Abstract
Objectives
Emerging evidence indicates that sleep duration is associated with health outcomes. However, the relationship of sleep duration with long-term health is unclear. This study was designed to determine the relationship of sleep duration with mortality as a parameter for long-term health in a large prospective cohort study in Korea.
Methods
The study population included 13 164 participants aged over 20 years from the Korean Multi-center Cancer Cohort study. Information on sleep duration was obtained through a structured questionnaire interview. The hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality were estimated using a Cox regression model. The non-linear relationship between sleep duration and mortality was examined non-parametrically using restricted cubic splines.
Results
The HRs for all-cause mortality showed a U-shape, with the lowest point at sleep duration of 7 to 8 hours. There was an increased risk of death among persons with sleep duration of ≤5 hours (HR, 1.21; 95% CI, 1.03 to 1.41) and of ≥10 hours (HR, 1.36; 95% CI, 1.07 to 1.72). In stratified analysis, this relationship of HR was seen in women and in participants aged ≥60 years. Risk of cardiovascular disease-specific mortality was associated with a sleep duration of ≤5 hours (HR, 1.40; 95% CI, 1.02 to 1.93). Risk of death from respiratory disease was associated with sleep duration at both extremes (≤5 and ≥10 hours).
Conclusions
Sleep durations of 7 to 8 hours may be recommended to the public for a general healthy lifestyle in Korea.
Keywords: Sleep duration, Mortality, Prospective cohort
INTRODUCTION
Duration of sleep is an important factor in predicting not only the quality of sleep but also the quality of life and overall health [1-3]. Many studies have found associations between inappropriate sleep duration and health outcomes such as total mortality, type 2 diabetes (DM), cardiovascular disease (CVD), and general health [4-6].
The factors predicting or influencing an individual's sleep duration may vary between countries or cultures [7,8]. Socioeconomic status indicators, including marital status, financial stress, education level, menopausal status, and history of night-shift work, are related to sleep duration [9,10]. Although the evidence is inconsistent, other lifestyle factors have been found to affect sleep duration. This may confound the association between sleep duration and health risks and modulate the association between sleep duration and health risk across different characteristics. As an important lifestyle factor, sleep duration may be an indicator of behavior, quality of life, or other aspects of health in Koreans.
In this study, we investigated the association between sleep duration and measures of all-cause and disease-specific mortality through a community-based cohort study in Korea, where non-communicable diseases, including cancer and CVD, have been the leading causes of death for the last 20 years. We controlled for lifestyle factors and factors associated with the prevalence of metabolic syndrome and comorbidities, such as blood cholesterol, hypertension, DM, and body mass index (BMI). Our purpose was to suggest appropriate sleep duration for the general population, using mortality as a parameter for long-term health.
METHODS
Study Population
Eligible subjects were enrolled from the Korean Multi-center Cancer Cohort (KMCC) study, a community-based prospective cohort, and from participants recruited from urban and rural areas in Korea (Haman, Chungju, Uljin, and Youngil). The rationale and design of the KMCC is described in detail elsewhere [11]. Between 1993 and 2004, 20 257 subjects participated in the KMCC study. We excluded 235 participants because of missing information on locality, 1245 participants who were younger than 20 years of age at the time of enrollment, and 43 participants with a missing birth date. A total of 5570 subjects were excluded because of missing information on sleep duration. Finally, 13 164 participants were included in the analysis. Written informed consent was obtained from all participants, and the institutional review boards of Human Research at Seoul National University College of Medicine approved the study.
Data Collection and Follow-up
At baseline, all lifestyle factors, including sleep duration, were assessed through direct interview conducted by well-trained personnel using a structured questionnaire. People were asked about their average sleeping time per day: ≤5, 6, 7, 8, 9, or ≥10 hours. Height and weight were used to calculate BMI (as current weight divided by height squared [kg/m2]). Waist circumference was also measured. Participants were asked about their smoking status (never, current, and past) and use of alcoholic beverages (never, current, and past).
Factors used in the diagnosis of metabolic syndrome were also collected. Plasma samples obtained at recruitment were used for determining total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides. Low-density lipoprotein (LDL) cholesterol was calculated by the following equation: LDL cholesterol=total cholesterol-(HDL cholesterol+triglyceride/400). Metabolic syndrome was diagnosed if participants met >3 of the following criteria: total cholesterol level ≥200 mg/dL, HDL level ≤40 mg/dL, waist circumference ≥102 cm in men or ≥88 cm in women, fasting plasma glucose level ≥100 mg/dL, diastolic blood pressure ≥85 mmHg, or systolic blood pressure ≥130 mmHg (National Cholesterol Education Program Adult Treatment Panel III 2001).
Cause of death was classified according to the international classification of the 10th revision of the International Classification of Disease (ICD-10). Follow-up through the Death Certificate database of the National Statistical Office was completed on December 31, 2010. Cause of death was classified by ICD-10 as follows: all causes of death (A00-Z99), all types of cancer death (C00-C97), CVD death (I00-I99), respiratory disease death (J00-J99, A15-A19), external cause of death with injury (S00-S99, T00-T99, and V01-X59), and cause of death "not elsewhere classified" including senility (R00-R99). The total number of deaths during the study period was 1580: 526 were due to cancer, 363 due to CVD, 129 due to respiratory disease, and the remaining due to other causes.
