Abstract
Background
Intrathoracic impedance monitoring has emerged as a promising new technique for the detection of impending heart failure (HF). Although false positive episodes have been reported in case reports and clinical trials, the efficacy and false positive rate in real-world practice remain unclear.
Objective
The aim of this study is to investigate the utility and reliability of the OptiVol alert feature in clinical practice.
Methods
We continuously recruited patients who underwent implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy with defibrillator (CRT-D) implantation with feature of intrathoracic impedance monitoring system in our center from Sep. 2010 to Oct. 2012. Regular in-office follow-up were required of all patients and the following information was collected at each visit: medical history, device interrogation, N-terminal pro-brain natriuretic peptide (NT-proBNP) measurement and an echocardiogram. Worsening HF was defined as hospitalization or the presentation of signs or symptoms of HF.
Results
Forty three patients (male: 76.7%, mean age: 57 ± 15 years, left ventricular ejection fraction (LVEF): 33% ± 14%) were included in this observational study. Fifty four alert events and 14 adjudicated worsening HF were detected within 288 ±163 days follow-up. Eleven (20.4%) alert episodes were associated with acute cardiac decompensation in 9 patients with a positive predictive value of 78.6%. Forty three audible alerts showed no connection to worsening HF. The unexplained alerts rate was 79.6% and 1.27 per person-year. Thirty seven alarm alerts were detected in patients with EF < 45%, among which 9 accompanied with HF, 17 alerts detected in patients with LVEF ≥ 45% and 2 associated with HF. There was no significant difference between the two groups (9/37 vs. 2/17; P = 0.47).
Conclusions
Patients with normal or nearly normal left ventricular systolic function also exhibited considerable alert events. The OptiVol fluid index predicted worsening cardiac events with a high unexplained detection rate, and any alert must therefore be analyzed with great caution. Efforts to improve the specificity of this monitoring system represent a significant aspect of future studies.
Keywords: Heart failure, Intrathoracic impedance measurement, OptiVol fluid index, Left ventricular ejection fraction
1. Introduction
Congestive heart failure (CHF) is a major clinical syndrome and the ultimate result of multiple cardiovascular diseases. It is estimated that approximately 6.6 million people over the age of 18 years and 10 per 1000 people over the age of 65 years have heart failure in the US.[1] Despite the great advances that have been achieved in medication and instrument therapies, heart failure remains a challenging medical issue with substantial morbidity and mortality. The factors most often responsible for causing exacerbations of heart failure are fluid accumulation and water retention. In recent years, a new device-based method known as intrathoracic impedance measurement has been developed and integrated into implantable cardioverter-defibrillator (ICD) or with cardiac resynchronization therapy-defibrillator (CRT-D). Animal studies have demonstrated that changes in intrathoracic impedance are inversely correlated with ventricular filling pressure and the extravascular lung water index.[2]–[4] Clinical trials have suggested that the OptiVol alert feature could identify impending cardiac decompensation and reduce hospitalization.[5]–[8]
However, previous studies had enrolled patients with left ventricular dysfunction. In fact, many patients who underwent ICD implantation with normal left ventricular systolic function and super responders to CRT-D exhibited complete recovery in cardiac dimension and ventricular ejection function. Data with regard to the utility of this system in clinical practice are rare, especially data concerning the relationship between alert episodes and left ventricular function, which has not been previously described. The purpose of this study was to investigate the usefulness and reliability of the OptiVol alert feature in real practice.
2. Methods
2.1. Patients
We prospectively and continuously recruited patients who had received either a CRT-D (InSync Marquis 7298; Concerto C174AWK, Medtronic Inc, Minneapolis, MN, US) or an ICD (Virtuoso VR D164VWC; Virtuoso DR D164AWG, Medtronic Inc, Minneapolis, MN, US) implantation in our single center from Sep. 2010 to Oct. 2012. Patients who underwent CRT-D placement were with left ventricular ejection fraction (LVEF) ≤ 35%, sinus rhythm, QRS duration ≥120 ms, and with New York Heart Association (NYHA) class III-IV under optimal medical therapy for at least 3 months. Patients with reduced and preserved ejection fraction (EF) were implanted with ICD for primary or secondary prevention of sudden cardiac death (SCD).[9] Those patients with a life expectancy of less than one year or those having difficulty in completing follow-up were excluded. The institutional review board approved the study strategy and all patients gave their formal consent.
