Abstract
The recent increase in the number of cases of indigenous hepatitis E virus (HEV) infection highlights the importance of identifying the transmission routes for the prevention of such infections. Presented herein is the first case of acute HEV infection after ingesting wild roe deer meat in South Korea. A 43-year-old male presented with abdominal discomfort and jaundice. He had not recently traveled abroad, but had eaten raw roe-deer meat 6-8 weeks before the presentation. On the 7th day of hospitalization the patient was diagnosed with acute viral hepatitis E. Phylogenetic analysis of his serum revealed genotype-4 HEV. This case supports the possibility of zoonotic transmission of HEV because the patient appears to have been infected with genotype-4 HEV after ingesting raw deer meat.
Keywords: Genotype 4 hepatitis E, Roe deer, South Korea
INTRODUCTION
Hepatitis E virus (HEV) has four genotypes. Of these four types, genotype 1 has caused epidemic outbreaks in Asia and Africa and genotype 2 is usually discovered in western Africa. Both genotype 1 and 2 are found exclusively in humans.1 On the other hand, genotypes 3 and 4 are usually isolated from sporadic hepatitis E in developed countries, and also found in swine, deer, wild boar populations.2,3 Sporadic infection in non-endemic areas such as developed countries has been known to be due to an influx from foreign countries. However, some reports of locally acquired acute viral hepatitis E in people with no history of travel to endemic regions have recently increased in non-endemic areas.4,5 Autochthonous sporadic HEV infections in a non-endemic area mostly give no clue as to their sources despite the diagnosis of acute viral hepatitis E, rendering the transmission routes undecided.
Of the viruses that cause acute viral hepatitis, HEV is known to be the only virus to have animal reservoirs. Since the discovery of HEV in swine, HEV has also been isolated from chickens, deers, mongooses, rabbits and rats, supporting zoonotic transmission and prompting its investigation.6,7 We report here a case of acute viral hepatitis E that occurred after ingestion of raw meat of a wild roe deer in the absence of contact with another hepatitis patient or travel to an endemic area.
CASE REPORT
A 43-year-old male presented with abdominal discomfort for 3 weeks and jaundice lasting 1 week. He had a past history of diabetes mellitus, which had been diagnosed 3 years prior to admission to our hospital. However, he arbitrarily stopped taking hypoglycemic agents. The patient was a heavy alcohol drinker, with the consumption of 2 to 3 bottles of Soju, distilled liquor, 4 to 5 times a week. He denied any travel outside South Korea in the preceding years. About 6-8 weeks before hospitalization, he ingested raw meat (about 300 g) of a captured wild roe deer inhabiting in Gyeongnam province with his friends, who enjoyed hunting on a regular basis.
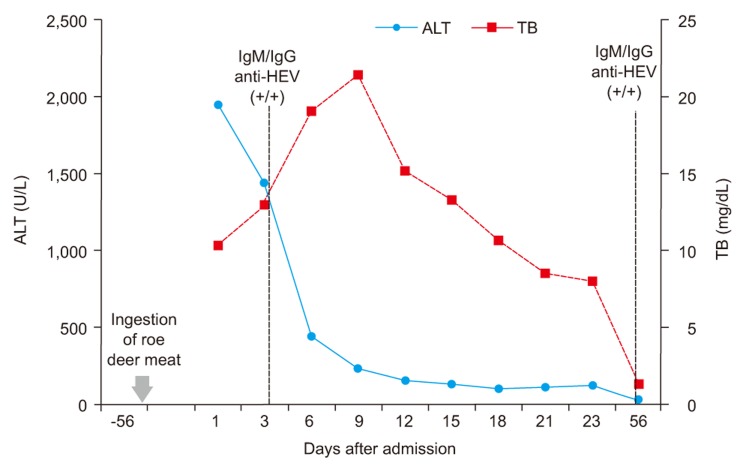
