Rosselli et al., 1998). NO is produced by the neuronal NO synthase (NOS) in nitrergic neurons. Nicotinamide adenine dinucleotide phosphate (NADPH) and calcium are required for this synthesis. Two techniques are used to identify and quantify nitrergic neurons: NADPH diaphorase (NADPH-d) histochemistry and NOS immunohistochemistry. Dawson et al. (1991) has previously reported that NOS staining is identical to NADPH-d staining. NADPH-d staining has been used in recent studies to qualify and quantify nitrergic neurons in the gut in different species: mice (Azzena and Mancinelli, 1999), rats (Sandgren et al., 2003), guinea-pigs (Wade et al., 2003), sheep (Lalatta-Costerbosa et al., 2007), rabbits (Junquera et al., 1998), dogs (Ward et al., 1992), pigs (Cserni et al., 2009b), cats (Feher and Montagnese, 1994), monkeys and humans (Degiorgio et al., 1994; Wittmeyer et al., 2010), pigs, guinea-pigs, and rats (Bodi et al., 2009), and chicks (O′Donnell et al., 2006). The whole-mount preparation technique allows the observation of the 3D meshwork of the myenteric plexus and quantitative and qualitative analyses of the myenteric neurons (Wester et al., 1999). Fertilized chicken eggs provide an easy access to embryos and therefore chicken models have been widely used to study age-related changes in the myenteric plexus of the prenatal period (Bagyanszki et al., 2000; O′Donnell et al., 2006; Sri Paran et al., 2009). Furthermore, such studies may provide us with some clues towards the periodic maturation of the human ENS. In previous literature, no studies have focused on the postnatal period of development of the myenteric nerves in chickens. Our aim was to perform qualitative and quantitative analyses of the nitrergic neurons in the myenteric plexus of developing chickens in the postnatal period using whole-mount preparations and NADPH-d histochemistry.
2. Materials and methods
All normal young and adult broiler chickens of either sex were obtained from a commercial poultry farm. They were housed in temperature-controlled rooms (20 °C) with natural light, and had free access to food and tap water. The chickens were sacrificed by cervical dislocation under ether anesthesia. All the experiments abide by the regulations of the Chinese Committee for Animal Use for Research and Education. Segments of the duodenum, jejunum, ileum, caecum, and colon were removed from 7-d old (90–100 g), 15-d old (400–420 g), and 40-d old (adult; around 1 800 g) broilers (5 chickens of each age group). Then these segments were washed with 0.1 mol/L phosphate buffered saline (PBS) (pH 7.3), ligated at the two ends, and fixed with 4% paraformaldehyde in 0.1 mol/L PBS for 1.5–2.5 h at 4 °C. After being fixed, the segments were cleared with two rinses in 0.1 mol/L of PBS for 10 min, and stored overnight in PBS at 4 °C. Lastly, the segments were opened along the mesenteric border, sheared into 10-cm long segments, and the mucous membrane, submucosa, and circular muscle were peeled back to produce a whole-mount preparation of gastrointestinal myenteric plexus and longitudinal muscle using fine forceps. The preparations were then put in PBS to prepare for staining. According to the method of Cserni et al. (2009a), the preparations were incubated in 0.5 mg/ml nitroblue tetrozolium (Sigma), 0.3% Triton X-100 (Sino-American), and 1 mg/ml NADPH (Sigma) in 0.1 mol/L PBS at 37 °C for 20–30 min; the reaction was terminated by washing in 0.1 mol/L PBS. Subsequently, the preparations were dehydrated and mounted on slides and examined with an Olympus microscope. Control experiments were performed in the same manner in the absence of NADPH.
The intensely blue-labeled cells and weakly positive cells were regarded as positive, while the cells of which the bodies had the same color as the background, and where the cell outlines were seen dimly although the nucleus was obvious, were regarded as negative cells. However, data were presented only for the positive cells. A standard line was calibrated to scale on the glass slide, and the pictures were taken at different magnifications (total length 1 mm; the smallest scale 10 μm). These pictures were then imported to the Image-Pro Plus (IPP) 6.0 software. This was used as a standard to measure the different size parameters, such as the cell body diameter and microscopic field. All statistical data were obtained by the software of IPP. NADPH-d-positive neurons were counted in randomly selected microscopic fields (1 microscopic field was 1.3222 mm2) and ganglia were counted at 100× magnification. In these ganglia, the area, length, and size of positive neurons were measured from randomly selected areas (1 microscopic field was 0.0846 mm2) at 400× magnification. The diameter of the neuron was measured as the shortest diagonal line that made the shortest straight line, which passed across the center of the nucleus between the membranes at 400× magnification. Data from 10 measured areas per intestinal segment or sample per age group, per animal (5 chickens of each age group), were included in this study. Statistical analysis was performed using the one-way analysis of variance (ANOVA) test on SPSS 18.0 software and the Student-Newman-Keuls (SNK) test to evaluate the effect of age on the quantitative distribution of NADPH-d-positive neurons of the same segment. A probability of P<0.05 was set as the level of significance in all analyses.
The ganglionic and neuronal densities were calculated as the numbers of positive ganglia and stained cells per mm2 of the whole-mount area, respectively. Data were expressed as mean±standard deviation (SD). The number of NADPH-d-positive neurons per ganglion and the size of NADPH-d-positive neurons were also calculated in relation to the developmental stage and location. Diagrams and tables were prepared with Microsoft Excel 7.0 computer program.