2.2. Device implantation and patient follow-up
Devices were successfully implanted by transvenous pathway in all cases. Retrograde coronary venography was conducted to determine the target vein. The left ventricular lead was implanted in the lateral, posterolateral, posterior, and the anterolateral vein in the preferred sequence. The right atrial lead was placed in the auricle and the right ventricular lead in the right ventricular apex. The pacing system was embedded in the left upper pectoral region. All patients were required to return for in-clinic follow-up in 3, 6, and 12 months, and every 12 months after that. Once an audible alert was reported, an unscheduled visit was arranged. During each visit, the patient's medical history and any signs and symptoms of HF were carefully evaluated. The parameters of echocardiography were collected from the parasternal long-axis view. LVEF was calculated by using biplane Simpson's method. Device interrogation and NT-proBNP measurement were carried out during follow-up. Worsening heart failure was defined as hospitalization or presenting signs or symptoms of HF in accordance with guideline recommendations.[10]
A two-week period of closely self-monitoring without medication adjustment was recommended in patients with a detectable alert and without evidence of decompensation. Agent modification was administered either for taking control of cardiac worsening or for terminating an alarm which had lasted over two weeks. Any alert without proof of cardiac decompensation was defined as an unexplained event.
2.3. Impedance measurement
The mechanism and the detailed process for intrathoracic impedance measurement have been specifically described in several papers.[5],[6],[11] In brief, the device can release a test impulse between the right ventricular defibrillating coil and the device case from noon to 5 p.m. in a day. The impedance between the two sites is measured and calculated as the actual impedance. Thirty four days after implantation, the reference impedance is initialized by averaging the measurements of the last 4 days. From then on, the reference impedance is calculated automatically by tracking the impedance trends and is compared to the actual measurement. Difference between the measured data and the ambulatory reference data are collected and the fluid index (FI) is calculated automatically. Once the OptiVol FI surpasses the reference threshold which is nominally set to 60Ω-d, an audible alert will be triggered.
2.4. Statistics
Data were analyzed using SPSS 18.0 statistical software (SPSS Institute, Chicago, IL, USA). Quantitative data were expressed as the mean ± SD. Qualitative data were presented as percentages and analyzed using the Fisher's exact test. P < 0.05 was considered statistically significant.
3. Results
There were 43 patients (male: 76.7%, mean age: 57 ± 15 years, LVEF: 33% ± 14%) enrolled in this observational study. Mean follow-up duration was 288 ± 163 days (90∼730 days). At baseline, 21 patients undertwent CRT-D implantation and 22 patients received ICD deployment. Seventeen patients with reduced LVEF and 5 patents with preserved systolic function received ICD. Patient demographics are listed in Table 1.
Table 1. Baseline patient characteristics.
| Items | Number or Percentage |
| Age (yrs) | 57 ± 15 |
| Males | 76.7% |
| LVEF | 33% ± 14% |
| Patients with LVEF < 35% | 76.7% |
| Patients with LVEF ≥ 45% | 16.2% |
| New York Heart Association class | |
| III or IV | 69.8% |
| I or II | 30.2% |
| Heart disease etiology | |
| Ischemic heart disease | 48.8% |
| Dilated cardiomyopathy | 39.6% |
| LQTS | 2.3% |
| ARVC | 2.3% |
| Idiopathic VT/VF | 7% |
| Implanted device | |
| CRT-D | 48.8% |
| ICD | 51.2% |
| SCD prevention | |
| Primary prevention | 60.5% |
| Secondary prevention | 39.5% |
| Medication | |
| Βeta blockers | 100% |
| ACEI/ARB | 83.7% |
| Digoxin | 72.1% |
| Diuretics | 86% |
| Aldosterone antagonist | 88.4% |
ACEI/ARB: angiotensin converting enzyme inhibitor/angiotensin receptor blocker; ARVC: arrhythmogenic right ventricular cardiomyopathy; CRT-D: cardiac resynchronization therapy-defibrillator; ICD: implantable cardioverter-defibrillator; LQTS: long QT syndrome; LVEF: left ventricular ejection fraction; SCD: sudden cardiac death; VT/VF: ventricular tachycardia/ventricular fibrillation.
Although a regular follow-up schedule was created for each patient, 13 patients failed to appear for periodic interviews. Only 69.7% of patients performed regular follow-up. In addition, 46.3% of alert events could not be perceived or reported by the patients due to hearing disorders, low alarm volume or a short alarm period. The relationship between undetected alerts and cardiac worsening was assessed retrospectively via medical history investigation.