Physical examination on admission was generally normal, except for jaundice. Mild tenderness was only noted in the epigastric area. Initial laboratory data showed white blood cell count of 4.88×103/mm3 (polymorphonuclear neutrophils, 57.2%; lymphocytes, 36.4%; and eosinophils, 1.5%), elevated serum total bilirubin level of 12.3 mg/dL, serum aspartate aminotransferase (AST) level of 1,637 IU/L, serum alanine aminotransferase (ALT) level of 1,949 IU/L, random glucose level of 220 mg/dL and HbA1c level of 10.8%. Hepatitis B surface (HBs) antigen, immunoglobulin M (IgM) anti-hepatitis B core antigen, anti-hepatitis C virus (HCV), HCV RNA (RT PCR) were all negative with positive anti-HBs. As a result of IgM anti-hepatitis A virus (HAV) was negative with positive immunoglobulin G (IgG) anti-HAV, acute hepatitis A could be excluded. Abdominal computed tomography showed findings compatible with secondary changes in acute hepatitis, and fatty infiltration with splenomegaly, which implied concurrent alcoholic liver disease. Seven days after admission, results of IgM anti-HEV and IgG anti-HEV were both positive, the optical density value of IgM anti-HEV of 3.656 (cut-off value: 0.276) and IgG anti-HEV of 3.384 (cut-off value: 0.375), which confirmed the diagnosis of acute viral hepatitis E. IgM anti-HEV and IgG anti-HEV were measured by a commercial immunoassay (HEV IgM and HEV IgG ELISA, Genelabs Diagnostic Pte. Ltd, Singapore). The serum total bilirubin peaked at 24.3 mg/dL and rapidly decreased. The levels of AST and ALT were highest at the time of admission and then showed a rapid decrease. Twenty-three days after admission, the patient was discharged with a total bilirubin of 8.06 mg/dL, AST of 130 IU/L and ALT of 133 IU/L (Fig. 1). Two months after discharge, IgM anti-HEV and IgG anti-HEV were both still positive with the optical density value of IgM anti-HEV of 3.315 (cut-off value: 0.282) and IgG anti-HEV of 2.753 (cut-off value: 0.375). Diagnosis of hepatitis E was confirmed by the detection of both IgM and IgG anti-HEV in serial samples and by the detection of serum HEV RNA.
Figure 1.
Patient's clinical course with changes in ALT, TB and results of IgM / IgG anti-HEV. ALT, alanine aminotransferase; TB, total bilirubin; HEV, hepatitis E virus.
Detection of HEV genome in patient's serum
Viral RNA was extracted from 140 µL of anti-HEV IgM-positive serum in phosphate-buffered saline using a QIAamp viral RNA mini-kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Purified RNA was used to generate the ORF2 of HEV using One-Step RT-PCR with a PLATINUM Taq Kit (Takara, Shiga, Japan). Briefly, RT-PCR was performed on 5 µL of purified RNA from serum in 50 µL of 2× reaction mix with 0.2 µM each primer (forward: 5' aggttggcgctctgtcgaga-3'; reverse: 5'-acagtcggctcgccattggc-3'). Reverse transcription was performed for 30 min at 50℃ and for 2 min at 94℃ followed by 40 cycles of 94℃ for 30s, 55℃ for 30s and 72℃ for 2 min, and a final extension step of 10 min at 72℃. The second round of PCR was performed under the same conditions as the first-round PCR with 0.2 µM each of the same primers.8
Phylogenetic analysis
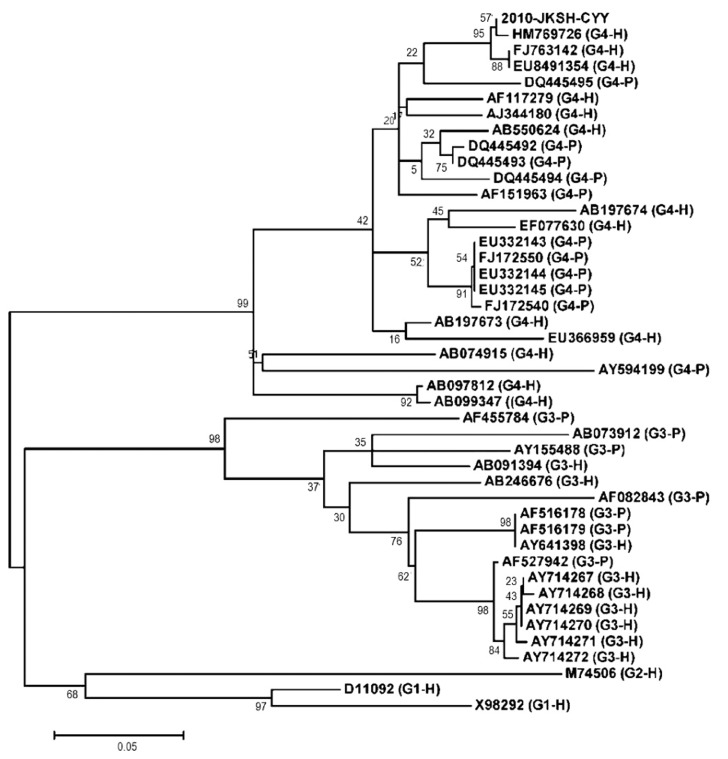
The relationship between the sequences is shown in the dendrogram. The length of each pair of branches represents the distance between the sequences. Phylogenetic trees were constructed by the neighbor-joining method based on the ORF2 sequence. All strains were separated into four groups according to their genotypes. Sequence analysis was conducted using the software VectorNTI and MEGA 4.1 beta. Bootstrap values are indicated for the major nodes as a percentage of the data obtained from 1000 resamplings.