3. Results
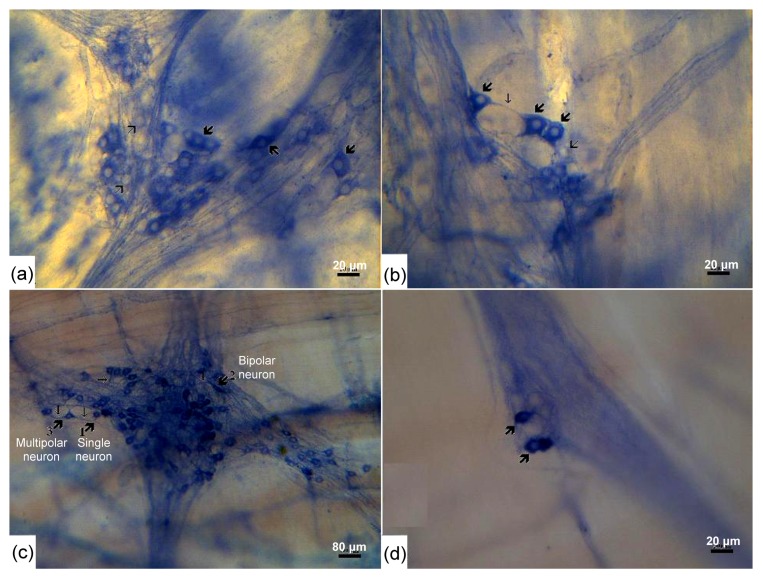
The NADPH-d histochemistry stained the NADPH-d-positive neurons blue with varying intensities. The length and thickness of the nerve fiber bundles connecting the ganglia increased with age, so that the meshwork appeared denser in the adult group. The ganglia appeared fusiform, showed a tendency of parallel arrangement, and were vertical to longitudinal smooth muscle (Fig. 1). The ganglia contained both positive and negative neurons (Fig. 2), and most of the positive ones were present within ganglia, but occasionally a single or a few neuron bodies could be found within the nerve fiber (axon) bundles (Fig. 2d). In the 7-d-old group, the diameters of positive cell bodies varied from 10 to 22 μm and most of them were between 12 and 18 μm. Their shape was mainly oval at the low magnification and the surface of cell bodies showed short processes at higher magnification (Fig. 2a). In the 15-d-old group, the diameters of positive cell bodies varied from 12 to 28 μm and most of them were between 13 to 19 μm. They were in oval, circular, fusiform, triangular or irregular shape and had many surface protrusions and small plate long processes (Fig. 2b). In the adult group, the diameters of positive cell bodies varied from 13 to 32 μm and most of them measured 15–24 μm and were oval, circular, fusiform, triangular or irregular. Single, bipolar and multipolar neurons have been identified (Fig. 2c). All the nuclei were plain and were situated on one side of the cytoplasm, which showed variable staining intensity.
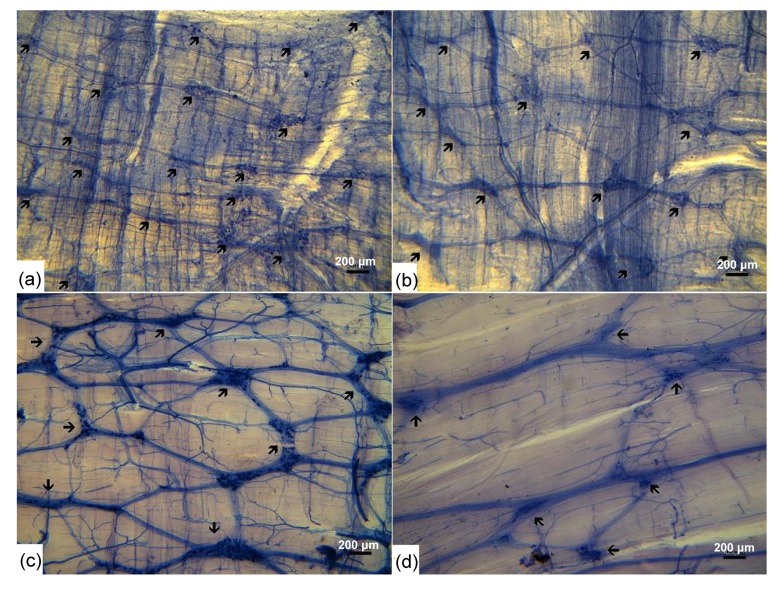
Fig. 1.
Whole-mount preparations of the myenteric plexus of chickens after NADPH-d staining in different age groups
(a) Jejunal myenteric plexus in 7-d-old chicken; (b) Jejunal myenteric plexus in 15-d-old chicken; (c) Ileal myenteric plexus in adult chicken; (d) Cecal myenteric plexus in adult chicken. The meshwork of nerve bundles with ganglia containing NADPH-d-positive neurons is clearly seen. The myenteric ganglia with different sizes (arrows) are interconnected by short nerve fiber strands
Fig. 2.
