Abstract
Obesity may be the consequence of various environmental or genetic factors, which may be highly correlated with each other. We aimed to examine whether grandmaternal and maternal obesity and environmental risk factors are related to obesity in daughters. Daughters (n = 182) recruited from female students, their mothers (n = 147) and their grandmothers (n = 67) were included in this study. Multivariable logistic regression was used to analyze the association between the daughter's obesity and maternal, grandmaternal, and environmental factors. Maternal heights of 161-175cm (OD: 8.48, 95% CI: 3.61-19.93) and 156-160 cm (2.37, 1.14-4.91) showed positive associations with a higher height of daughter, compared to those of 149-155 cm. Mothers receiving a university or a higher education had a significant OR (3.82, 1.27-11.50) for a higher height of daughter compared to those having a low education (elementary school). Mother having the heaviest weight at current time (59-80 kg, 3.78, 1.73-8.28) and the heaviest weight at 20 years of age (51-65 kg, 3.17, 1.53-6.55) had significant associations with a higher height of daughters, compared to those having the lightest weight at the same times. There was no association between the height, weight, and BMI of daughters and the characteristics and education of her grandmothers. In conclusion, although genetic factors appear to influence the daughter's height more than environmental factors, the daughter's weight appears to be more strongly associated with individual factors than the genetic factors.
Keywords: Trans generational, body mass index, daughter, mother, grandmother
Introduction
Obesity has been a major public health problem worldwide for decades, not only in Western countries, but also in Asian countries, including Korea. The 2010 Korean National Health and Nutrition Examination Survey (KNHANES) reported that the prevalence of obesity in aged 19 years and older in 2010 was 36.3% among men and 24.8% among women [1]. Obesity may be the consequence of various environmental or genetic factors. Potential risk factors for obesity in early life include genetic, physical, lifestyle, and environmental conditions. These factors may be highly correlated and may interact with each other. Therefore, the particular causal pathways involved remain unclear to a certain extent [2,3].
Some studies have shown that a strong association exists between parent and offspring obesity over two generation. [3-21]. Although controversy surrounds whether the mother's BMI plays a more powerful role than the father's BMI in the offspring's BMI, most studies have found that the former has a strong association with offspring BMI than the latter [6-8,19,22-26]. A positive association has also been reported between birth weight and overweight, with individuals tending to be overweight aged 6 to 7 years [17] and also aged 7 to 18 years when their birth weight was high [4].
Several studies have investigated obesity over three generations, and most of these have reported familial patterns of birth weight, food availability, and long life [19,20,27-29]. Murrin et al. [19] found evidence of BMI transmission over three generations through the maternal line (grandmother, mother, and child aged 5 to7 years). Another three generation study in the U.S. among subjects aged 5 to 19 years with their father and grandfather found an association of child weight status with grand-paternal obesity, distinct from paternal obesity [20]. Guillaume et al. [30] found familial factors (parents and grandparents) more influential than environmental factors on the BMI of children aged 6 to 12 years in Belgium.
In relation to the effect of environmental factors on offspring, studies have shown associations between offspring obesity and household income, educational level of the mother, meals, smoking, drinking, menarche age, sleeping hours, energy intake, macronutrients, and physical activity [31-36]. Some studies showed a negative association between offspring being overweight and socioeconomic status [3,10,14,31,37]. In contrast, other studies showed that high socioeconomic status exhibited a great with being overweight than low socioeconomic status [38,39]. Overweight parents affect children's television viewing, and that parental overweight had a positive influence on the child being overweight [11] and watching television was related to adult obesity [40]. Children who skipped breakfast [13], had a high energy intake [17,18], and were exposed to parental smoking [7,17] were more likely to be overweight and obese. Lower menarche age [36,41], reduced number of sleeping hours [8,16,17] and low physical activity [40,42] were also associated with being overweight.
Few studies have examined associations of the height, weight, and BMI of the daughter with various factors of three generations of mother, grandmother, and daughter in Korea. Therefore, we examined how grandmaternal and maternal obesity and environmental risk factors are related to the daughter's height, weight, and BMI among Korean female college students.
Subjects and Methods
Subjects
Study subjects were female students of the Department of Food and Nutrition from three national universities in Korea, and mothers and grandmothers of the female students (182 daughters, 147 mothers, and 67 grandmothers). Questionnaires were distributed through each university during lectures, and daughters who received the questionnaires were asked to forward them to mothers and grandmothers and then submit the completed forms to staff at each university. A total of 396 participants completed the survey. Missing answers or logistical errors were checked. When necessary, the students were asked to complete the questionnaires again. The mean ages of the daughters, mothers, and grandmothers were 21.1, 47.8, and 77.2 years, respectively. The present study was conducted between April and June 2011.
Survey contents
The self-administered questionnaires for the different age groups consisted of demographic and anthropometric items. The demographic data included the age, household income, and educational level. The anthropometric data included the current height (cm) and the weight (kg) of the three generations and the weight at age 20 years of the mother and the grandmother, Data were not collected on their height at 20 years. All the participants' BMIs [weight (kg) / height (m2)] were calculated from self-reported height and weight. The BMI was used as a continuous or categorical variable. The cut-off point for "underweight" was < 18.5 kg/m2; "normal" was 18.5-22.9 kg/m2; "overweight" was 23.0-24.9 kg/m2; and "obese" was ≥ 25.0 kg/m2 according to the decision of WHO criteria pertaining to obesity [43]. The term overweight refer to both overweight and obesity. In the lifestyle survey, we obtained information on frequencies of meals/day, smoking (non-, former-, and current- smoker), frequencies of passive smoking at home and outdoor (non-, former-, and current-smoker), drinking (non-, former-, and current-drinker), physical activity (metabolic equivalent of task values [METs]), menarche age (years), sleeping hours (min), dieting attempts (yes or no), age of dieting attempts, and frequency of dieting attempt. The METs were used to assess the physical activity of participants. METs calculation for "vigorous physical activity" was hour/day × day × 7, "moderately physical activity" was hour/day × day × 5, "walking" was hour/day × day × 3, and "sedentary activity" was hour/day × day × 2. We assessed dietary intake for the previous year using a 13-page self-administered semiquantitative (food frequency questionnaire). Dietary intake was measured for 95 food and beverage items, and the energy and nutrients were calculated.