A total of 54 threshold-crossing episodes and 14 heart failure worsening events were observed. Through detailed medical history investigations or clinical symptoms and plasma NT-proBNP evaluations, 11 alert episodes had been found to be associated with acute cardiac decompensation in 9 patients. The sensitivity of the alert episodes was 78.6% and the positive predictive value was 20.4%. Forty three threshold-crossing events showed no relation to an acute worsening episode. The unexplained events rate was 79.6% and 1.27 per person-year (Figure 1).
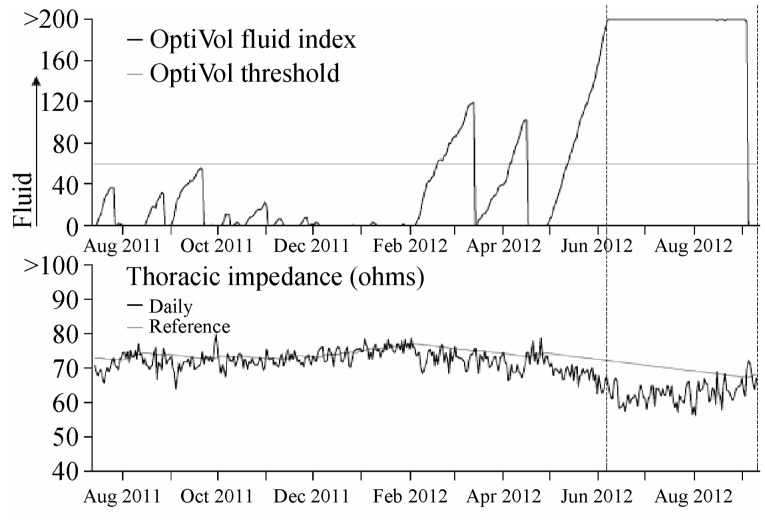
Figure 1. Thoracic impedance and OptiVol FI trend recording.
OptiVol fluid index is an accumulation of the difference between the daily and reference impedance.
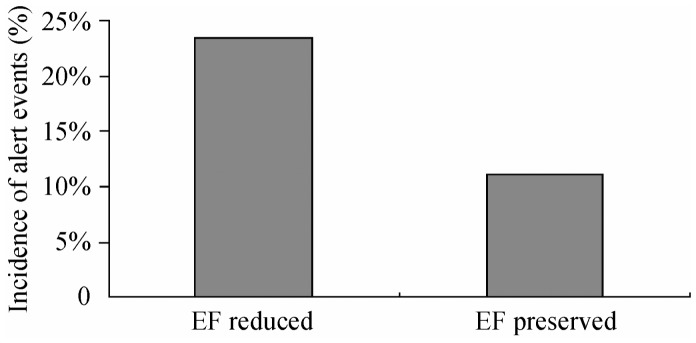
Fifty four threshold-crossing events occurred in 26 patients, of whom 17 patients had LVEF < 45% and 9 had LVEF ≥ 45% (2 were preserved and 7 were due to restored EF). The incidence of alert events in relation to cardiac decompensation in the reduced EF group was up to 47.1% (8 in 17) and was 11.1% (1 in 9) in the preserved EF group. Although the proportion was apparently higher in the decreased EF group, there was a substantial number of patients in the normal EF group also presenting with alert events. Thirty seven alarms were detected in patients with EF < 45%, among which 9 were accompanied by HF, and 17 alerts were detected in patients with LVEF ≥ 45%, of which 2 were associated with HF. There was no significant difference between the two groups (24.3% vs. 11.8%, P = 0.47), (Figure 2).
Figure 2. Incidence of alert events in relation to heart failure in different ejection fraction (EF) groups.
This recording was from a patient diagnosed as arrhythmic right ventricular cardiomyopathy (ARVC) without evidence of HF. As shown here, the decrease in thoracic impedance was accompanied by an increase in OptiVol FI. There were three threshold-crossing events but none with evidence of overt heart failure, so there was no medication adjustment in this patient.
There was no significant difference in the alert rate between the reduced EF and the preserved EF group (P = 0.47).
4. Discussion
In this study, we found that the intrathoracic impedance monitoring system had a high sensitivity, but also a substantial false positive rate. Patients with preserved or restored EF could also present with an OptiVol alarm without an HF event.