NADPH-d-positive neurons in the myenteric plexus of the chicken intestine in different age groups
Note that NADPH-d-positive neurons increase their sizes during development. (a) The ganglia contain both NADPH-d-positive neurons (thick big arrows) and NADPH-d-negative neurons (thin big arrows) in the duodenum of the 7-d-old chicken. The positive neurons show variant sizes and shapes. (b) The ganglia contain both positive neurons (thick big arrows) and negative neurons (thin big arrows) in the jejunum of 15-d-old chickens. Most NADPH-d-positive neurons (big thick arrows) have many surface protrusions and thin long processes (thin short arrows). (c) NADPH-d-positive neurons (thick big arrows) with different shapes could be distinguished in the jejunum of the adult chicken. (d) Occasionally, a limited number of positive neuron cells are seen in the nerve bundles connecting different ganglia
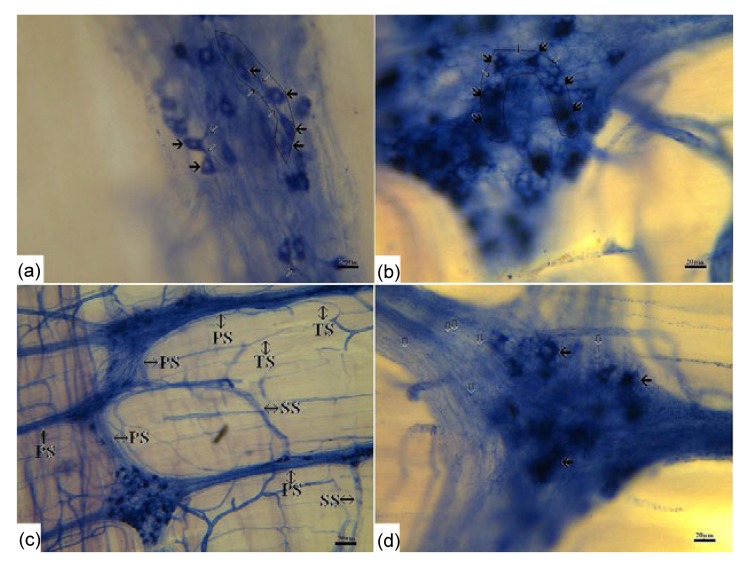
The positive neurons extended both long processes and short processes towards other negative or positive neurons (Figs. 2b and 2c). Some neurons that were linked together formed bead-shaped (Fig. 3a) and U-shaped ganglia (Fig. 3b). The positively stained nerve fibers arose from the positive cell bodies and branched to the secondary and tertiary bundles forming a complex network. The trend of fibers coincided with that of the longitudinal muscle (Fig. 3c). Most of the nerve fibers had varicosities (Fig. 3d).
Fig. 3.
Different connections between NADPH-d-positive ganglia and neurons
(a) Neurons connected with each other form a bead shape (boxed area) (bar=20 μm); (b) Neurons connected with each other form a U shape (boxed area) (bar=20 μm); (c) The primary NADPH-d-positive nerve fibers (PS) branch to secondary (SS) and tertiary (TS) bundles (bar=80 μm); (d) Most of the NADPH-d-positive nerve fibers have varicosities (hollow arrows) (bar=20 μm)
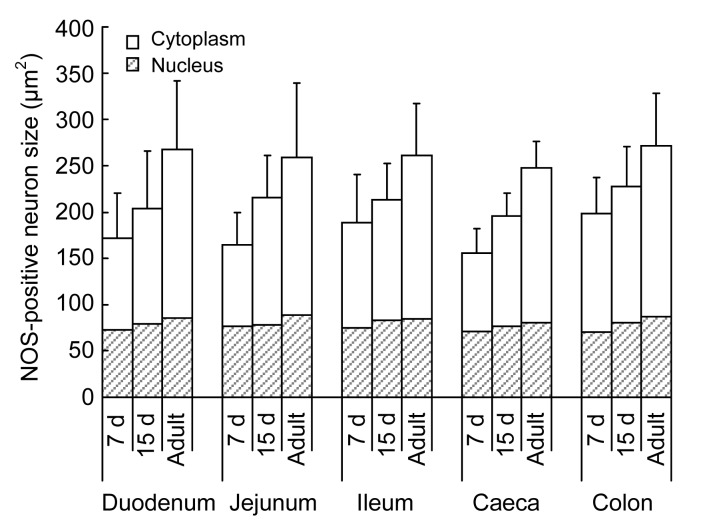
In each group, there was little regional variation along the gastrointestinal axis in the morphological type, sizes of ganglia and labeled cells, as well as the branched form of nerve fibers. However, the spatial densities of ganglia and positive neurons in each segment were variable so that the form of meshwork was different (Figs. 1c and 1d). The sizes of NADPH-d-positive neurons were similar in different segments in the same age group. The growth of NADPH-d-positive neurons was evident with age (Table 1). The size of the cytoplasm increased markedly, while the sizes of nuclei only increased slightly in different segments throughout the development (Figs. 2a, 2b, and 4).
Table 1.