We identified genotype 4 human HEV from the 43-year-old Korean male patient with acute hepatitis, who had never been abroad. He was negative for serum markers of hepatitis A, B and C viruses and positive for anti-hepatitis E virus as IgM class leading to the diagnosis of hepatitis E, whose ORF2 nucleotide sequence from the patient was isolated. The identified Korean HEV strain named 2010-JKSH-CKK (HM769726) belonged to genotype 4, based on comparison with previously reported HEV strains (Table 1, Fig. 2). When comparing the ORF2 region, HM769726 was closely related to the two strains EU849134 and FJ763142 with a nucleotide similarity of 97 and 98%, respectively (Table 1). The two human strains by HEV genome from Korean patients, who had never been aboard, were isolated in Gyeonggi-do, Korea. Among swine HEV isolates, HM769726 was closely related to EU332143-45 from Heilongjiang, China (Table 1).9
Table 1.
Comparison of the HEV isolates obtained in the present study (2010-JKSH-CYY) with 42 human and swine HEV reference strains, based on ORF2 sequence
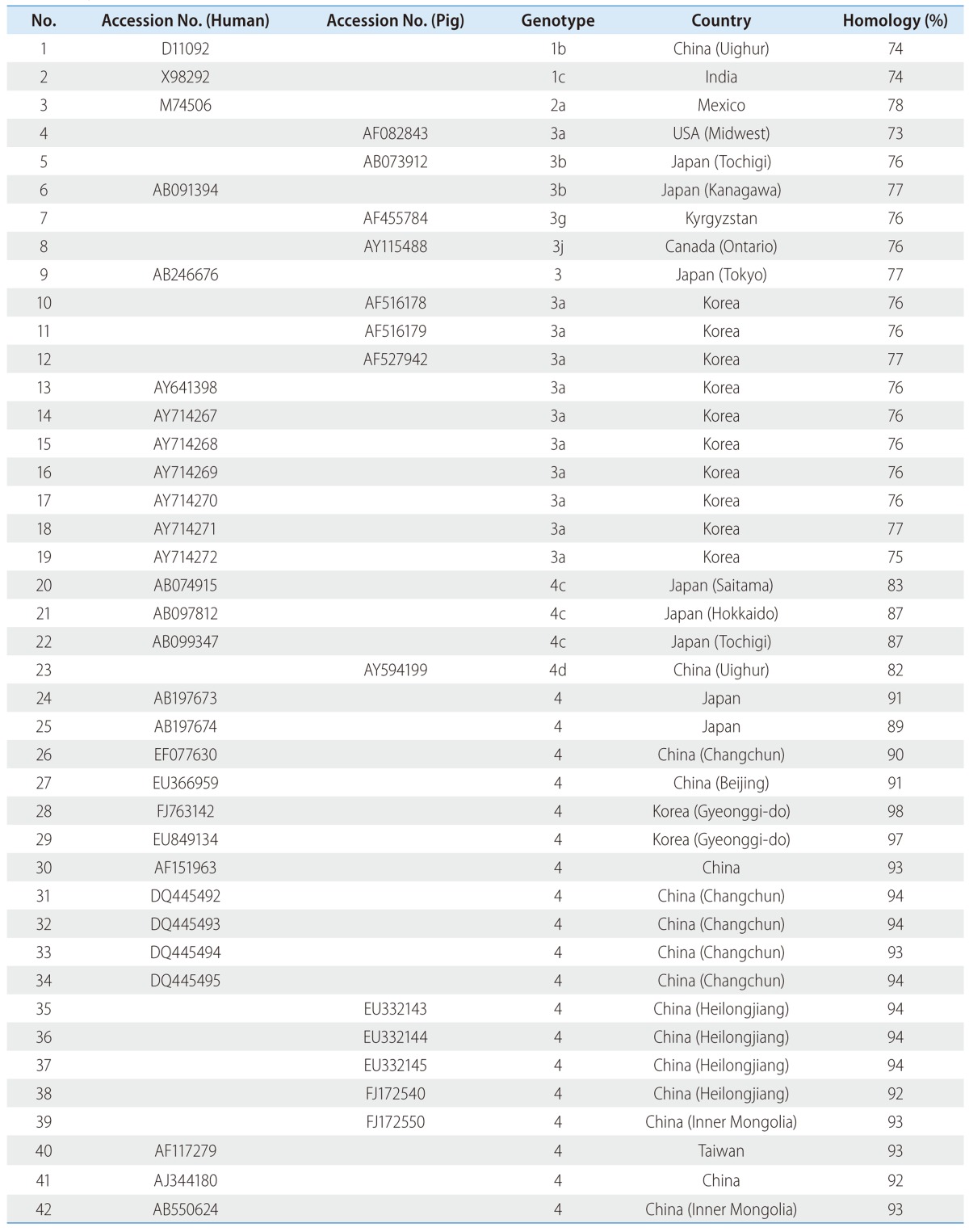
Figure 2.
