Abstract
The flow properties of blood play significant roles in tissue perfusion by contributing to hydrodynamic resistance in blood vessels. These properties are influenced by pathophysiological processes, thereby increasing the clinical relevance of blood rheology information. There is well-established clinical evidence for impaired blood fluidity in humans of advanced age, including enhanced plasma and whole blood viscosity, impaired red blood cell (RBC) deformability and enhanced RBC aggregation. Increased plasma fibrinogen concentration is a common finding in many studies owing to the pro-inflammatory condition of aged individuals; this finding of increased fibrinogen concentration explains the higher plasma viscosity and RBC aggregation in elderly subjects. Enhanced oxidant stress in advanced age is also known to contribute to altered blood fluidity, with RBC deformability being an important determinant of blood viscosity. Several studies have shown that physical activity may improve the hemorheological picture in elderly subjects, yet well-designed observational and mechanistic studies are required to determine the specific effects of regular exercise on hemorheological parameters in healthy and older individuals.
Keywords: Aggregation, Deformability, Elderly, Erythrocyte, Red blood cell, Viscosity
1. Introduction
Since the first half of the twentieth century, the contribution of the flow properties of blood to in vivo blood flow and tissue perfusion has been the subject of extensive research.[1] Experimental findings indicating the significant impact of blood fluidity on tissue perfusion, especially in organs with blood vessels affected by atherosclerosis, provide a firm basis for clinical evaluations.[2],[3] A vast number of clinical studies also confirm the relationship between blood fluidity and severity of circulatory problems, although the cause-effect relationship may not always be very obvious. This uncertainty fuels the debate on the exact position of blood fluidity alterations in chronic disease: sub-optimal blood fluidity might contribute to the impairment of tissue perfusion, or alternatively, result from such impairments,[4] thus creating a “chicken or egg” question.[5] Although this controversy places blood rheology onto slippery ground from a clinical point of view, it does not reduce the clinical significance of findings indicating alterations in factors related to blood fluidity. This article reviews the experimental and clinical data relevant to the role of blood rheology in circulatory efficiency, with special emphasis on age-related pathophysiological conditions.
2. Brief definition of flow properties of blood
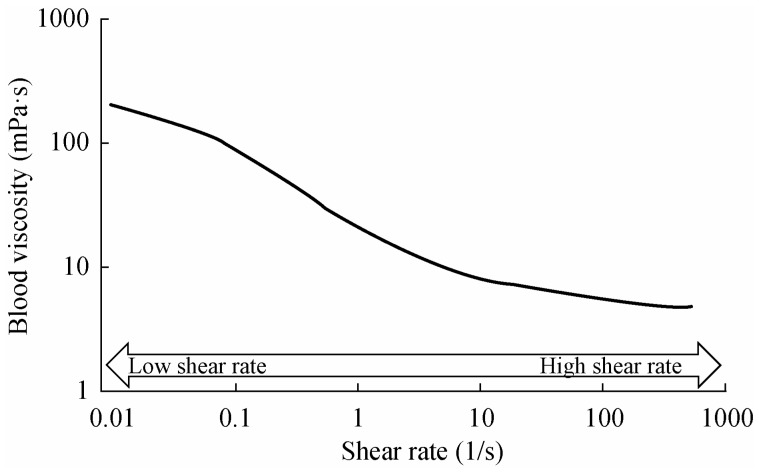
From a physical point of view, blood can be defined as a “non-Newtonian, shear thinning fluid,” reflecting its composition (i.e., a suspension of blood cells in plasma), and the special behavior of red blood cells (RBC) that constitute 99.9% of the cellular elements.[6] As suggested by this physical definition, the viscosity of blood decreases with increasing shear forces (i.e., increasing flow rate in cylindrical tubes or blood vessels). The magnitude of the change in blood viscosity may be on the order of 101 to 102 when measured at flow rates corresponding to different parts of the circulatory system (e.g., arterial vs. venous blood vessels).[7] This relationship explains why blood viscosity approaches a minimum at arterial flow rates in vivo, while it reaches maximum levels in the venous circulation (Figure 1).