Sizes of the body and nuclei of NADPH-d-positive neurons in small and large intestines of different age groups
| Group | Size (μm2) |
|
| Body | Nuclei | |
| Duodenum | ||
| 7 d | 171.27±49.25 | 72.43±16.21 |
| 15 d | 203.74±62.21 | 79.29±17.82 |
| Adult | 267.45±74.61 | 85.48±30.36 |
| Jejunum | ||
| 7 d | 164.65±35.29 | 76.35±19.42 |
| 15 d | 215.42±45.42 | 78.43±21.25 |
| Adult | 258.57±80.54 | 88.26±22.98 |
| Ileum | ||
| 7 d | 188.32±51.77 | 75.39±12.56 |
| 15 d | 213.29±38.76 | 82.87±20.56 |
| Adult | 260.92±55.65 | 84.73±21.76 |
| Caeca | ||
| 7 d | 155.79±26.16 | 70.71±11.52 |
| 15 d | 195.49±24.52 | 76.48±19.23 |
| Adult | 247.68±28.35 | 80.29±18.22 |
| Colon | ||
| 7 d | 198.32±38.74 | 69.89±19.22 |
| 15 d | 227.32±43.37 | 80.29±16.72 |
| Adult | 271.49±56.36 | 87.34±19.52 |
Data are expressed as mean±SD (n=5)
Fig. 4.
Size of NADPH-d-positive neurons in different segments of the intestine in 7-d-old, 15-d-old, and adult chickens
Data are expressed as mean±SD (n=5). The increase in size of NADPH-d-positive neurons was evident with age. However, this increase is predominantly because of the growth of the cytoplasm
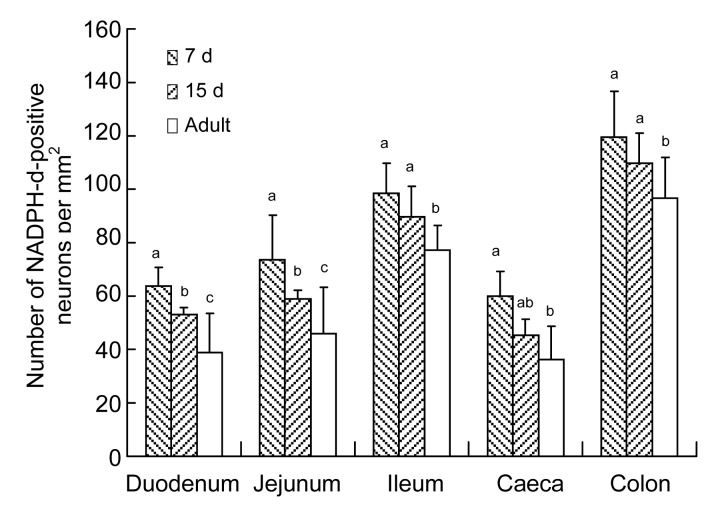
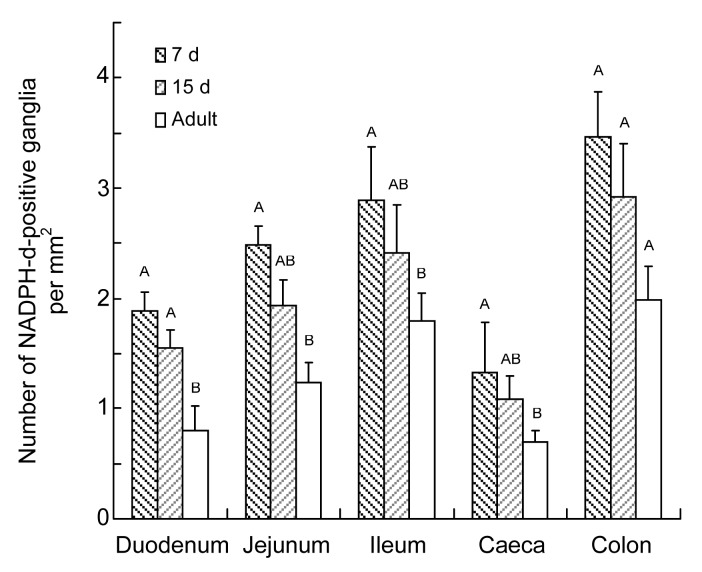
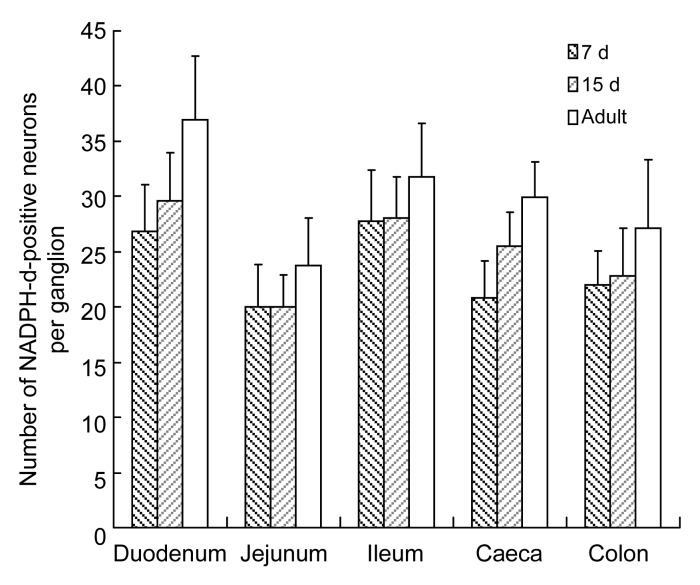
The number of NADPH-d-positive neurons per area was highest in the colon, followed by the ileum, jejunum, duodenum, and caecum (Fig. 5). The density of ganglia followed the same pattern (Fig. 6). However, the number of neurons per ganglion in the 7-d-old group was higher in the ileum than in the duodenum, followed by the colon, caecum, and jejunum. In the 15-d-old and adult groups, a higher number of neurons per ganglion was found in the duodenum than in the ileum, followed by the colon, caecum, and jejunum (Fig. 7). Table 2 shows the number of NADPH-d-positive ganglia and neurons per unit area, and the neurons per ganglion in small and large intestines of chickens.