A phylogenetic tree constructed by the neighbor-joining method based on the ORF2 sequence of the Korean HEV in question, 2010-JKSH-CYY (HM769726), and 42 HEV reference strains with genotypes 1 - 4 (G1-G4). All strains were separated into four groups according to their genotype. H: isolated from humans; P: isolated from pigs. Sequence analysis was conducted using the software VectorNTI and MEGA 5.04. Bootstrap values are indicated for the major nodes as a percentage of the data obtained from 1000 resamplings.
DISCUSSION
In 1997, an animal strain of HEV was isolated from pig, characterized as swine HEV, which was revealed to be genetically associated with human HEV in the United States.6 Subsequently, a number of swine HEVs were isolated and proven to be closely associated genetically with human HEV.10,11 Unlike genotype 1 and 2 HEV, genotype 3 and 4 HEV are isolated from both human and animals. Recently, sporadic cases of genotype 3 and 4 hepatitis E has been reported in non endemic area, such as France,12 Germany,13,14 and Japan.6,11,15,16 In Germany, an autochthonous genotype 3 HEV infection was reported even in a 5-month-old female child.14 In Japan where raw fish and uncooked or undercooked meat are part of the traditional diet, there have been some case reports of zoonotic food-borne transmission, though domestic pig, wild boar and wild deer are the main routes for autochthonous HEV infection.10,11 For example, in 2003, hepatitis E was diagnosed in a family who had previously ingested raw meat of a wild roe deer. HEV genotype 3 was isolated from the remnant raw meat and the nucleotide sequence homology of HEV was verified between the family and the meat.7 Following this event, there were some published articles about zoonotic transmission of HEV in Japan.16
In South Korea, There have been case reports of HEV with some reports of HEV serologically diagnosed since 2002; however, only three of them including our case could detect serum HEV RNA (Table 2).17-20 In the interpretation of HEV serological test, we should consider the possibility of false-positive result especially in nonendemic areas. The detection of IgM anti-HEV in serial samples as well as serum or stool HEV RNA detection is helpful to confirm diagnosis.21 Two Korean cases of genotype 4 hepatitis E reported in 2010 and 2011 are similar to our case with a nucleotide similarity of 97 and 98%, respectively.8,22 Our case is the first human case of autochthonous genotype 4 hepatitis E from a roe deer, while most of the HEV infections previously reported were by zoonotic transmission from a domestic pig or a wild boar in South Korea. Accumulating data supports the notion that zoonotic transmission is the main route of autochthonous infection of HEV, which is an increasingly interesting issue.
Table 2.