Figure 1. The effect of shear rate on whole blood viscosity.
Shear rate is the velocity gradient between adjacent layers of blood, is expressed as 1/s and is proportional to flow rate in a tube (e.g., blood vessel). The lower shear rates correspond to the circulatory conditions in the venous circulation while higher shear rates characterize the arterial vessels. The unit of viscosity is mPa.s, numerically equal to centiPoise (cP); water has a viscosity of about 1 mPa.s at 25°C and is independent of shear rate.
Two distinct properties of RBC contribute to blood viscosity at high and low shear rates (i.e., the velocity gradient during laminar flow which is a function of flow rate in blood vessels): RBC deformability is the determinant of blood viscosity at high shear rates, while low shear viscosity reflects RBC aggregation.[8]
2.1. RBC deformability
RBC are the most “flexible” cells of the mammalian organism: they can change their shape to ellipsoidal structures under the influence of external forces more easily compared to other cells.[9] Such alterations facilitate their orientation to flow stream lines in blood vessels, resulting in decreased blood viscosity. This change in shape and orientation is optimized at high shear rates.[8] The ability of RBC to change shape and orientation when subjected to shear forces is common for all mammalian species and reflects the early evolutionary adaptations related to cell shape and composition.[10] The biconcave-discoid shape and lack of nucleus and organelles are the key features of RBC, which in addition to its specially-organized membrane skeleton, represent the major determinants of the deformability of this cell. The cell membrane skeleton is obviously intrinsically involved in the active regulation of RBC deformability.[11]
2.2. RBC aggregation
In contrast to high-shear behavior, RBC regain their biconcave-discoid shape under low-shear conditions, or at stasis and aggregate into specially-shaped structures resembling a stack of coins (Figure 2). This reversible process is known as rouleaux formation under which these aggregates are dispersed into individual RBC upon increases of shear forces. The aggregates tend to increase the frictional resistance between flow-streamlines and thus elevate blood viscosity under low-shear conditions.[8] RBC aggregation is induced by sufficiently large macromolecules (e.g., proteins, such as fibrinogen), while cellular properties determine the efficiency of a given concentration of such aggregating macromolecules in inducing aggregation.[12] Collectively, the contribution of cellular properties to rouleaux formation defines the aggregability of RBC.[13]
Figure 2. Red blood cell aggregates in autologous plasma.
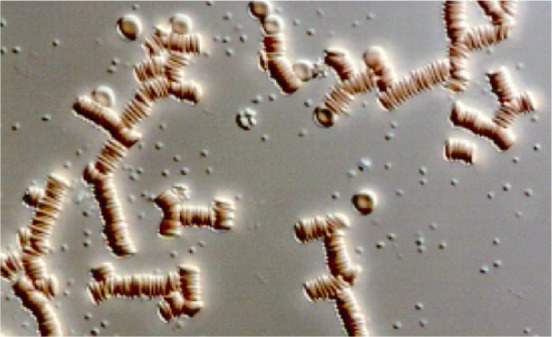
Small round particles are blood platelets.
2.3. Influence of blood rheology on blood flow and tissue perfusion
Earlier experimental studies indicated that the in vivo flow behavior of blood might be quite different from that studied in vitro.[1] Measurements based on in vivo flow resistance indicated a limited influence of blood viscosity, generating much controversy about the significance of blood rheology alterations in terms of tissue perfusion. There are a number of physiological mechanisms to explain the lower apparent viscosity of blood in vivo, including plasma skimming, reduced microvascular hematocrit, the Fahraeus effect, all of which are related to the axial migration of RBC in tube flow, and also the Fahraeus-Lindqvist effect (i.e., decreasing viscosity with decreasing tube diameter). These hemodynamic factors are not discussed here in detail, and further discussions of them can be found elsewhere.[14]–[16] Another important factor that distinguishes in vitro studies from those that are observed in living organs/tissues is vasomotor control (i.e., in vivo changes of vessel diameter), which is an efficient compensator for alterations in tissue perfusion due to pathophysiological alterations, including impaired blood fluidity. It has been demonstrated that experimental abolishment of vasomotor control may result in prominently increased sensitivity of perfused organs/tissues to altered blood fluidity factors (e.g., RBC deformability or aggregation).[2],[15]
3. Blood rheology and disease processes
There is a large collection of data related to alterations of RBC mechanical properties and the flow behavior of blood during disease processes. Full coverage of pathophysiological changes in blood rheology parameters can be found elsewhere,[17]–[20] and thus only a brief discussion is presented below.