Fig. 5.
Number of NADPH-d-positive neurons per mm2 in 7-d-old, 15-d-old, and adult chickens in different segments of the intestine
Data are expressed as mean±SD (n=5). Different superscripts above the bars represent the significant differences among the number of NADPH-d-positive neurons per mm2 in the same segment of different age groups (P<0.05)
Fig. 6.
Number of NADPH-d-positive ganglia per mm2 in 7-d-old, 15-d-old, and adult chickens in different segments of the intestine
Data are expressed as mean±SD (n=5). Superscripts above the bars represent the comparison between the numbers of NADPH-d-positive neurons per mm2 in same segment of different age groups. Different letters mean the difference was significant (P<0.05)
Fig. 7.
Number of NADPH-d-positive neurons per ganglion in 7-d-old, 15-d-old, and adult chickens in different segments of the intestine
Data are expressed as mean±SD (n=5)
Table 2.
Number of NADPH-d-positive ganglia and neurons per unit area, and the neurons per ganglia in small and large intestines of different age groups
| Group | Number per mm2
|
Neuron/ganglion | |
| Ganglia | Neuron | ||
| Duodenum | |||
| 7 d | 1.89±0.17A | 63.64±7.18a | 26.79±4.29 |
| 15 d | 1.55±0.16A | 52.94±2.55b | 29.67±4.29 |
| Adult | 0.80±0.22B | 38.79±14.91c | 36.90±5.78 |
| Jejunum | |||
| 7 d | 2.48±0.18A | 73.47±16.99a | 20.03±3.83 |
| 15 d | 1.94±0.23AB | 58.88±3.16b | 20.07±2.82 |
| Adult | 1.24±0.18B | 45.92±17.51c | 23.72±4.38 |
| Ileum | |||
| 7 d | 2.89±0.49A | 98.32±11.22a | 27.77±4.68 |
| 15 d | 2.41±0.44AB | 89.62±11.36a | 28.09±3.67 |
| Adult | 1.79±0.26B | 77.52±9.03b | 31.84±4.76 |
| Caeca | |||
| 7 d | 1.33±0.45A | 59.75±9.30a | 20.87±3.24 |
| 15 day | 1.08±0.22AB | 45.38±5.86ab | 25.45±3.09 |
| Adult | 0.70±0.10B | 36.18±12.37b | 29.89±3.29 |
| Colon | |||
| 7 d | 3.47±0.40A | 119.49±17.19a | 21.96±3.11 |
| 15 d | 2.92±0.49A | 109.66±11.34a | 22.85±4.28 |
| Adult | 1.99±0.30A | 96.81±14.96b | 27.10±6.24 |
Data are expressed as mean±SD (n=5). Different superscripts in the same column between age groups of each part of intestine are significantly different (P<0.05)
Comparing the three age groups, the densities of NADPH-d-positive neurons and ganglia were highest in the 7-d-old group, following by the 15-d-old and the adult groups.
4. Discussion
Our qualitative and quantitative analyses of NADPH-d-positive neurons in the myenteric plexus of developing chickens show significant age-related changes in the postnatal period. These changes are similar to those previously reported in chicken embryos (Balaskas et al., 1995; Bagyanszki et al., 2000; O′Donnell et al., 2006), and other species like guinea pigs (Nichols et al., 1992), rabbits (Junquera et al., 1998), rats (Cracco and Filogamo, 1994), pigs (Sri Paran et al., 2009), and monkeys and humans (Degiorgio et al., 1994; Román et al., 2004).
Variance in staining intensity, shape and size of the NADPH-d-positive neurons observed in our study suggests the existence of different subpopulations of nitrergic neurons. The most well known functional subgroup of nitrergic neurons is the group of inhibitory motor neurons that mediate relaxation of the smooth muscle of the gastrointestinal tract (Vanden Berghe et al., 1999). Some elements of the sensory innervation of the circular musculature may derive from nitrergic neurons as well. The rest of the nitrergic neurons may serve as inhibitory interneurons and control other (probably excitatory) motor neurons in the myenteric plexus (Cserni et al., 2009a). The staining intensity of the positive neurons may be related to their NADPH-d content, activity, and isoenzymes (Cracco and Filogamo, 1994). Cserni et al. (2009a) hypothesized that neurons containing the higher amount of NOS/NADPH-d and stained darker are most likely to be inhibitory motor neurons. The cells stained weaker may be interneurons, sensory neurons, or glial cells (Cserni et al., 2009a).
The positive neurons stretched processes towards other positive or negative neurons (Llewellyn-Smith et al., 1992). The bead-shaped and U-shaped neuronal arrangements suggest the existence of nitrergic neural circuits; however, the synaptic contacts need further exploration.
Most of the NADPH-d-positive nerve fibers showed varicosities in our specimens. It would be easy to hypothesize that these varicosities are for storage of NO or NOS. NO is known as a gaseous messenger and it is released immediately as soon as it is synthesized, and is not stored in specific organelles or vesicles. However, NOS may be stored in these varicosities. Xiao et al. (1996) demonstrated that NADPH-d either covers the surface of synaptic vesicles or exists in their insides, instead of being their specific contents.