Statistical analysis
The statistical analysis was performed with SAS software (version 9.1, Cary, NC, USA). All the variables of the mother and the grandmother corresponded to the daughter's variables. Frequencies of underweight, normal weight, and overweight (including obesity) stratified by categorical variables were analyzed. First, the χ2 test was used to compare differences in the categorical variables between the three groups (< 18.5 kg/m2, 18.5-22.9 kg/m2 and 23.0 kg/m2). Second, the analysis of variance (ANOVA) was used to determine the significance of the differences between the three groups for continuous variables. Post hoc analysis was performed by using the Duncan's multiple range test. Third, a multivariable logistic regression analysis was conducted with height, weight, and BMI as the dependent variables adjusted for age, energy intake, and physical activity as continuous variables. Energy intake, sleeping hours, and the age at menarche of the daughters were treated in tertiles. The lowest tertile was used as a reference in each variable, except for menarche age, where the highest tertile was used as a reference. The heights and the weights of the mothers and the grandmothers were also treated in tertiles. Underweight mothers and grandmothers were excluded from the categories of their BMI due to their very little proportion. The physical activity of the daughters was divided into two groups and low physical activity was considered as a reference. P-values and p for linear trend of < 0.05 were considered to be statistically significant.
Results
Characteristics of study subjects
Table 1 shows the characteristics of the daughters with different BMI status (underweight-, normal weight-, and overweight). The frequency of eating two meals per day was higher than three meals per day, regardless of their BMI. Most daughters were non-smokers and current-drinkers. Smoking and drinking were not associated with the BMI status of the daughters. Although the frequencies of passive smoking at home and outdoor were higher in overweight daughters, there were no significant differences in the BMI status. Daughters with dieting attempt experiences were significantly more likely to be overweight than those with no dieting attempt experiences. Daughters with low household income were significantly more likely to be overweight than those with high household income. While most mothers had graduated from middle or high school, most grandmothers had graduated only from elementary school.
Table 1.
General characteristics of study subjects (daughters) according to obesity
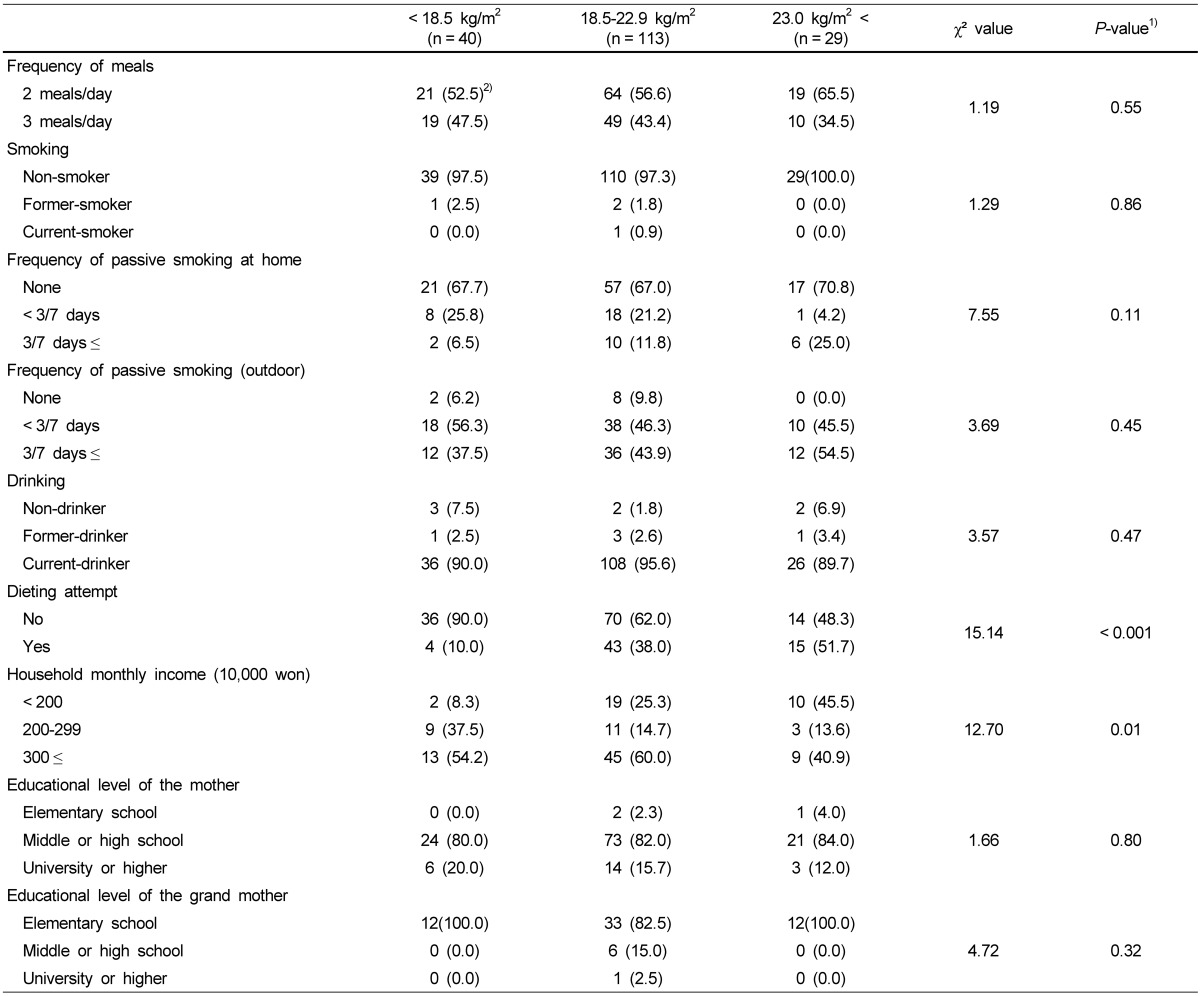
1)Different between the three BMI groups at α = 0.05 by chi-squared test
2)N (%)
Macronutrient intake, sleeping hours, physical activity, menarche age of daughters, and anthropometric measurements of daughters, mothers, and grandmothers according to obesity
The daughter's heights were not different among three groups of BMI status (Table 2). There were no significant differences in intakes of total energy and macronutrients, sleeping hours, and menarche age among the BMI status groups, but physical activity showed significant differences between the three BMI groups. The anthropometric measurements of the mothers and the grandmothers also showed no significant differences in the different BMI status groups.