Clinical conditions characterized by impaired blood fluidity are known as hyperviscosity syndromes.[18],[21] As the two phases contributing to the composition of blood (i.e., plasma and RBC) are the main determinants of blood viscosity, any changes in the flow properties of plasma and/or RBC directly influence the flow properties of blood. The relative contribution of the cellular content to blood volume (i.e., hematocrit) is also a major determinant of blood viscosity at both low and high shear rates.[6]
3.1. Hematocrit change
Increased RBC mass or decreased plasma volume generally lead to higher levels of hematocrit with consequent increases in whole blood viscosity. A major cause of enhanced RBC production relates to a myoproliferative disease known as polycythemia vera.[22] Increased RBC production may also be secondary to hypoxemia or tissue hypoxia resulting from various primary causes (e.g., cyanotic heart diseases, pulmonary insufficiency).[18] Hematocrit increases occurring secondary to plasma volume changes are known as relative polycythemia; conditions inducing such alterations include endocrine disorders leading to water and salt depletion, capillary leakage (e.g., allergic reactions, anaphylaxis), acute contraction of vascular capacity due to sudden release of vasoactive agents, and iatrogenic causes (e.g., improper diuretic treatment).[18],[23]
3.2. Alteration of plasma composition
The protein content of plasma determines its viscosity, and consequently is a major determinant of blood viscosity.[8] A group of plasma proteins, including fibrinogen, are known as acute phase reactants that are increased during inflammatory reactions. Fibrinogen concentration in inflammatory conditions may rise five-fold, with significant increases in plasma viscosity.[24] The fibrinogen concentration of plasma is also an important determinant of RBC aggregation as discussed in the next section. Increased immunoglobulin concentrations in plasma cell dyscrasias (e.g., multiple myeloma) may reach to very high levels (e.g., 10 g/dL), resulting in serious hyperviscosity and intense RBC aggregation.[25]
3.3. Blood cells
RBC are the dominant blood cells due to their relative population in blood, while white blood cells (WBC) and platelets have non-significant contributions to the fluidity of blood under physiological conditions. WBC may only influence blood fluidity if their number per unit volume of blood reaches extremes (e.g., in leukemic patients).[26] Platelets have prominent influences on thrombotic processes, which might include interference with blood rheology factors.[23] Furthermore, RBC mechanical behavior, such as cellular deformability, is significantly influenced by disease processes.
3.3.1. Alterations of RBC deformability
Significant alterations of RBC deformability often accompany pathophysiological changes of RBC cytoplasmic and membrane properties. Sickle cell disease is an illustrative example of alterations in cytoplasmic viscosity due to a genetic disorder causing a single amino acid change in the β-globin chain and hemoglobin S is formed, which undergoes sol-gel transformation under hypoxic conditions.[27] Other hemoglobinopathies have milder influences on cytoplasmic properties, but may affect the overall biochemical integrity of RBC.[28] The second major group of factors affecting RBC deformability is related to membrane protein functions, including the special membrane skeleton and its regulators, ion pumps and channels.[29] There is a well-documented collection of genetic disorders which affect membrane structure and properties and hence, membrane mechanical properties.[30]