Statistical Analysis
Baseline characteristics of the study population for sleep duration were compared by the chi-square test for categorical variables and the Student's t-test or ANOVA for continuous variables. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) of risk factors for all-cause and disease-specific mortality were obtained based on the regression coefficients and standard errors from the Cox's proportional hazards regression models with follow-up time as the time scale. The proportional hazard assumptions for different durations of sleep were examined by inspecting log minus-log survival plots. All models were adjusted for age (20-29, 30-39, 40-49, 50-59, 60-69, and ≥70 years), sex, and history of chronic disease including hypertension, DM, or heart disease. Educational attainment (none, 1-11, and ≥12 years) and BMI (<21, 21-22.9, 23-24.9, and ≥25 kg/m2) were also included in the models. We evaluated how the association between sleep duration and mortality was modified by variables such as age and sex. Participants were divided into two age groups (<60 and ≥60 years) and stratified by sex.
To explore the possibly non-linear shape of the risk function, we examined the relationship between sleep duration and mortality non-parametrically with restricted cubic splines [12]. Non-linearity was tested using the likelihood ratio, comparing the model with only the linear term to the model with the linear and the cubic spline terms. We fitted a Cox proportional hazards model with restricted cubic splines for sleep duration treated as continuous variables [13,14]. We specified 4 knot positions at 6, 7, 8, and 9 hours of sleep, because the data for sleep duration were collected as nominal variables. HRs for all-cause mortality were assessed by comparison to subjects who reported fixed sleep duration of 7 hours, which corresponded to the lowest risk of death. HRs for all-cause mortality were stratified by gender and age (<60 and ≥60 years). Statistical significance was assumed at a p-value of <0.05. All statistical analyses were performed using SAS version 9.3 (SAS Inc., Cary, NC, USA).
RESULTS
During the mean follow-up of 9.44 years, corresponding to 124 267 person-years, 1580 deaths were observed. Cancer was the leading cause of death (33.3%), followed by CVD (23.0%). The general characteristics of the study population are shown in Table 1. Participants with sleep durations of ≤5 hours were more likely to be women and less educated. Participants with sleep durations of ≤5 or ≥10 hours had a higher proportion of comorbidities associated with metabolic syndrome, such as central obesity, hypertension, DM, high plasma triglycerides, or lower HDL cholesterol.
Table 1.
General characteristics of the study population at the time of enrollment in the Korean Multi-center Cancer Cohort study according to sleep duration (n=13 164)
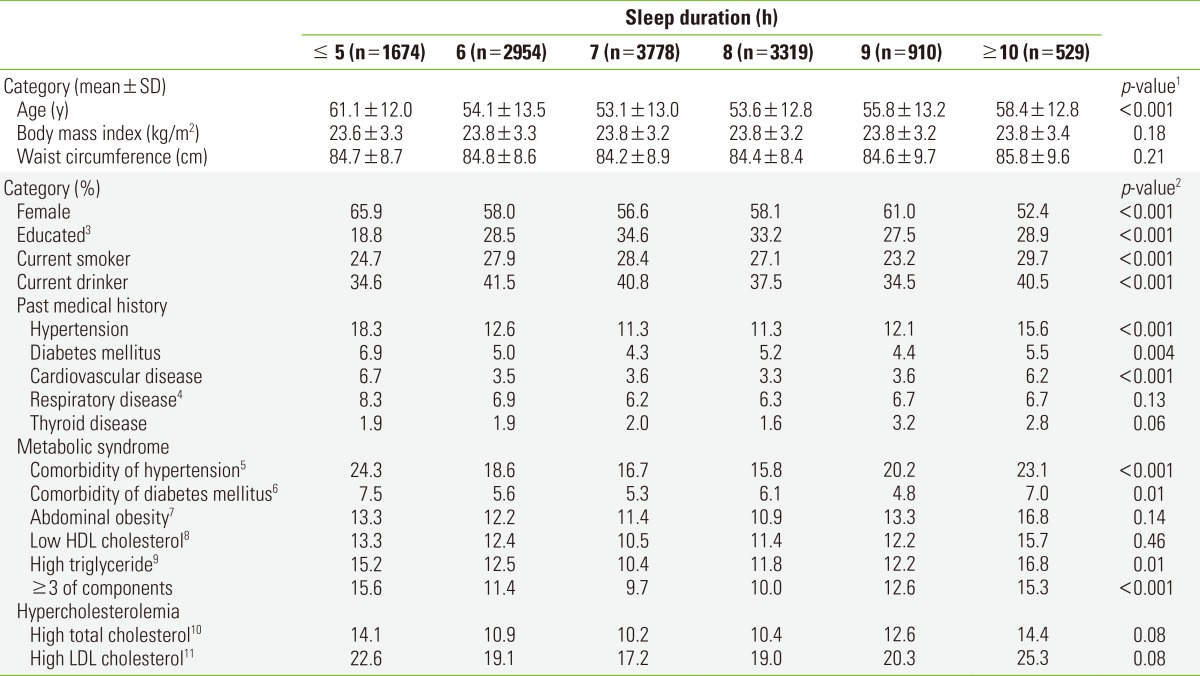
HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure.