Table 2.
Macronutrient intake, sleeping hours, physical activity, menarche age of daughters, and anthropometric measurements of daughters, mothers, and grand mothers according to obesity
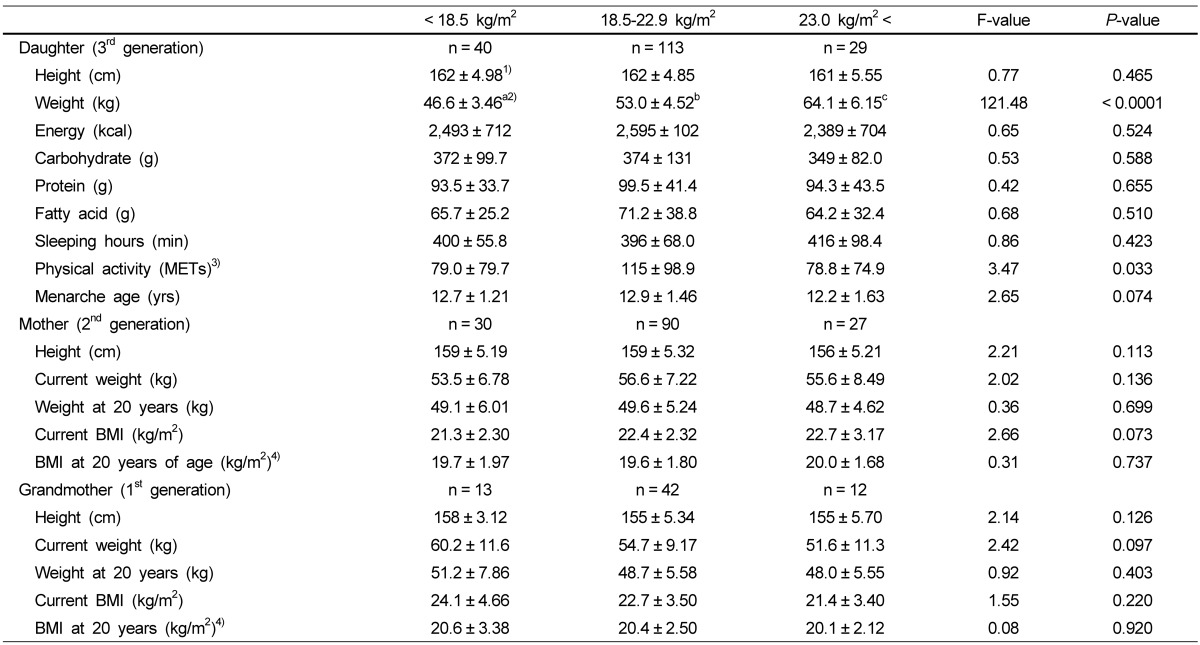
1)Mean ± SD.
2)Superscripts with different alphabets in a row are significantly different by Duncan's multiple range test.
3)METs; vigorous physical activity: hour/day × day × 7; moderately physical activity: hour/day × day × 5; walk: hour/day × day × 3; sedentary activity: hour/day × day × 2
4)Height of at 20 years was not researched; now height was used.
Association with the daughter's height, weight, and body mass index and lifestyle characteristics
As shown in Table 3, there were no significant associations between the daughter's height and energy intake, macronutrients, frequencies of meals, dieting attempt, sleeping hours, physical activity, menarche ages, and household monthly income. Weight of daughters was associated with their dieting attempts and menarche age. Daughters having a menarche age of under 12 years did not show a significant association (OD: 2.75, 95% CI: 0.89-8.46) with their overweight (BMI > 23.0 kg/m2). But daughters having dieting attempts (3.77, 1.82-7.82) and menarche age of under 12 years (3.44, 1.34-8.83) had a significant association with their higher weight.
Table 3.