Gabella (1971) observed in developing rats that the neuronal density decreased with age. Similar observations have been made in guinea-pigs, sheep, horses, pigs, and chick embryos (Gabella, 1987; Young et al., 1993; Doxey et al., 1995; Zhang et al., 2004; O′Donnell et al., 2006). Decreasing NADPH-d-positive neuron density in the developing pig model has been used to explain why primary intussusception does not occur in adults (Cserni et al., 2007).
Our study showed similar changes in the spatial density of NADPH-d-positive neurons in the myenteric plexus of chickens as has been shown in other species as mentioned above. This may explain the occurrence of intussusception in young fowls (Okoye, 1985).
The decrease in the neuron density may be caused by the rapid growth of the bowel. Neuronal cell migration into ganglia or fusion of ganglia may be an explanation for ganglion size growth, but cell division or proliferation cannot be ruled out.
The significant regional variation in the density of NADPH-d-positive neurons along the intestine may represent functional differences. The highest proportion of NADPH-d-positive cells has been found in the colon in our study. This is in accordance with the literature and may be responsible for long-term relaxation of the organ in the avian species (An et al., 2003).
In mammals, however, the caecum contains more relaxing neurons (Belai et al., 1992). This may be explained by different motility patterns of the cecum in different tribes, or it is possible that the thinner caecal wall in the chicken may require fewer neurons to achieve relaxation.
In the small intestine, we found nitrergic hyperinnervation of the ileum. This is similar in mammals (Nichols et al., 1992; Pearson, 1994). In the chicken, the muscle wall of the terminal ileum is relatively thick compared to other segments. This, along with the nitrergic hyperinnervation, suggests that the ileum plays a crucial role in digestion and absorption in the chicken. The food of the poultry is usually dry and eaten in large amounts. The intestine is able to adapt to these conditions. Nitrergic innervation regulates this adaptation by regulating motility and expanding capacity (Rand, 1992).
The density of NADPH-d-positive neurons of the chicken small intestine is significantly higher than that of the goat or pig (Liu et al., 2007), but lower than that of the rat (An et al., 2003; Bodi et al., 2009). This suggests that smaller animals have more neuronal density in the myenteric plexus. The significance of this, however, is not clear.
Balaskas et al. (1995) and Bagyanszki et al. (2000) working on chicken embryos reported that the overall density of NADPH-d-positive neurons was higher in embryos along the gastrointestinal tract, especially in the small intestine and colon, than that in the postnatal period revealed in the present study. In addition, the density of positive neurons in the colon was higher on gestational Day 14 than that in the ileum, but this trend changed to the opposite situation by Day 19 of development. In our study, we observed regional differences in relation to increasing age. However, the regional differences remain constant in the postnatal development of the chicken.
5. Conclusions
Developmental changes in the myenteric plexus of chickens continue in the postnatal period, indicating that the maturation process of the gastrointestinal function is gradual. In addition, no significant difference is happening among different intestinal segments during postnatal development, suggesting that the function of different intestinal segments had been determined after birth.
Acknowledgments
We are grateful to Mark PINES (Institute of Animal Science, the Volcani Center, Bet Dagan, Israel) for his critical comments on the manuscript.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 31172282 and 31272521), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Natural Science Foundation of Jiangsu Province for Youths (No. BK20130681), China
Compliance with ethics guidelines: Ping YANG, Jameel Ahmed GANDAHI, Qian ZHANG, Lin-li ZHANG, Xun-guang BIAN, Li WU, Yi LIU, and Qiu-sheng CHEN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.An S, Xu C, Xu J, Liu M. Comparison of NOS distribution in myenteric plexus of various segments of gatrointestinal tract of the mandarin vole, Microtus mandarinus . Chin J Neurosci. 2003;19(5):313–317. (in Chinese) [Google Scholar]
- 2.Azzena GB, Mancinelli R. Nitric oxide regenerates the normal colonic peristaltic activity in mdx dystrophic mouse. Neurosci Lett. 1999;261(1-2):9–12. doi: 10.1016/S0304-3940(98)00993-8. [DOI] [PubMed] [Google Scholar]
- 3.Bagyanszki M, Roman V, Fekete E. Quantitative distribution of NADPH-diaphorase-positive myenteric neurons in different segments of the developing chicken small intestine and colon. Histochem J. 2000;32(11):679–684. doi: 10.1023/A:1004167416731. [DOI] [PubMed] [Google Scholar]