1Significance tests for the categories of sleep time based on t-test and ANOVA test for continuous characteristics.
2Significance tests for the categories of sleep time based on the chi-square test for contingency table analysis of categorical characteristics.
3Person who ever been educated for ≥12 years.
4Including tuberculosis, chronic bronchitis, pneumonia, asthma and chronic obstructive pulmonary disease.
5Person who ever diagnosed of hypertension or systolic blood pressure≥130, diastolic blood pressure≥85 (mmHg).
6Person who ever diagnosed of diabetes mellitus or fasting plasma glucose level ≥100 (mg/dL).
7Person whose waist circumference >102 in men, or >88 in women (cm).
8Person whose level of fasting plasma HDL level <40 in men, or <50 in women (mg/dL).
9Person whose level of fasting plasma triglyceride level ≥150 (mg/dL).
10Person whose level of fasting plasma total cholesterol level ≥200 (mg/dL).
11Person whose level of fasting plasma LDL level ≥100 (mg/dL).
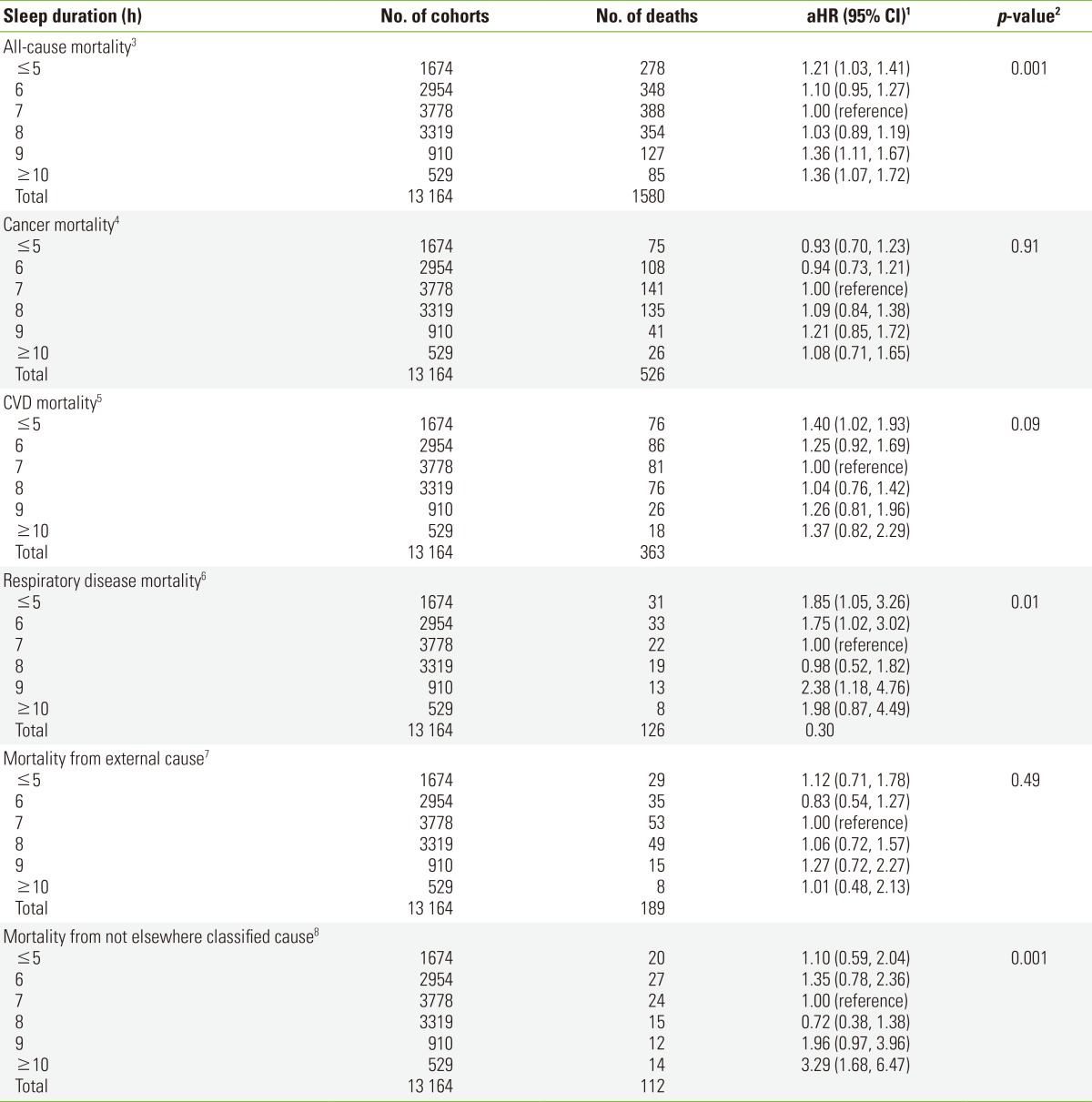
The HRs for all-cause mortality according to sleep duration are shown in Table 2. The sleep durations associated with the lowest risk of death from any cause or a disease-specific cause, were 7 and 8 hours. The HR for a sleep duration of 8 hours was very similar to that for a duration of 7 hours (the comparison number), with a <10% increase (HR, 1.03) in all-cause mortality. The relationship of all-cause mortality with sleep duration showed a U-shape (Pcurvilinearity=0.001) (Figure 1). As compared with a sleep duration of 7 hours, the HR for a sleep duration of ≤5 hours was 1.21 (95% CI, 1.03 to 1.41) and the HR for a sleep duration of ≥10 hours was 1.36 (95% CI, 1.07 to 1.72).