Lifestyle characteristics and other information of daughters in related to height, weight, and body mass index: results of multivariate logistic regression
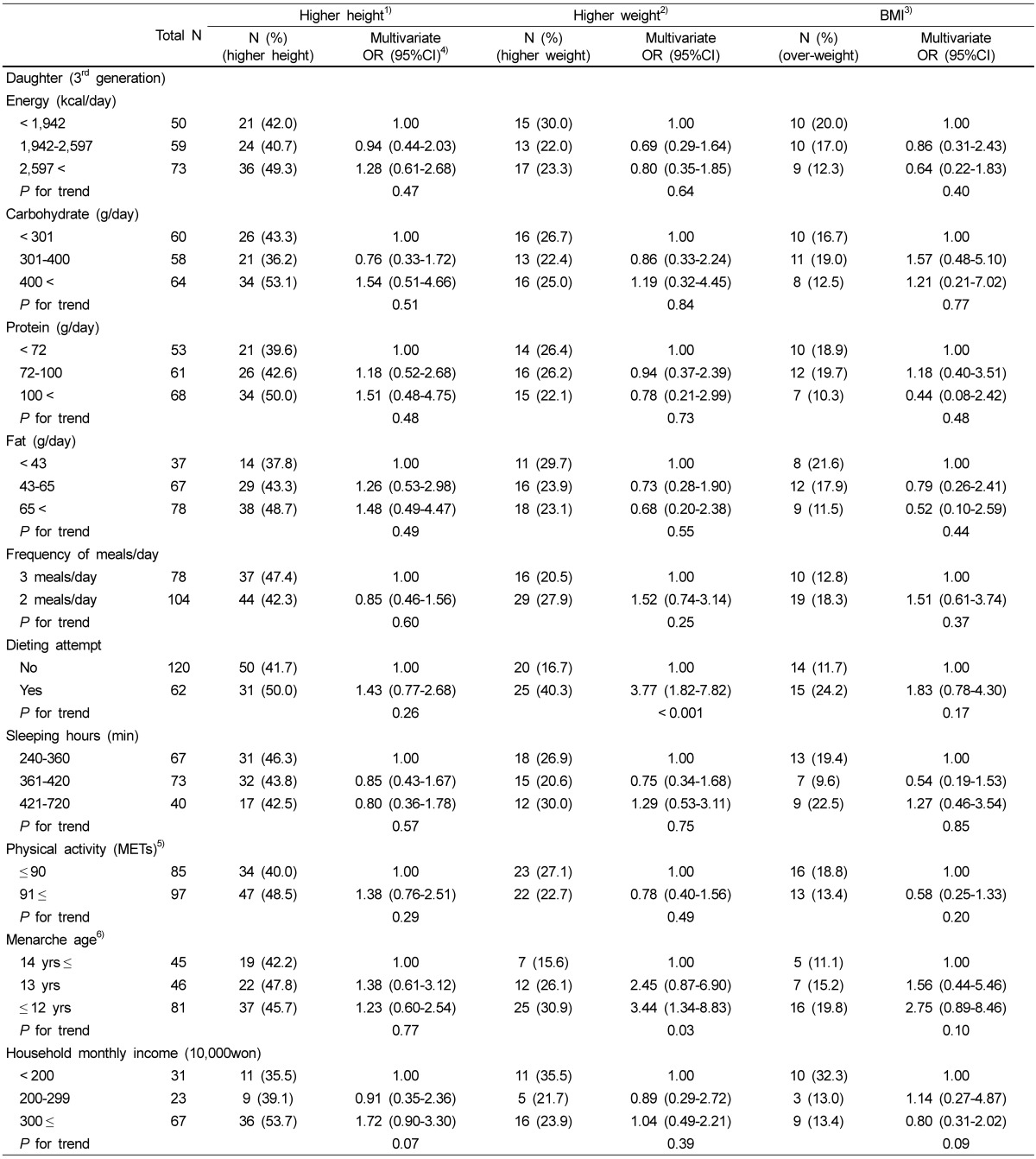
1)Higher height was defined as the highest tertile of height among the daughters.
2)Higher weight was defined as the highest tertile of weight among the daughters.
3)Obesity was defined as ≥ 23 kg/m2 of the BMI.
4)OR: odds ratio, 95%CI: 95% confidence interval Multivariate ORs were adjusted for age (continuous), energy intake (kcal), physical activity (METs).
5)METs; vigorous physical activity: hour/day × day × 7; moderately physical activity: hour/day × day × 5; walk: hour/day × day × 3; sedentary activity: hour/day × day × 2
6)The age at menarche of the daughters were divided into tertile.
Association with the daughter's height, weight, and body mass index and the anthropometric and educational level of their grandmother and mother
As shown in Table 4, we found positive associations between the height of daughters and the current weight, the weight at 20 years, and a high education of her mother. Maternal heights of 161-175 cm (OD: 8.48, 95% CI: 3.61-19.93) and 156-160 cm (2.37, 1.14-4.91) showed positive associations with a higher height of daughter, compared to those of 149-155 cm. Mothers receiving a university and a higher education had a significant OR (3.82, 1.27-11.50) for a higher height of daughter compared to those having a low education (elementary school). Mother having the heaviest weight at current time (59-80 kg: 3.78, 1.73-8.28) and the heaviest weight at 20 years of age (51-65 kg: 3.17, 1.53-6.55) had significant associations with a higher height of daughters, compared to those having the lightest weight at the same times. In addition, we could not find the association between the characteristics and education of grandmothers and the height, weight, and BMI of daughters.
Table 4.