RBC deformability is also affected during various acquired disease processes, including infections, circulatory disorders (e.g., ischemia and reperfusion), metabolic diseases (e.g., diabetes), and pulmonary disorders.[20] Alterations during disease processes may include one or more of the following changes of the RBC:[29] (1) cytoplasmic hemoglobin concentration and viscosity alterations, reflecting either osmotic influences or metabolic challenges affecting the water/electrolyte balance across the membrane; (2) shape change from biconcave-discoid to a more spherical morphology, thus reducing the relatively high surface area advantage of a biconcave disc; (3) oxidative stress-induced alterations in protein and lipid structures of the RBC membrane (e.g., increased crosslinking among membrane skeletal and integral proteins and/or hemoglobin); (4) changes of RBC membrane lipid composition, thereby affecting membrane fluidity and protein function; and (5) interference with intracellular signaling mechanisms that regulate protein-protein relations and the mechanical properties of the membrane.[31],[32] Recent experimental evidence suggests that RBC deformability may be actively regulated via the phosphorylation/dephosphorylation of various proteins contributing to the membrane skeletal network and its interactions with the RBC membrane.[31],[32]
It should be noted that alterations of RBC deformability may be a part of a hemorheological vicious cycle: impaired deformability may result from disturbed tissue perfusion which causes enhanced formation of oxygen free radicals either by ischemia-reperfusion processes and/or activated leukocytes, thereby reducing blood fluidity and further impairing tissue perfusion.[29]
3.3.2. Alterations of RBC aggregation
An inflammatory response is the major cause of enhanced RBC aggregation. This enhancement is related to increased plasma concentrations of acute phase reactants, fibrinogen being the most prominent (Figure 3), since these plasma proteins are directly involved in the aggregation process.[33] RBC aggregation is the physical factor affecting erythrocyte sedimentation,[34] yet more detailed measures of aggregation (e.g., microscopy, aggregate formation in a fluid flow field) have been proposed as an alternative and more appropriate method to monitor the course of inflammation rather than measuring the erythrocyte sedimentation rate (ESR).[35] RBC aggregation has been reported to be increased in infections, chronic inflammatory conditions, malignant diseases and circulatory disorders,[36],[37] and hence, aggregation mainly reflects the acute phase reactants under such clinical conditions. It has been reported that RBC aggregation indices may have a prognostic value in ischemic diseases, with higher aggregation being related to a poor clinical outcome.[38]
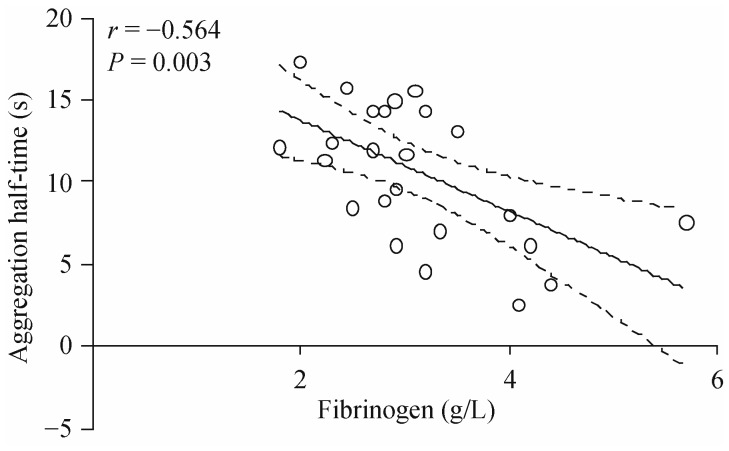
Figure 3. The relationship between plasma fibrinogen concentration and the half-time for red blood cell (RBC) aggregation.
A decreased half-time means a faster rate of RBC aggregation. Data are values determined with blood from 25 donors aged 23–79 years. The solid line represents linear regression and the dashed lines indicate the associated 95% confidence intervals; the slope of the line is highly significant (P = 0.003).