Table 2.
HRs and 95% CIs for disease-specific mortalities according to sleep duration in the Korean Multi-center Cancer Cohort study
HR, hazard ratio; CI, confidence interval; aHR, adjusted hazard ratio; CVD, cardiovascular disease; ICD-10, the 10th revision of the International Classification of Disease.
1HRs and 95% CIs were based on Cox's proportional hazard model, adjusting for age, sex, educational attainment, body mass index, cigarette smoking, alcohol consumption, past history of hypertension, type 2 diabetes, CVD, and metabolic syndrome.
2p-value for the non-linear relation between sleep duration and mortality tested by restricted cubic splines.
3ICD-10 codes of A00-Z99.
4ICD-10 codes of I00-I99.
5ICD-10 codes of C00-C99.
6ICD-10 codes of J00-J99, A15-A19.
7ICD-10 codes of S00-S99, T00-T99, and V01-X59.
8ICD-10 codes of R00-R99.
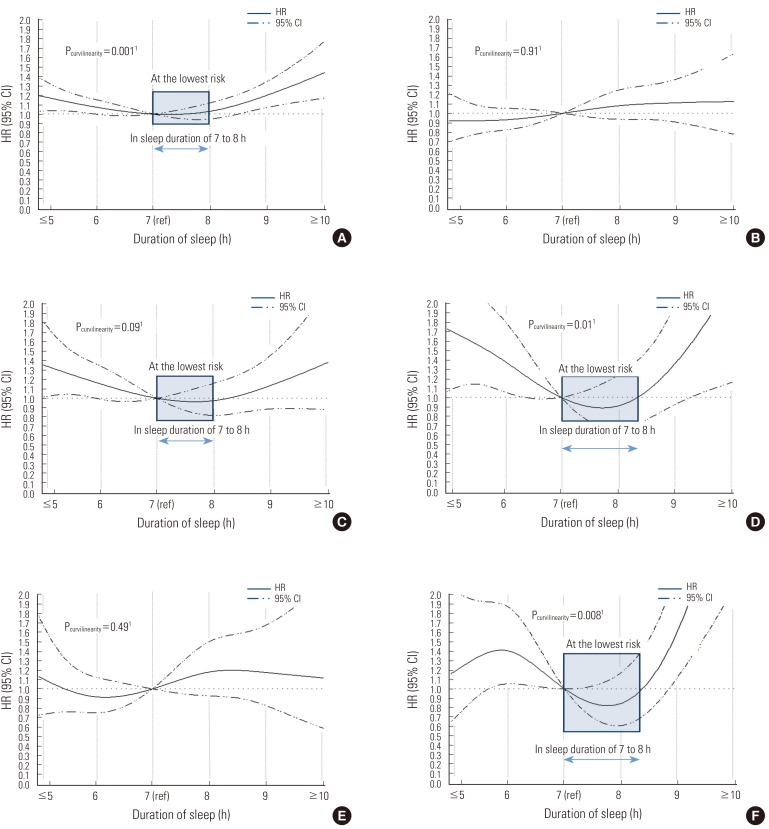
Figure 1.
Nonparametric regression curve for the relation of sleep duration with all-cause and disease-specific mortality, the Korean Multi-center Cancer Cohort (KMCC) study. (A) All-cause mortality. ICD-10 codes of A00-Z99. (B) Cancer mortality. ICD-10 codes of C00-C99. (C) CVD mortality. ICD-10 codes of I00-I99. (D) Respiratory disease mortality. ICD-10 codes of J00-J99, A15-A19. (E) Mortality from external cause. ICD-10 codes of S00-S99, T00-T99, V01-X59. (F) Mortality from not elsewhere classified cause. ICD-10 codes of R00-R99.1p-value for the non-linear relation between sleep duration and mortality tested by restricted cubic splines. HR, hazard ratio; CI, confidence interval.