Reported human cases of hepatitis E in Korea
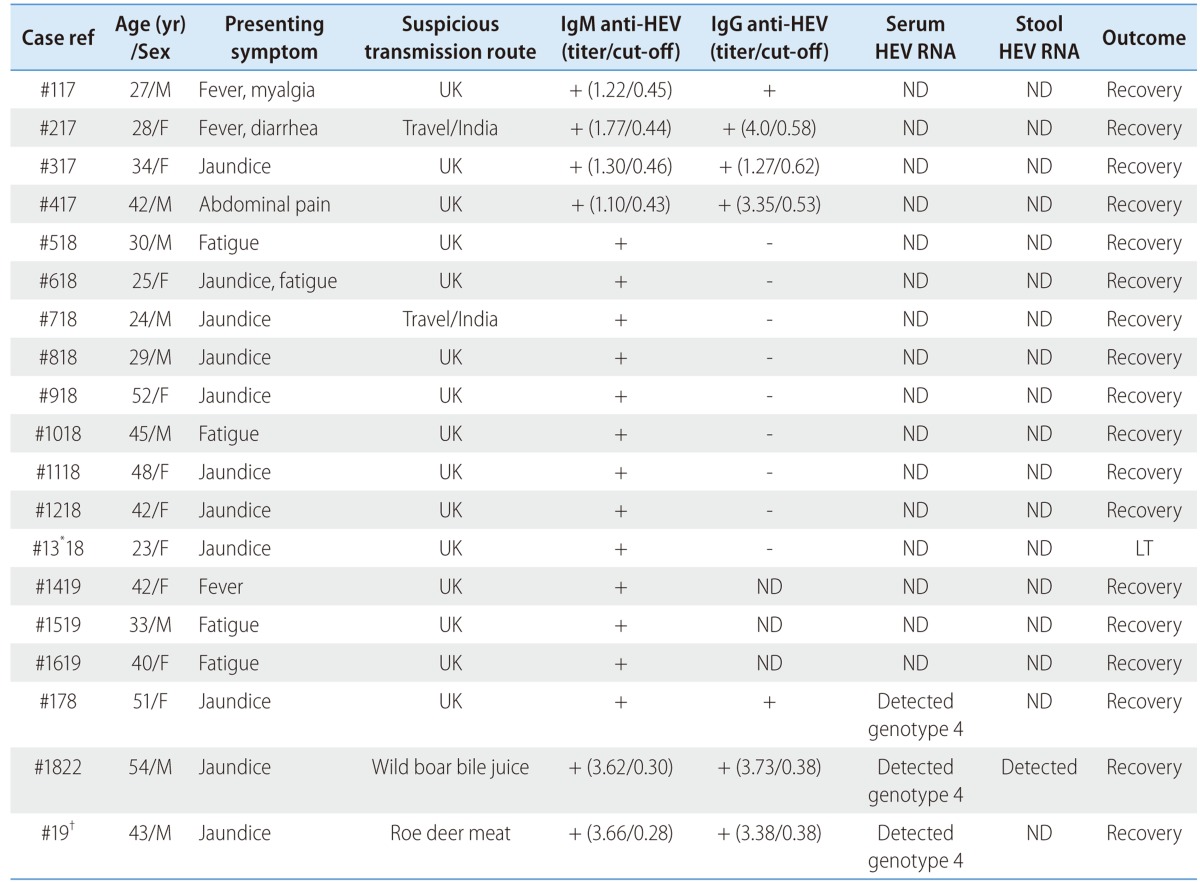
Ref, reference; HEV, hepatitis E virus; UK, unknown; ND, not detected; LT, liver transplantation.
*underlying autoimmune hepatitis.
†present case.
Even with an increasing interest in acute hepatitis E, HEV is still regarded as an infrequent cause of acute viral hepatitis. Perhaps this is because most asymptomatic patients with merely elevated levels of aminotransferase receive supportive care without a definitive diagnosis, which can possibly underestimate the incidence of hepatitis E. Therefore, physicians should be on the alert for HEV infection in patients with acute hepatitis of unknown etiology in developed countries. Furthermore, it is important to check whether they intake the raw meat of wild animals in patients without a travel history of HEV endemic area.
Abbreviations
- HEV
hepatitis E virus
- LT
liver transplantation
Footnotes
The authors have no conflicts to disclose.