- 4.Balaskas C, Saffrey MJ, Burnstock G. Distribution of NADPH-diaphorase activity in the embryonic chicken gut. Anat Embryol. 1995;192(3):239–245. doi: 10.1007/BF00184748. [DOI] [PubMed] [Google Scholar]
- 5.Belai A, Schmidt H, Hoyle C, Hassall C, Saffrey M, Moss J, Förstermann U, Murad F, Burnstock G. Colocalization of nitric oxide synthase and NADPH-diaphorase in the myenteric plexus of the rat gut. Neurosci Lett. 1992;143(1-2):60–64. doi: 10.1016/0304-3940(92)90233-W. [DOI] [PubMed] [Google Scholar]
- 6.Bodi N, Battonyai I, Talapka P, Fekete E, Bagyanszki M. Spatial pattern analysis of nitrergic neurons in the myenteric plexus of the duodenum of different mammalian species. Acta Biol Hung. 2009;60(4):347–358. doi: 10.1556/ABiol.60.2009.4.2. [DOI] [PubMed] [Google Scholar]
- 7.Boeckxstaens GE, Pelckmans PA, Bult H, Deman JG, Herman AG, van Maercke YM. Non-adrenergic non-cholinergic relaxation mediated by nitric oxide in the canine ileocolonic junction. Eur J Pharmacol. 1990;190(1-2):239–246. doi: 10.1016/0014-2999(90)94132-H. [DOI] [PubMed] [Google Scholar]
- 8.Burleigh DE. Ng-nitro-L-arginine reduces nonadrenergic, noncholinergic relaxations of human gut. Gastroenterology. 1992;102(2):679–683. doi: 10.1016/0016-5085(92)90120-n. [DOI] [PubMed] [Google Scholar]
- 9.Cracco C, Filogamo G. Quantitative study of the NADPH-diaphorase positive myenteric neuron of the rat ileum. Neuroscience. 1994;61(2):351–359. doi: 10.1016/0306-4522(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 10.Cserni T, Paran S, Puri P. New hypothesis on the pathogenesis of ileocecal intussusception. J Pediatr Surg. 2007;42(9):1515–1519. doi: 10.1016/j.jpedsurg.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Cserni T, O′Donnel A, Paran S, Puri P. Correlation of enteric NADPH-d positive cell counts with the duration of incubation period in NADPH-d histochemistry. Pathol Oncol Res. 2009;15(1):103–107. doi: 10.1007/s12253-008-9081-5. [DOI] [PubMed] [Google Scholar]
- 12.Cserni T, Paran S, Kanyari Z, O′Donnell AM, Kutasy B, Nemeth N, Puri P. New insights into the neuromuscular anatomy of the ileocecal valve. Anat Rec. 2009;292(2):254–261. doi: 10.1002/ar.20839. [DOI] [PubMed] [Google Scholar]
- 13.Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. PNAS. 1991;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degiorgio R, Parodi JE, Brecha NC, Brunicardi FC, Becker JM, Go VLW, Sternini C. Nitric oxide producing neurons in the monkey and human digestive system. J Comp Neurol. 1994;342(4):619–627. doi: 10.1002/cne.903420409. [DOI] [PubMed] [Google Scholar]
- 15.Doxey DL, Pearson GT, Milne EM, Gilmour JS, Chisholm HK. The equine enteric nervous system-neuron characterization and disribution in adults and juveniles. Vet Res Commun. 1995;19(6):433–449. doi: 10.1007/BF01839331. [DOI] [PubMed] [Google Scholar]
- 16.Feher E, Montagnese C. Distribution and morphological features of nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) activity in intrinsic neurons of the oddi sphincter the cat. Neurosci Lett. 1994;170(1):114–116. doi: 10.1016/0304-3940(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 17.Gabella G. Neuron size and number in the myenteric plexus of the newborn and adult rat. J Anat. 1971;109(1):81. [PMC free article] [PubMed] [Google Scholar]
- 18.Gabella G. The number of neurons in the small intestine of mice, guinea pigs and sheep. Neuroscience. 1987;22(2):737–752. doi: 10.1016/0306-4522(87)90369-1. [DOI] [PubMed] [Google Scholar]
- 19.Hanani M, Louzon V, Udassin R, Freund HR, Karmeli F, Rachmilewitz D. Nitric oxide-containing nerves in bowel segments of patients with Hirschsprung’s disease. J Pediatr Surg. 1995;30(6):818–822. doi: 10.1016/0022-3468(95)90756-4. [DOI] [PubMed] [Google Scholar]
- 20.Hirakawa H, Kobayashi H, Obriain DS, Puri P. Absence of NADPH-diaphorase activity in internal anal sphincter (IAS) achalasia. J Pediatr Gastr Nutr. 1995;20(1):54–58. doi: 10.1097/00005176-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Junquera C, Martinez-Ciriano C, Blasco J, Aisa J, Peg MT, Azanza MJ. Distribution of NADPH diaphorase-positive neurons in the enteric nervous system of the rabbit intestine. Neurochem Res. 1998;23(10):1233–1240. doi: 10.1023/A:1020783830811. [DOI] [PubMed] [Google Scholar]
- 22.Lalatta-Costerbosa G, Mazzoni M, Clavenzani P, Di Guardo G, Mazzuoli G, Marruchella G, de Grossi L, Agrimi U, Chiocchetti R. Nitric oxide synthase immunoreactivity and NADPH-d histochemistry in the enteric nervous system of sarda breed sheep with different PrP genotypes in whole-mount and cryostat preparations. J Histochem Cytochem. 2007;55(4):387–401. doi: 10.1369/jhc.6A7052.2007. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Chen Y, Wang Z. Differences of AchE and NOS-positive neuron number and distribution in goat small intestine. J China Agric Univ. 2007;12(2):10–14. (in Chinese) [Google Scholar]