Sleep duration was not associated with cancer mortality, which was the most common cause of death in this study (Table 2). In contrast, CVD-specific mortality, the second most common cause of death, showed a borderline significant relationship with sleep duration (Pcurvilinearity=0.09), where the HR for a sleep duration of ≤5 hours was 1.40 (95% CI, 1.02 to 1.93). A sleep duration of 8 hours showed a risk of CVD death (HR, 1.04), which is similar to the risk associated with a sleep duration of 7 hours. The group reporting ≥10 hours sleep duration also showed an increased risk of CVD death (HR, 1.37), even though the risk was not statistically significant when all variables were included in the model. There was a strikingly positive association between sleep duration and the risk of death due to respiratory diseases. The HR for death due to respiratory disease was found to have a U-shape (Pcurvilinearity=0.01), with the highest risk at both extremes (≤5 and ≥10 hours of sleep) (Table 2). For external causes of death, including all types of injury, no specific changes in HRs with sleep duration were observed. There was no significant trend when we included all other related factors in the model (Pcurvilinearity=0.49). Meanwhile, the risk of unclassified cause of death, including senility, increased with a sleep duration of ≥10 hours (HR, 3.29; 95% CI, 1.68 to 6.47) (Table 2). HRs for "not elsewhere classified" causes had a significant non-linear relationship with sleep duration, but not a U-shape as we expected (Figure 1).
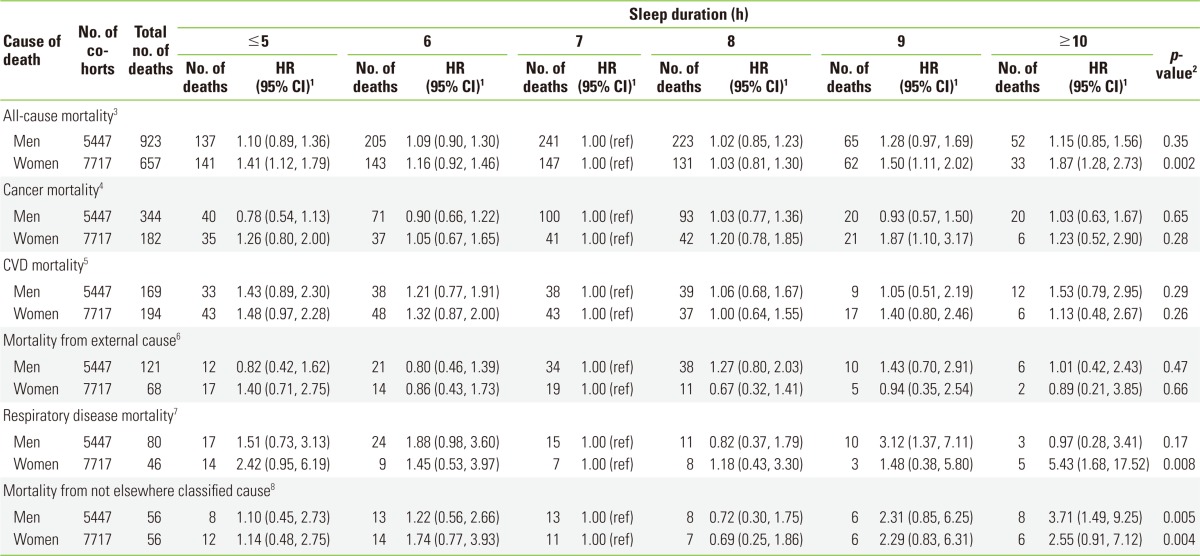
The relationship between sleep duration and risk of death was prominent in women (Pcurvilinearity=0.002) (Table 3). HRs in CVD mortality according to sleep duration in both men and women had a U-shape, even though it was not statistically significant in the model (Pcurvilinearity=0.29 and 0.26, respectively). A sleep duration of ≤5 hours increased the mortality due to CVD death, with a HR of 1.48 (95% CI, 0.97 to 2.28) in women (Table 3). Female participants with a sleep duration of ≥10 hours did not show any increased risk of death by CVD, but this might be due to a lack of power in the study. Among deaths from CVD, an increase for stroke mortality with sleep duration of ≤5 hours was prominent in men (HR, 2.12; 95% CI, 1.06 to 4.26) (data not shown). In contrast, an increased risk of death due to heart disease with a sleep duration of ≤5 hours was found only in women (HR, 1.81; 95% CI, 0.97 to 3.39). An increased risk of death due to heart disease with a sleep duration of ≥10 hours was prominent in men; however, it was not statistically significant (HR, 1.82; 95% CI, 0.83 to 4.03) (data not shown). The risk of death due to respiratory disease increased with sleep duration of ≤5 hours in both men (HR, 1.51; 95% CI, 0.73 to 3.13) and women (HR, 2.42; 95% CI, 0.95 to 6.19), although this was not statistically significant. An increased risk of death from respiratory disease with sleep duration of ≥10 hours was found only in women (HR, 5.43; 95% CI, 1.68 to 17.52) (Table 3). The curvilinear patterns of HR for "not elsewhere classified" causes were significant in both men and women in the model (Pcurvilinearity=0.005 and Pcurvilinearity=0.004, respectively) (Table 3).
Table 3.
HRs and 95% CIs for all-cause and disease-specific mortalities by sleep duration in gender-stratified analyses within the Korean Multi-center Cancer Cohort study
HR, hazard ratio; CI, confidence interval; CVD, cardiovascular disease; ICD-10, the 10th revision of the International Classification of Disease.
1HRs and 95% CIs were based on Cox's proportional hazard model, adjusting for age, educational attainment, body mass index, cigarette smoking, alcohol consumption, past history of hypertension, type 2 diabetes, CVD and metabolic syndrome.
2p-value for the non-linear relation between sleep duration and mortality tested by restricted cubic splines.