References
- 1.van Cuyck-Gandré H, Zhang HY, Tsarev SA, Clements NJ, Cohen SJ, Caudill JD, et al. Characterization of hepatitis E virus (HEV) from algeria and chad by partial genome sequence. J Med Virol. 1997;53:340–347. doi: 10.1002/(sici)1096-9071(199712)53:4<340::aid-jmv5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Arankalle VA, Chobe LP, Joshi MV, Chadha MS, Kundu B, Walimbe AM. Human and swine hepatitis E viruses from western india belong to different genotypes. J Hepatol. 2002;36:417–425. doi: 10.1016/s0168-8278(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 3.Shukla P, Chauhan UK, Naik S, Anderson D, Aggarwal R. Hepatitis E virus infection among animals in northern india: An unlikely source of human disease. J Viral Hepat. 2007;14:310–317. doi: 10.1111/j.1365-2893.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 4.Kwo PY, Schlauder GG, Carpenter HA, Murphy PJ, Rosenblatt JE, Dawson GJ, et al. Acute hepatitis E by a new isolate acquired in the united states. Mayo Clin Proc. 1997;72:1133–1136. doi: 10.4065/72.12.1133. [DOI] [PubMed] [Google Scholar]
- 5.Kabrane-Lazizi Y, Zhang M, Purcell RH, Miller KD, Davey RT, Emerson SU. Acute hepatitis caused by a novel strain of hepatitis E virus most closely related to united states strains. J Gen Virol. 2001;82:1687–1693. doi: 10.1099/0022-1317-82-7-1687. [DOI] [PubMed] [Google Scholar]
- 6.Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- 8.Yun H, Kim JS, Lee HJ, Jeong SH, Kim JS, Park SJ, et al. The complete genome sequence and molecular analysis of human hepatitis E virus genotype IV identified from a korean patient. Arch Virol. 2010;155:1003–1008. doi: 10.1007/s00705-010-0661-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhu G, Qu Y, Jin N, Sun Z, Liu T, Lee H, et al. Seroepidemiology and molecular characterization of hepatitis E virus in jilin, china. Infection. 2008;36:140–146. doi: 10.1007/s15010-007-7130-8. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda H, Okada K, Takahashi K, Mishiro S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J Infect Dis. 2003;188:944. doi: 10.1086/378074. [DOI] [PubMed] [Google Scholar]
- 11.Tamada Y, Yano K, Yatsuhashi H, Inoue O, Mawatari F, Ishibashi H. Consumption of wild boar linked to cases of hepatitis E. J Hepatol. 2004;40:869–870. doi: 10.1016/j.jhep.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Tessé S, Lioure B, Fornecker L, Wendling MJ, Stoll-Keller F, Bigaillon C, et al. Circulation of genotype 4 hepatitis E virus in europe: First autochthonous hepatitis E infection in france. J Clin Virol. 2012;54:197–200. doi: 10.1016/j.jcv.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Brost S, Wenzel JJ, Ganten TM, Filser M, Flechtenmacher C, Boehm S, et al. Sporadic cases of acute autochthonous hepatitis E virus infection in southwest germany. J Clin Virol. 2010;47:89–92. doi: 10.1016/j.jcv.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Krüttgen A, Scheithauer S, Hausler M, Kleines M. First report of an autochthonous hepatitis E virus genotype 3 infection in a 5 month old female child in germany. J Clin Virol. 2011;50:175–176. doi: 10.1016/j.jcv.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Ishida S, Yoshizumi S, Miyoshi M, Okui T, Ishida A, Abe S, et al. A cluster of hepatitis E virus infection in hokkaido, japan. Jpn J Infect Dis. 2006;59:135–136. [PubMed] [Google Scholar]
- 16.Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, et al. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol. 2003;84:2351–2357. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- 17.Jeong SH. Current status of hepatitis e virus infection in Korea. Gut Liver. 2011;5:427–431. doi: 10.5009/gnl.2011.5.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim JW, Park CS, Ahn JM, Yu MH, Kim TS, Lim YS, et al. Nine cases of sporadic acute hepatitis E in Korea. Korean J Hepatol. 2006;12:230–236. [PubMed] [Google Scholar]
- 19.Kim DH, Park H, Moon SW, Jeong JH, Yang HS, Kim do H, et al. Three sporadic cases of acute hepatitis E. Korean J Gastroenterol. 2007;50:121–125. [PubMed] [Google Scholar]
- 20.Kim SE, Kim MY, Kim DG, Song YJ, Jeong HJ, Lee SW, et al. Determination of fecal shedding rates and genotypes of swine hepatitis E virus (HEV) in Korea. J Vet Med Sci. 2008;70:1367–1371. doi: 10.1292/jvms.70.1367. [DOI] [PubMed] [Google Scholar]
- 21.Jang JH, Jung YM, Kim JS, Lee SH, Kim JW, Hwang SG, et al. Coexistence of IgM antihepatitis A virus and IgM antihepatitis E virus in acute viral hepatitis: a prospective, multicentre study in Korea. J Viral Hepat. 2011;18:e408–e414. doi: 10.1111/j.1365-2893.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim YM, Jeong SH, Kim JY, Song JC, Lee JH, Kim JW, et al. The first case of genotype 4 hepatitis E related to wild boar in South Korea. J Clin Virol. 2011;50:253–256. doi: 10.1016/j.jcv.2010.11.005. [DOI] [PubMed] [Google Scholar]