Anthropometric characteristics and educational level of grandmother and mother for daughters in related to height, weight, and body mass index: results of multivariate logistic regression
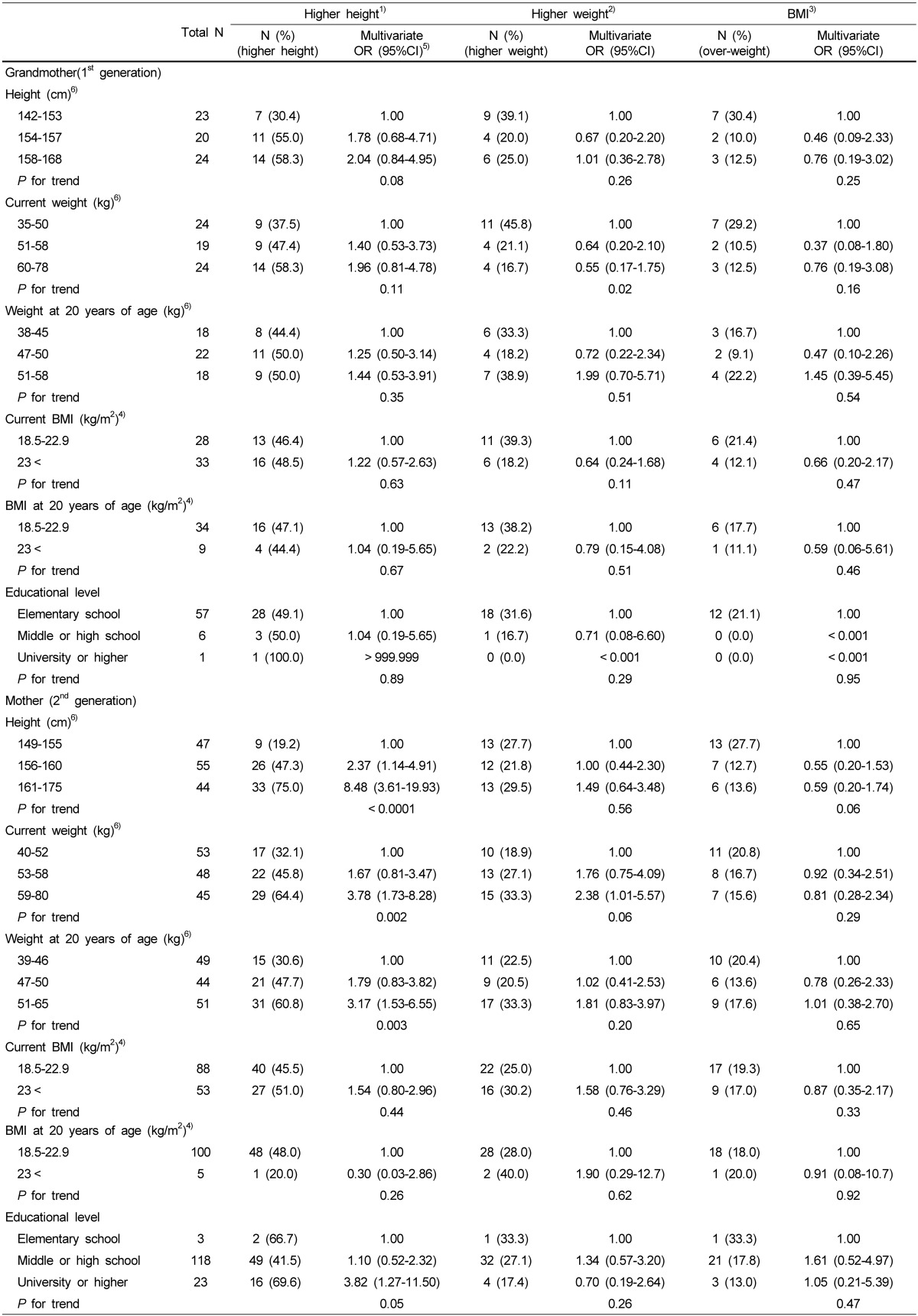
1)Higher height was defined as the highest tertile of height among the daughters.
2)Higher weight was defined as the highest tertile of weight among the daughters.
3)Obesity was defined as ≥ 23 kg/m2 of the BMI.
4)Normal weight: 18.5-22.9 kg/m2; overweight: 23.0-24.9 kg/m2; obese: ≥ 25.00 kg/m2
5)OR: odds ratio, 95%CI: 95% confidence interval Multivariate ORs were adjusted for age (continuous), energy intake (kcal), physical activity (METs).
6)Height and weight of the mother were divided into tertile.
Discussion
The present study found that the daughter's height is related to the current height, weight, weight at 20 years, and educational level of her mother. The daughter's weight was associated with her age at menarche and dieting attempts and the current weight of her mother.
Many studies have revealed a strong association between parental and offspring BMI [3-15,18-21]. Results from studies showing a stronger association of the mother than the father with the offspring's BMI may be explained by woman's crucial role of shopping and cooking within a family and by the maternal uterine environment [5,24]. However, some studies have shown no differences between the maternal-offspring and the paternal - offspring BMI [23,44]. This suggests that the influence of the intra-uterine environment is not decisive [23], whereas parents and offspring may share similar meals and exercise habits [44]. In this study, only the influence of the maternal line on the daughter's obesity was evaluated. Many studies have shown an association between parent and child obesity, with studies of obesity over three generations revealing an association between parent and child obesity and between grandparent and grandchild obesity [8,11,13,14,20,29]. This was not confirmed in our study, possibly due to the insufficient numbers of grandmothers surveyed compared with the numbers of mothers and daughters. Cooper et al. [15] found a strong association between parental and child BMI that continued until middle age. Although our results showed a positive association between maternal current weight and the weight of the daughters, there was no association between the mother's and the daughter's BMI.