3ICD-10 codes of A00-Z99.
4ICD-10 codes of I00-I99.
5ICD-10 codes of C00-C99.
6ICD-10 codes of J00-J99, A15-A19.
7ICD-10 codes of S00-S99, T00-T99, and V01-X59.
8ICD-10 codes of R00-R99.
An increased risk of death with a decrease or increase in the sleep duration as compared with a sleep duration of 7 hours was found only in adults aged ≥60 years (Pcurvilinearity=0.001) (Table 4). In those aged <60 years, the relationship of HR with sleep duration did not show any specific shape because of the small number of deaths due to disease-specific causes. Among disease-specific causes, the relationship between sleep duration and respiratory disease mortality was prominent in those aged ≥60 years. The HRs for mortality from "not elsewhere classified" causes in those aged ≥60 years also had a non-linear relationship with an increased risk toward sleep duration of ≥10 hours (Pcurvilinearity=0.001) (Table 4).
Table 4.
HRs and 95% CIs for all-cause and disease-specific mortalities by sleep duration in age-stratified analyses within the Korean Multi-center Cancer Cohort study
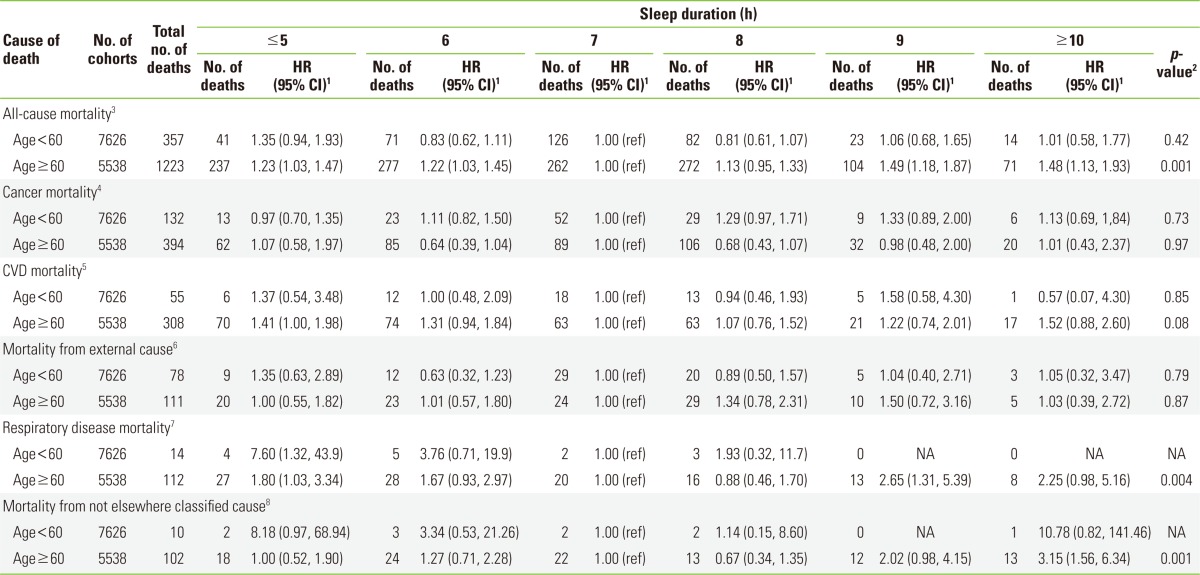
HR, hazard ratio; CI, confidence interval; CVD, cardiovascular disease; NA, not available; ICD-10, the 10th revision of the International Classification of Disease.
1HRs and 95% CIs were based on Cox's proportional hazard model, adjusting for sex, educational attainment, body mass index, cigarette smoking, alcohol consumption, past history of hypertension, type 2 diabetes, CVD and metabolic syndrome.
2p-value for the non-linear relation between sleep duration and mortality tested by restricted cubic splines.
3ICD-10 codes of A00-Z99.
4ICD-10 codes of I00-I99.
5ICD-10 codes of C00-C99.
6ICD-10 codes of J00-J99, A15-A19.
7ICD-10 codes of S00-S99, T00-T99, and V01-X59.
8ICD-10 codes of R00-R99.
DISCUSSION
We found that the risk of all-cause, CVD, and respiratory disease mortality increased in participants who slept ≤5 hours as compared with participants who slept 7 hours. Particularly noteworthy was the finding that the risk of dying also increased with a sleep duration ≥10 hours. Sleep durations of 7 and 8 hours showed the lowest risk of death due to any cause, even though the results suggest that disease-specific mortality should be differently associated with sleep duration.
The U-shaped association between sleep duration and all-cause mortality with the lowest risk at 7 or 8 hours has been reported in many studies [3,15-19]. A meta-analysis supported the U-shaped association between sleep duration and all-cause mortality [20]. However, the association between sleep duration and disease-specific mortality is still controversial, because studies examining these relationships have yielded less consistent results. In our study, CVD-specific mortality showed a significant association with sleep duration ≤5 hours.