Animal studies and clinical trials have provided evidence of the usefulness of intrathoracic impedance monitoring in the detection cardiac worsening,[5],[12] with sensitivity ranging between 76%–83%.[5],[13],[14] Similar to the early studies, we found that the sensitivity was 78.6%, which suggested that OptiVol FI could serve as a useful tool in managing HF patients. Timely interventions may avoid subsequent worsening events.
The practicality of impedance monitoring remains controversial, however. Van Veldhuisen, et al.[15] reported that impedance monitoring and the alert function did not improve the prognosis of HF patients, but that they did increase hospitalizations and outpatient visits. Ypenburg, et al.[16] found that the OptiVol FI alert was not specific in predicting HF. Only 33% of the alarm events associated with cardiac worsening events in patients received CRT-D implantation. Until recently, nearly all of the published data regarding impedance monitoring related to patients with left ventricular malfunction. In clinical practice, patients who undergo devices placement may present with preserved or improved left ventricular function.
In the current investigation, we mainly focused on the relationship between LVEF and OptiVol alert events by recruiting patients with preserved and restored EF. During follow-up, 16.3% of patients exhibited improvement in left ventricular function. OptiVol alerts were observed in patients with preserved and improved EF. Of the 17 episodes observed in nine patients with normal EF, only two alerts were proved to be associated with a cardiac exacerbation in patients with previously symptomatic HF. No difference was observed in the alert rate in preserved EF group and the decreased EF group (P = 0.47). Previously, individual cases have been published regarding false alerts.[17]–[19] We report that a large number of patients with normal left ventricular systolic function could also display unexplained alerts in clinical practice. The positive predictive value of the impedance measurement feature in the diagnosis of worsening HF was 20.4%. This value is far below those reported previously.[20] In addition, the number of unexplained threshold crossing events was much higher in this study comparing with previous studies, Catanzariti, et al.[8] reported 0.25 per patient-year. The difference may be attributed to restrictive patient selection in these studies. In our patient cohort, the false positive rate was 79.6% and 1.27 per person-year.
Nevertheless, intrathoracic impedance monitoring still represents a new and promising way to detect acute cardiac deterioration, despite the pitfalls. However, device-based diagnostics could not take the place of careful clinical evaluation, but could be helpful as an adjunct tool. Attempts to improve the specificity of this system have been reported, including resetting the alarm threshold,[14] combining of multiple device diagnostics into the analysis[21] and modification of the algorithm of intrathoracic impedance measurement.[22] The effectiveness of these methods, however, needs to be tested further in future studies.
4.1. Limitations
This was a single center study with limited cases. A proportion of the patients failed to comply with regular follow-up. Many of the alerts were neglected and were not reported to the physician. Retrospective investigation was applied in this setting to determine the correlation between alert events and clinical worsening events. This condition might potentially influence the conclusions drawn from inaccurate patient memory. In addition, we did not further investigate for the causes of false alert events.
4.2. Conclusions
OptiVol FI predicted the acute worsening of heart failure with high sensitivity and a high rate of unexplained events. Any alert event must be analyzed carefully. Efforts to improve the specificity of this monitoring system represent a significant requirement for future studies.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:E2–E220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Lahtinen S, Lentz L, et al. et al. Feasibility of using an implantable system to measure thoracic congestion in an ambulatory chronic heart failure canine model. Pacing Clin Electrophysiol. 2005;28:404–411. doi: 10.1111/j.1540-8159.2005.40009.x. [DOI] [PubMed] [Google Scholar]
- 3.Ganion V, Rhodes M, Stadler RW. Intrathoracic impedance to monitor heart failure status: a comparison of two methods in a chronic heart failure dog model. Congest Heart Fail. 2005;11:177–181, 211. doi: 10.1111/j.1527-5299.2005.04443.x. [DOI] [PubMed] [Google Scholar]