Previous studies, which examined associations between socioeconomic status and offspring obesity, showed that offspring with low socioeconomic status have an increased risk of being overweight [3,31,33-37]. Other studies have reported that offspring with low socioeconomic status in industrialized countries [31,37] and/or in urban areas [4] are more likely to be overweight than offspring with high socioeconomic status. Drewnowski et al. [45] suggested that high household income could provide the economic capability to purchase more expensive food with high levels of nutrients and be associated with a balanced diet that contains more whole grains, low-fat dairy products, fruits, and vegetables. On the other hand, the prevalence of offspring being overweight has been reported to be positively related with socioeconomic status in developing countries [38] and in rural area [39]. Despite the many studies reporting that socioeconomic status is related to the offspring being overweight, only the daughter's height was positively associated with the educational level of her mother in the present study. This may be because most mothers surveyed graduated only middle or high schools. High energy intake is widely known to be linked to being overweight [17,18], but our results did not conform this. This may be because 34% of the daughters had experiences in dieting attempts, and 57% of the daughters ate only two meals per day. Although sleeping hours showed no significant association with obesity in the present study, other studies showed that offspring with short sleeping hours have an increased risk of being overweight [8,16,17,40]. There are several possible explanations for the relationship between sleeping hours and obesity. A short number of sleeping hours could cause a decrease in the secretion of the nigh-time growth hormone, which is mostly secreted during the first half of the night and functions in controlling lipolysis during the night [8,46]. And other explanation may be that sleeping for limited periods could cause an increase in sympathetic nerve activity, cortisol secretion, and hyperinsulinemia, all of which are known to be associated with the development of obesity [47].
In the present study, physical activity was lowest among those who were overweight, but it was not associated with daughters being overweight in multivariate logistic regression analysis. This finding dose not coincide with results reported in other studies, which showed that low physical activity is related to obesity [35,40,42]. The adjusted OR for overweight with early menarche, which was menarche aged under 12 years, was 3.44. There is no consensus as to whether early puberty causes later obesity or obesity causes early puberty [36,41].
The main strength of the present study was in conducting a survey of young adult daughters and their mothers and grandmothers, enabling us to identify influences of genetic and environmental factors. Several limitations of the present study should be taken into account. First, the population was not a representative sample of Korean women in the general population. Second, we could not analyze using only the subjects having perfect data composed to daughter, her mother and her grandmother due to their small sample size. The grandmother sample size was relatively small compared with the daughter and mother sample sizes. Therefore, the statistical power may have been insufficient to allow the identification and stabilization of the association between the daughter's obesity and the grandmother's obesity. Third, using self-reported height and weight data may result in some inaccuracies. As shown in other studies, the use of self-reported weight and BMI tends to underestimate actual values, particularly in overweight women [48]. In addition, the self-reported weight at age 20 years relied on the mother's and the grandmother's memories, thereby increasing the risk of inaccuracy. However, self-reported height and weight have been reported to be reasonably valid measures for identifying relationships in epidemiological studies [49]. Fourth, most participants belonged to the normal weight range, making is difficult to generalize the results of this study.
In conclusion, the present study of Korean women showed that young adult daughter's height was associated with her mother's height and her mother's current weight, weight at 20 years, and educational level. The daughter's weight was related to dieting attempts, age at menarche, and to maternal current weight. Therefore, genetic factors may exert a great influence on the daughter's height than environmental factors. The daughter's weight might show a stronger association with environmental factors than with genetic factors. Further large-scale studies with sufficient numbers of obese participants and with all family members of three generations are required to confirm the roles of various genetic and environmental factors at each stage of life (birth, childhood, adolescence, and adulthood) and to determine obesity.
Footnotes
This research was supported by a grant from the National Cancer Center in Korea (1110320, 1310361).
References
- 1.Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea Health Statistics 2010: Korea National Health and Nutrition Examination Survey (KNHANES V-1) Cheongwon: Korea Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2.Barness LA, Opitz JM, Gilbert-Barness E. Obesity: genetic, molecular, and environmental aspects. Am J Med Genet A. 2007;143A:3016–3034. doi: 10.1002/ajmg.a.32035. [DOI] [PubMed] [Google Scholar]