The association between longer sleep durations (9 hours to ≥10 hours) and increased risk of death across studies is still unclear. Long sleep duration may be a consequence of medical conditions and age-related sleep changes rather than a contributor to increased risk of mortality [21]. In this study, we found that mortality from respiratory disease and "not elsewhere classified" causes was significantly increased with longer sleep duration. Longer sleep durations may mediate inflammatory, metabolic, or immune responses related to the risk of CVD or respiratory diseases [2,22]. However, there is little evidence to indicate that sleep duration of >7-8 hours has adverse health effects. No other studies have demonstrated possible mechanisms for identifying long sleep duration as a cause of mortality [23]. In this study, we accounted for possible comorbidities; however, we could not discern psychological abnormalities that might affect quality or duration of sleep. Furthermore, as we found an increased risk of death in those ≥60 years of age, the causes of death might not have been recorded accurately. For example, "non-specific" or "general illness" in elderly adults might have led to longer sleep durations due to age-related aspects of their health status.
The results from studies that have analyzed the association of baseline sleep duration with mortality separately in both genders have been inconsistent. An increased risk of death with short sleep durations was found in both genders [24-26] or only among women [27]. An increased risk of death with long sleep durations was also found in both genders [26,28] or only among women [29]. Previous studies did not find any change in the risk of death in either gender [30,31]. Several methodological explanations that might account for these inconsistencies, such as binary or tertiary scales of sleep duration, relatively short-term follow-up, or the small number of participants, have been reported [8]. In this study, the scale of sleep duration was not binary or tertiary, and there was a relatively long-term follow-up period, but the number of male participants was relatively small compared with female participants. Additional research might be needed to confirm the effects of gender on the relationship between sleep duration and mortality.
Several studies reported that the relationship between sleep duration and mortality is largely influenced by deaths in elderly subjects and by the measurement of sleep durations close to death [8,27]. This might be caused by the geographic locality of the study, which was based in rural areas where the proportion of elderly adults is high. Subjects aged ≥60 years who reported sleep durations of ≤5 hours or of ≥10 hours were more likely to die within the follow-up period. As expected, most deaths occurred among those aged ≥60 years at recruitment (n=1223 for all causes of death and 308 for CVD death), whereas comparatively few deaths occurred among middle-aged subjects (n=357 for all causes of death and 55 for CVD death). Therefore, the lack of association between sleep duration and mortality in middle-aged subjects could be due to lack of statistical power.
Biological plausibility for the association of sleep duration and mortality might derive from the fact that sleep deprivation can cause alterations in cortisol secretion and altered growth hormone metabolism [32]. Another mechanism relating sleep duration to adverse health outcomes is reciprocal changes in circulating levels of leptin and ghrelin, which in turn would increase appetite and caloric intake, reduce energy expenditure, and facilitate development of obesity and impaired glycemic control [33], leading to increased CVD risk. Several studies have reported that chronic inflammation is sustained in short-duration sleepers, as evidenced by markers such as C-reactive protein, and could explain how health status is influenced by sleep patterns [34]. The hypothesis that there is a curvilinear association between sleep duration and markers of inflammation was partly supported. All these mechanisms could be involved in the U-shape of the relationship between HR and sleep duration in this study.
This study had some limitations. First, we did not have data to classify sleep quality, sleep apnea, or sleep-disordered breathing, which could influence the health of long-duration sleepers. There may also be differences between self-reported sleep duration and values obtained through actigraphic monitoring. Although some studies have found good agreement between self-reported sleep durations and those measured through actigraphic monitoring [35], other studies have found self-reported sleep durations to overestimate sleep duration when compared with actigraphic and polysomnographic monitoring [36]. Second, a cause-and-effect relationship of sleep duration with mortality is not clear. In this study, changes in all-cause mortality according to sleep duration appeared to be explained by the presence of an increase in CVD or respiratory disease mortality. However, the results were still complex in stratified analysis, and there is no other plausibility to explain these results, especially with longer sleep durations (9 hours or ≥10 hours). We classified other specific causes of death and found increased mortality from respiratory disease and "not elsewhere classified" causes in longer-duration sleepers. This pattern was prominent in elderly adults, a population where the general health status and accompanying comorbidities associated with aging might affect the results. Third, because each lifestyle factor was assessed at baseline only, we could not consider the changes in sleep duration over time. Changes in sleep patterns over the follow-up period could have weakened the association between sleep duration reported at baseline and mortality. Fourth, the number of subjects and follow-up duration were limited in the analysis of disease-specific mortality stratified by gender and age.
This prospective cohort study found a U-shaped relationship of sleep duration with an increased risk of death at both extremes (≤5 and ≥10 hours of sleep duration). This relationship was also revealed in death due to CVD, respiratory disease, and "not elsewhere classified" causes. Sleep durations of 7 and 8 hours showed the lowest risk of death due to all causes and disease-specific causes. The results from this study suggest that sleep duration of 7 to 8 hours be recommended to the public for a general healthy lifestyle in Korea. Further studies are needed to investigate the influence of biological mechanisms or gender differences on the association between sleep duration and risk of death.
Footnotes
The authors have no conflicts of interest with the material presented in this paper.