- 24.Llewellyn-Smith IJ, Song ZM, Costa M, Bredt DS, Snyder SH. Ultrastructural localization of nitric oxide synthese immunoreactivity in guinea-pig enteric neurons. Brain Res. 1992;577(2):337–342. doi: 10.1016/0006-8993(92)90294-J. [DOI] [PubMed] [Google Scholar]
- 25.Nichols K, Krantis A, Staines W. Histochemical localization of nitric oxide-synthxizing neurons and vascular sites in the guinea-pig intestine. Neuroscience. 1992;51(4):791–799. doi: 10.1016/0306-4522(92)90520-C. [DOI] [PubMed] [Google Scholar]
- 26.O′Donnell AM, Bannigan J, Puri P. Differences in nitrergic innervation of the developing chick cloaca and colorectum. Pediatr Surg Int. 2006;22(1):90–94. doi: 10.1007/s00383-005-1590-7. [DOI] [PubMed] [Google Scholar]
- 27.Okoye JOA. Cases of intestinal intussusception in young fowls. Avian Pathol. 1985;14(2):275–279. doi: 10.1080/03079458508436230. [DOI] [PubMed] [Google Scholar]
- 28.Pearson GT. Structural organization and neuropeptide distributions in the equine enteric nervous system: an immunohistochemical study using whole-mount preparations from the small intestine. Cell Tissue Res. 1994;276(3):523–534. doi: 10.1007/BF00343949. [DOI] [PubMed] [Google Scholar]
- 29.Rand MJ. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuroeffector transmission. Clin Exp Pharmacol Physiol. 1992;19(3):147–169. doi: 10.1111/j.1440-1681.1992.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 30.Román V, Bagyánszki M, Krecsmárik M, Horvath A, Resch BÁ, Fekete É. Spatial pattern analysis of nitrergic neurons in the developing myenteric plexus of the human fetal intestine. Cytom Part A. 2004;57A(2):108–112. doi: 10.1002/cyto.a.10112. [DOI] [PubMed] [Google Scholar]
- 31.Rosselli M, Keller R, Dubey RK. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum Reprod Update. 1998;4(1):3–24. doi: 10.1093/humupd/4.1.3. [DOI] [PubMed] [Google Scholar]
- 32.Sandgren K, Lin Z, Svenningsen AF, Ekblad E. Vasoactive intestinal peptide and nitric oxide promote survival of adult rat myenteric neurons in culture. J Neurosci Res. 2003;72(5):595–602. doi: 10.1002/jnr.10612. [DOI] [PubMed] [Google Scholar]
- 33.Sri Paran T, Rolle U, Puri P. Age-related changes in the myenteric plexus of the porcine bowel. J Pediatr Surg. 2009;44(9):1771–1777. doi: 10.1016/j.jpedsurg.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Stark ME, Bauer AJ, Sarr MG, Szurszewski JH. Nitric oxide mediates inhibitory nerve input in human and canine jejunum. Gastroenterology. 1993;104(2):398–409. doi: 10.1016/0016-5085(93)90407-4. [DOI] [PubMed] [Google Scholar]
- 35.Vanden Berghe P, Coulie B, Tack J, Mawe G, Schemann M, Janssens J. Neurochemical coding of myenteric neurons in the guinea-pig antrum. Cell Tissue Res. 1999;297(1):81–90. doi: 10.1007/s004410051335. [DOI] [PubMed] [Google Scholar]
- 36.Vanderwinden JM, Mailleux P, Schiffmann SN, Vanderhaeghen JJ, Delaet MH. Nitric oxide synthase activity in infantile hypertrophic pyloric stenosis. New Engl J Med. 1992;327(8):511–515. doi: 10.1056/NEJM199208203270802. [DOI] [PubMed] [Google Scholar]
- 37.Wade PR, Gulbransen B, Lieb J. Age-related changes in motility and in nitrergic myenteric neurons in guinea pig distal colon. Gastroenterology. 2003;124(3):A545–A545. [Google Scholar]
- 38.Ward SM, Xue C, Shuttleworth CW, Bredt DS, Snyder SH, Sanders KM. NADPH-diaphorase and nitric oxide synthase colocalization in enteric neurons of canine proximal colon. Am J Physiol. 1992;263(2):G277–G284. doi: 10.1152/ajpgi.1992.263.2.G277. [DOI] [PubMed] [Google Scholar]
- 39.Wester T, O′Briain DS, Puri P. Notable postnatal alterations in the myenteric plexus of normal human bowel. Gut. 1999;44(5):666–674. doi: 10.1136/gut.44.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wittmeyer V, Merrot T, Mazet B. Tonic inhibition of human small intestinal motility by nitric oxide in children but not in adults. Neurogastroent Motil. 2010;22(10):1078–e1282. doi: 10.1111/j.1365-2982.2010.01532.x. [DOI] [PubMed] [Google Scholar]
- 41.Xiao L, Cai WQ, Sun Y. A light and electron microscope observation of NADPH-diaphorase in the jejumun myenteric plexus of rats. Acta Anat Sin. 1996;27(1):85–87. (in Chinese) [Google Scholar]
- 42.Young HM, Furness JB, Sewell P, Burcher EF, Kandiah CJ. Total numbers of neurons in myenteric ganglia of the guinea-pig small intestine. Cell Tissue Res. 1993;272(1):197–200. doi: 10.1007/BF00323587. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Teng K, Zhang H, Zhao Q, Wang R, Li L. The morphological features of NADPH-diaphorase positive myenteric neurons of the postnatal piglets small intestine. Acta Vet Zoot Sin. 2004;35(6):705–710. (in Chinese) [Google Scholar]