- 3.Kleiser C, Schaffrath Rosario A, Mensink GB, Prinz-Langenohl R, Kurth BM. Potential determinants of obesity among children and adolescents in Germany: results from the cross-sectional KiGGS study. BMC Public Health. 2009;9:46. doi: 10.1186/1471-2458-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andegiorgish AK, Wang J, Zhang X, Liu X, Zhu H. Prevalence of overweight, obesity, and associated risk factors among school children and adolescents in Tianjin, China. Eur J Pediatr. 2012;171:697–703. doi: 10.1007/s00431-011-1636-x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson PC, Logue J, McConnachie A, Abu-Rmeileh NM, Hart C, Upton MN, Lean M, Sattar N, Watt G. Intergenerational change and familial aggregation of body mass index. Eur J Epidemiol. 2012;27:53–61. doi: 10.1007/s10654-011-9639-5. [DOI] [PubMed] [Google Scholar]
- 6.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Pastor EM, Metcalf BS, Hosking J, Jeffery AN, Voss LD, Wilkin TJ. Assortative weight gain in mother-daughter and father-son pairs: an emerging source of childhood obesity. Longitudinal study of trios (EarlyBird 43) Int J Obes (Lond) 2009;33:727–735. doi: 10.1038/ijo.2009.76. [DOI] [PubMed] [Google Scholar]
- 8.Sekine M, Yamagami T, Hamanishi S, Handa K, Saito T, Nanri S, Kawaminami K, Tokui N, Yoshida K, Kagamimori S. Parental obesity, lifestyle factors and obesity in preschool children: results of the Toyama birth cohort study. J Epidemiol. 2002;12:33–39. doi: 10.2188/jea.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Law C, Lo Conte R, Power C. Intergenerational influences on childhood body mass index: the effect of parental body mass index trajectories. Am J Clin Nutr. 2009;89:551–557. doi: 10.3945/ajcn.2008.26759. [DOI] [PubMed] [Google Scholar]
- 10.Danielzik S, Czerwinski-Mast M, Langnäse K, Dilba B, Müller MJ. Parental overweight, socioeconomic status and high birth weight are the major determinants of overweight and obesity in 5-7 y-old children: baseline data of the Kiel Obesity Prevention Study (KOPS) Int J Obes Relat Metab Disord. 2004;28:1494–1502. doi: 10.1038/sj.ijo.0802756. [DOI] [PubMed] [Google Scholar]
- 11.Steffen LM, Dai S, Fulton JE, Labarthe DR. Overweight in children and adolescents associated with TV viewing and parental weight: project heartbeat. Am J Prev Med. 2009;37:S50–S55. doi: 10.1016/j.amepre.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safer DL, Agras WS, Bryson S, Hammer LD. Early body mass index and other anthropometric relationships between parents and children. Int J Obes Relat Metab Disord. 2001;25:1532–1536. doi: 10.1038/sj.ijo.0801786. [DOI] [PubMed] [Google Scholar]
- 13.Maddah M, Nikooyeh B. Factors associated with overweight in children in Rasht, Iran: gender, maternal education, skipping breakfast and parental obesity. Public Health Nutr. 2010;13:196–200. doi: 10.1017/S1368980009990589. [DOI] [PubMed] [Google Scholar]
- 14.Svensson V, Jacobsson JA, Fredriksson R, Danielsson P, Sobko T, Schiöth HB, Marcus C. Associations between severity of obesity in childhood and adolescence, obesity onset and parental BMI: a longitudinal cohort study. Int J Obes (Lond) 2011;35:46–52. doi: 10.1038/ijo.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper R, Hyppönen E, Berry D, Power C. Associations between parental and offspring adiposity up to midlife: the contribution of adult lifestyle factors in the 1958 British birth cohort study. Am J Clin Nutr. 2010;92:946–953. doi: 10.3945/ajcn.2010.29477. [DOI] [PubMed] [Google Scholar]
- 16.Peña MM, Taveras EM. Preventing childhood obesity: wake up, it's time for sleep. J Clin Sleep Med. 2011;7:343–344. doi: 10.5664/JCSM.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui LL, Nelson EA, Yu LM, Li AM, Fok TF. Risk factors for childhood overweight in 6- to 7-y-old Hong Kong children. Int J Obes Relat Metab Disord. 2003;27:1411–1418. doi: 10.1038/sj.ijo.0802423. [DOI] [PubMed] [Google Scholar]
- 18.Poston L. Maternal obesity, gestational weight gain and diet as determinants of offspring long term health. Best Pract Res Clin Endocrinol Metab. 2012;26:627–639. doi: 10.1016/j.beem.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Murrin CM, Kelly GE, Tremblay RE, Kelleher CC. Body mass index and height over three generations: evidence from the lifeways cross-generational cohort study. BMC Public Health. 2012;12:81. doi: 10.1186/1471-2458-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis MM, McGonagle K, Schoeni RF, Stafford F. Grandparental and parental obesity influences on childhood overweight: implications for primary care practice. J Am Board Fam Med. 2008;21:549–554. doi: 10.3122/jabfm.2008.06.070140. [DOI] [PubMed] [Google Scholar]
- 21.Patel R, Martin RM, Kramer MS, Oken E, Bogdanovich N, Matush L, Smith GD, Lawlor DA. Familial associations of adiposity: findings from a cross-sectional study of 12,181 parental-offspring trios from Belarus. PLoS One. 2011;6:e14607. doi: 10.1371/journal.pone.0014607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danielzik S, Langnäse K, Mast M, Spethmann C, Müller MJ. Impact of parental BMI on the manifestation of overweight 5-7 year old children. Eur J Nutr. 2002;41:132–138. doi: 10.1007/s00394-002-0367-1. [DOI] [PubMed] [Google Scholar]
- 23.Davey Smith G, Steer C, Leary S, Ness A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC) Arch Dis Child. 2007;92:876–880. doi: 10.1136/adc.2006.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitaker KL, Jarvis MJ, Beeken RJ, Boniface D, Wardle J. Comparing maternal and paternal intergenerational transmission of obesity risk in a large population-based sample. Am J Clin Nutr. 2010;91:1560–1567. doi: 10.3945/ajcn.2009.28838. [DOI] [PubMed] [Google Scholar]