References
- 1.Groeger JA, Zijlstra FR, Dijk DJ. Sleep quantity, sleep difficulties and their perceived consequences in a representative sample of some 2000 British adults. J Sleep Res. 2004;13(4):359–371. doi: 10.1111/j.1365-2869.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- 2.Parish JM. Sleep-related problems in common medical conditions. Chest. 2009;135(2):563–572. doi: 10.1378/chest.08-0934. [DOI] [PubMed] [Google Scholar]
- 3.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27(3):440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 4.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71(5):1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 5.Magee CA, Caputi P, Iverson DC. Relationships between self-rated health, quality of life and sleep duration in middle aged and elderly Australians. Sleep Med. 2011;12(4):346–350. doi: 10.1016/j.sleep.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Kripke DF, Langer RD, Elliott JA, Klauber MR, Rex KM. Mortality related to actigraphic long and short sleep. Sleep Med. 2011;12(1):28–33. doi: 10.1016/j.sleep.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR, et al. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007;30(9):1085–1095. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kronholm E, Härmä M, Hublin C, Aro AR, Partonen T. Self-reported sleep duration in Finnish general population. J Sleep Res. 2006;15(3):276–290. doi: 10.1111/j.1365-2869.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 9.Sudo N, Ohtsuka R. Sleep patterns and sleep disorders among female shift workers in a computer factory of Japan. J Hum Ergol (Tokyo) 1999;28(1-2):39–47. [PubMed] [Google Scholar]
- 10.Kalleinen N, Polo-Kantola P, Himanen SL, Alhola P, Joutsen A, Urrila AS, et al. Sleep and the menopause: do postmenopausal women experience worse sleep than premenopausal women? Menopause Int. 2008;14(3):97–104. doi: 10.1258/mi.2008.008013. [DOI] [PubMed] [Google Scholar]
- 11.Yoo KY, Shin HR, Chang SH, Lee KS, Park SK, Kang D, et al. Korean Multi-center Cancer Cohort Study including a Biological Materials Bank (KMCC-I) Asian Pac J Cancer Prev. 2002;3(1):85–92. [PubMed] [Google Scholar]
- 12.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 13.Steenland K, Deddens JA. A practical guide to dose-response analyses and risk assessment in occupational epidemiology. Epidemiology. 2004;15(1):63–70. doi: 10.1097/01.ede.0000100287.45004.e7. [DOI] [PubMed] [Google Scholar]
- 14.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54(3):201–208. doi: 10.1016/s0169-2607(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 15.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59(2):131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 16.Tamakoshi A, Ohno Y JACC Study Group. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27(1):51–54. [PubMed] [Google Scholar]
- 17.Gottlieb DJ, Schulman DA, Nam BH, D'agostino RA, Kannel WA. Sleep duration predicts mortality: the Framingham Study. Sleep. 2002;25:A108. [Google Scholar]
- 18.Kojima M, Wakai K, Kawamura T, Tamakoshi A, Aoki R, Lin Y, et al. Sleep patterns and total mortality: a 12-year follow-up study in Japan. J Epidemiol. 2000;10(2):87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 19.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30(10):1245–1253. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18(2):148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 21.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Opler MG, et al. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31(8):1087–1096. [PMC free article] [PubMed] [Google Scholar]
- 22.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farré R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186(2):190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knutson KL, Turek FW. The U-shaped association between sleep and health: the 2 peaks do not mean the same thing. Sleep. 2006;29(7):878–879. doi: 10.1093/sleep/29.7.878. [DOI] [PubMed] [Google Scholar]
- 24.Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30(12):1659–1666. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eguchi K, Pickering TG, Schwartz JE, Hoshide S, Ishikawa J, Ishikawa S, et al. Short sleep duration as an independent predictor of cardiovascular events in Japanese patients with hypertension. Arch Intern Med. 2008;168(20):2225–2231. doi: 10.1001/archinte.168.20.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankar A, Koh WP, Yuan JM, Lee HP, Yu MC. Sleep duration and coronary heart disease mortality among Chinese adults in Singapore: a population-based cohort study. Am J Epidemiol. 2008;168(12):1367–1373. doi: 10.1093/aje/kwn281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kronholm E, Laatikainen T, Peltonen M, Sippola R, Partonen T. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 2011;12(3):215–221. doi: 10.1016/j.sleep.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki E, Yorifuji T, Ueshima K, Takao S, Sugiyama M, Ohta T, et al. Sleep duration, sleep quality and cardiovascular disease mortality among the elderly: a population-based cohort study. Prev Med. 2009;49(2-3):135–141. doi: 10.1016/j.ypmed.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Amagai Y, Ishikawa S, Gotoh T, Doi Y, Kayaba K, Nakamura Y, et al. Sleep duration and mortality in Japan: the Jichi Medical School Cohort Study. J Epidemiol. 2004;14(4):124–128. doi: 10.2188/jea.14.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251(3):207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 31.Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Med. 2002;3(4):305–314. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 32.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99(5):2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 34.Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011;21(11):799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8(3):175–183. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 36.Walsleben JA, Kapur VK, Newman AB, Shahar E, Bootzin RR, Rosenberg CE, et al. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep. 2004;27(2):293–298. doi: 10.1093/sleep/27.2.293. [DOI] [PubMed] [Google Scholar]