- 4.Becher J, Kaufmann SG, Paule S, et al. et al. Device-based impedance measurement is a useful and accurate tool for direct assessment of intrathoracic fluid accumulation in heart failure. Europace. 2010;12:731–740. doi: 10.1093/europace/eup413. [DOI] [PubMed] [Google Scholar]
- 5.Yu CM, Wang L, Chau E, et al. et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–848. doi: 10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 6.Luthje L, Drescher T, Zenker D, et al. et al. Detection of heart failure decompensation using intrathoracic impedance monitoring by a triple-chamber implantable defibrillator. Heart Rhythm. 2005;2:997–999. doi: 10.1016/j.hrthm.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Small RS, Wickemeyer W, Germany R, et al. et al. Changes in intrathoracic impedance are associated with subsequent risk of hospitalizations for acute decompensated heart failure: clinical utility of implanted device monitoring without a patient alert. J Card Fail. 2009;15:475–481. doi: 10.1016/j.cardfail.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Catanzariti D, Lunati M, Landolina M, et al. et al. Monitoring intrathoracic impedance with an implantable defibrillator reduces hospitalizations in patients with heart failure. Pacing Clin Electrophysiol. 2009;32:363–370. doi: 10.1111/j.1540-8159.2008.02245.x. [DOI] [PubMed] [Google Scholar]
- 9.Epstein AE, DiMarco JP, Ellenbogen KA, et al. et al. ACC/AHA/ HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. Heart Rhythm. 2008;5:e1–e62. doi: 10.1016/j.hrthm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Dickstein K, Cohen-Solal A, Filippatos G, et al. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 11.Wang L. Fundamentals of intrathoracic impedance monitoring in heart failure. Am J Cardiol. 2007;99:3G–10G. doi: 10.1016/j.amjcard.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Maines M, Catanzariti D, Cemin C, et al. et al. Usefulness of intrathoracic fluids accumulation monitoring with an implantable biventricular defibrillator in reducing hospitalizations in patients with heart failure: a case-control study. J Interv Card Electrophysiol. 2007;19:201–207. doi: 10.1007/s10840-007-9155-4. [DOI] [PubMed] [Google Scholar]
- 13.Abraham WT, Compton S, Haas G, et al. et al. Intrathoracic impedance vs daily weight monitoring for predicting worsening heart failure events: results of the Fluid Accumulation Status Trial (FAST) Congest Heart Fail. 2011;17:51–55. doi: 10.1111/j.1751-7133.2011.00220.x. [DOI] [PubMed] [Google Scholar]
- 14.Soga Y, Ando K, Arita T, et al. et al. Efficacy of fluid assessment based on intrathoracic impedance monitoring in patients with systolic heart failure. Circ J. 2011;75:129–134. doi: 10.1253/circj.cj-10-0730. [DOI] [PubMed] [Google Scholar]
- 15.van Veldhuisen DJ, Braunschweig F, Conraads V, et al. et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–1726. doi: 10.1161/CIRCULATIONAHA.111.043042. [DOI] [PubMed] [Google Scholar]
- 16.Ypenburg C, Bax JJ, van der Wall EE, et al. et al. Intrathoracic impedance monitoring to predict decompensated heart failure. Am J Cardiol. 2007;99:554–557. doi: 10.1016/j.amjcard.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 17.Kramer DB, Maisel WH. An unusual cause of abnormal intrathoracic impedance in a patient with arrhythmogenic right ventricular cardiomyopathy. Pacing Clin Electrophysiol. 2011;34:E60–E63. doi: 10.1111/j.1540-8159.2010.02774.x. [DOI] [PubMed] [Google Scholar]
- 18.Turkoglu C, Aliyev F, Celiker C, et al. et al. An unusual cause of OptiVol alarm: increased intra-abdominal pressure associated with irritable bowel syndrome. Turk Kardiyol Dern Ars. 2009;37:403–406. [PubMed] [Google Scholar]
- 19.Timperley J, Mitchell AR, Brown P, et al. et al. Changes in intrathoracic impedance from a pneumothorax: insights from an implanted monitoring system. Pacing Clin Electrophysiol. 2005;28:1109–1111. doi: 10.1111/j.1540-8159.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 20.Maines M, Catanzariti D, Cirrincione C, et al. et al. Intrathoracic impedance and pulmonary wedge pressure for the detection of heart failure deterioration. Europace. 2010;12:680–685. doi: 10.1093/europace/eup419. [DOI] [PubMed] [Google Scholar]
- 21.Whellan DJ, Ousdigian KT, Al-Khatib SM, et al. et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol. 2010;55:1803–1810. doi: 10.1016/j.jacc.2009.11.089. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar S, Hettrick DA, Koehler J, et al. et al. Improved algorithm to detect fluid accumulation via intrathoracic impedance monitoring in heart failure patients with implantable devices. J Card Fail. 2011;17:569–576. doi: 10.1016/j.cardfail.2011.03.002. [DOI] [PubMed] [Google Scholar]