- 25.Lawlor DA, Timpson NJ, Harbord RM, Leary S, Ness A, McCarthy MI, Frayling TM, Hattersley AT, Smith GD. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med. 2008;5:e33. doi: 10.1371/journal.pmed.0050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawlor DA, Smith GD, O'Callaghan M, Alati R, Mamun AA, Williams GM, Najman JM. Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol. 2007;165:418–424. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- 27.Lumey LH, Stein AD. Offspring birth weights after maternal intrauterine undernutrition: a comparison within sibships. Am J Epidemiol. 1997;146:810–819. doi: 10.1093/oxfordjournals.aje.a009198. [DOI] [PubMed] [Google Scholar]
- 28.Emanuel I, Filakti H, Alberman E, Evans SJ. Intergenerational studies of human birthweight from the 1958 birth cohort. 1. Evidence for a multigenerational effect. Br J Obstet Gynaecol. 1992;99:67–74. doi: 10.1111/j.1471-0528.1992.tb14396.x. [DOI] [PubMed] [Google Scholar]
- 29.Hyppönen E, Smith GD, Power C. Effects of grandmothers' smoking in pregnancy on birth weight: intergenerational cohort study. BMJ. 2003;327:898. doi: 10.1136/bmj.327.7420.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillaume M, Lapidus L, Beckers F, Lambert A, Björntorp P. Familial trends of obesity through three generations: the Belgian-Luxembourg child study. Int J Obes Relat Metab Disord. 1995;19(Suppl 3):S5–S9. [PubMed] [Google Scholar]
- 31.Gnavi R, Spagnoli TD, Galotto C, Pugliese E, Carta A, Cesari L. Socioeconomic status, overweight and obesity in prepuberal children: a study in an area of Northern Italy. Eur J Epidemiol. 2000;16:797–803. doi: 10.1023/a:1007645703292. [DOI] [PubMed] [Google Scholar]
- 32.Greenlund KJ, Liu K, Dyer AR, Kiefe CI, Burke GL, Yunis C. Body mass index in young adults: associations with parental body size and education in the CARDIA study. Am J Public Health. 1996;86:480–485. doi: 10.2105/ajph.86.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ball K, Mishra GD. Whose socioeconomic status influences a woman's obesity risk: her mother's, her father's, or her own? Int J Epidemiol. 2006;35:131–138. doi: 10.1093/ije/dyi216. [DOI] [PubMed] [Google Scholar]
- 34.Saunders MR, Watson KT, Tak HJ. Social factors in childhood and adulthood associated with adult obesity in African American and white women. ISRN Public Health. 2012;2012:10. [Google Scholar]
- 35.Thibault H, Contrand B, Saubusse E, Baine M, Maurice-Tison S. Risk factors for overweight and obesity in French adolescents: physical activity, sedentary behavior and parental characteristics. Nutrition. 2010;26:192–200. doi: 10.1016/j.nut.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Laitinen J, Power C, Järvelin MR. Family social class, maternal body mass index, childhood body mass index, and age at menarche as predictors of adult obesity. Am J Clin Nutr. 2001;74:287–294. doi: 10.1093/ajcn/74.3.287. [DOI] [PubMed] [Google Scholar]
- 37.Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J Psychosom Res. 2002;53:891–895. doi: 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 38.Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Soc Sci Med. 1997;44:809–819. doi: 10.1016/s0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- 39.Kang HT, Ju YS, Park KH, Kwon YJ, Im HJ, Paek DM, Lee HJ. Study on the relationship between childhood obesity and various determinants, including socioeconomic factors, in an urban area. J Prev Med Public Health. 2006;39:371–378. [PubMed] [Google Scholar]
- 40.Vioque J, Torres A, Quiles J. Time spent watching television, sleep duration and obesity in adults living in Valencia, Spain. Int J Obes Relat Metab Disord. 2000;24:1683–1688. doi: 10.1038/sj.ijo.0801434. [DOI] [PubMed] [Google Scholar]
- 41.Currie C, Ahluwalia N, Godeau E, Nic Gabhainn S, Due P, Currie DB. Is obesity at individual and national level associated with lower age at menarche? Evidence from 34 countries in the health behaviour in school-aged children study. J Adolesc Health. 2012;50:621–626. doi: 10.1016/j.jadohealth.2011.10.254. [DOI] [PubMed] [Google Scholar]
- 42.Frieden TR, Dietz W, Collins J. Reducing childhood obesity through policy change: acting now to prevent obesity. Health Aff (Millwood) 2010;29:357–363. doi: 10.1377/hlthaff.2010.0039. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization; International Association for the Study of Obesity; International Obesity Task Force. The Asia-Pacific Perspective: Redefining Obesity and its Treatment. Balmain: Health Communications Australia Pty Ltd.; 2000. p. 18. [Google Scholar]
- 44.Kivimäki M, Lawlor DA, Smith GD, Elovainio M, Jokela M, Keltikangas-Järvinen L, Viikari JS, Raitakari OT. Substantial intergenerational increases in body mass index are not explained by the fetal overnutrition hypothesis: the cardiovascular risk in young Finns study. Am J Clin Nutr. 2007;86:1509–1514. doi: 10.1093/ajcn/86.5.1509. [DOI] [PubMed] [Google Scholar]
- 45.Drewnowski A, Darmon N. The economics of obesity: dietary energy density and energy cost. Am J Clin Nutr. 2005;82:265S–273S. doi: 10.1093/ajcn/82.1.265S. [DOI] [PubMed] [Google Scholar]
- 46.Scheen AJ, Byrne MM, Plat L, Leproult R, Van Cauter E. Relationships between sleep quality and glucose regulation in normal humans. Am J Physiol. 1996;271:E261–E270. doi: 10.1152/ajpendo.1996.271.2.E261. [DOI] [PubMed] [Google Scholar]
- 47.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 48.Tokmakidis SP, Christodoulos AD, Mantzouranis NI. Validity of self-reported anthropometric values used to assess body mass index and estimate obesity in Greek school children. J Adolesc Health. 2007;40:305–310. doi: 10.1016/j.jadohealth.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106:52–58. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]