In addition to plasma factors that reflect acute phase reactions, RBC aggregation may also be influenced by cellular factors,[12] with these factors also affected during pathophysiological processes. The importance of cellular factors can be monitored by the assessment of RBC aggregation in a standard aggregation medium;[39] comparisons between different cell populations obtained by such measurements indicate the intrinsic ability of RBC to aggregate and is termed RBC aggregability.[13] Note that RBC aggregation reflects the effects of both cellular and suspending medium factors, while RBC aggregability reflects solely cellular factors. Clinical conditions with altered aggregability include circulatory disorders (e.g., stroke, myocardial ischemia/infarct, peripheral arterial diseases), metabolic disorder (e.g., diabetes), hematological disorders (e.g., hemoglobinopathies) and infections (e.g., sepsis).[36],[37] There is also experimental evidence indicating that RBC aggregability can be modulated, via intracellular signaling mechanisms, by alterations of the surface exposure of phosphatidylserine.[32]
4. Altered hemorheology in aging and related mechanisms
Close associations between impaired hemorheology and chronic diseases, and the increased incidence of the latter with aging, have been an obvious stimulus for investigating the role of aging in hemorheological health. It has been well-documented that blood rheology factors are significantly affected by aging.[40] Feher, et al.,[41] demonstrated that hemorheological parameters, including whole blood and plasma viscosity, RBC aggregation, hematocrit and plasma fibrinogen concentration, were all strongly correlated with age in a large group of patients with cardio- and cerebro-vascular diseases. However, when the analysis was repeated in a carefully selected group with matched disease risk factors, there was no relationship between the hemorheological parameters and aging.[41] They thus concluded, that the reported age-related decline in hemorheological health mainly reflects the influence of disease progression rather than the aging process itself.[42]
4.1. Human aging increases blood viscosity
Whole blood viscosity has been reported to be increased with aging. In most reports, the viscosity was measured at relatively higher shear rates, and therefore, the reported positive correlations indicate the influence of RBC deformability[41],[43] rather than the influence of RBC aggregation. It has been suggested that increased blood viscosity can be explained by increased plasma viscosity,[44],[45] particularly among woman.[46] Most of the literature reports exploring the relationship of aging and hemorheological parameters are cross-sectional studies, comparing different populations in various age ranges. Carallo, et al.,[45] provided insights into the longitudinal changes that occur with aging – these authors investigated a number of hemorheological parameters in the same population over an 11.6 year period. Whole blood viscosity at high shear rate and RBC rigidity were found to be significantly increased, while plasma viscosity was not altered.[45] It is not clear why these longitudinal findings are incongruent with previous cross-sectional reports demonstrating an age-related increase in plasma viscosity.
4.2. Aging increases plasma viscosity via increased fibrinogen concentration
Plasma viscosity closely reflects the concentration of its protein content, although certain proteins (e.g., fibrinogen) have a more predominant influence due, in part, to their relatively large molecular weight and fibrous structure. Human aging does not appear to directly alter the molecular properties of proteins, and thus, increased plasma viscosity in advanced age cannot be attributed to the altered molecular properties of plasma proteins. Human aging does, however, increase the plasma concentration of fibrinogen, although the possible causes that explain this phenomenon are complex.
Data collected from large population studies indicate that the increase in plasma fibrinogen is the most prominent hemorheologically-relevant alteration with age.[47]–[51] In large scale epidemiological studies, increased plasma fibrinogen concentration was also identified as the most powerful predictor of vascular problems (e.g., myocardial infarction, stroke) which increase with age.[52] Two important hemorheological variables – plasma viscosity and RBC aggregation – are directly influenced by plasma fibrinogen concentration, and therefore it is not surprising to observe increased plasma viscosity and RBC aggregation with aging.[41],[53],[54] Indeed, Avellone, et al.,[53] reported a significant, positive correlation between fibrinogen concentration and plasma viscosity.
The plasma concentration of a given protein represents the net balance between the rate of synthesis and rate of degradation of the protein. Fu and Nair[55] investigated the synthesis rates of fibrinogen among young (∼25 yrs), middle-aged (∼50 yrs) and older (∼70 yrs) adults, and reported that the increased plasma concentration of fibrinogen could not be explained by increased protein synthesis. Rather, the fractional synthesis rate of fibrinogen actually decreased in those aged > 50 years old. Whereas understanding the age-related decline in the rate of synthesis of various liver-produced proteins (e.g., fibrinogen) is relatively straightforward, the multiple pathways involved in protein degradation are complex and not completely understood. Nevertheless, it is generally viewed that the rate of protein degradation also declines with aging.[56] Higher rates of turnover are considered to be a protective mechanism that enables recently synthesized and functional proteins to replace those that are damaged and dysfunctional.[57] It is plausible that the concomitant decrease in the rates of synthesis and degradation of fibrinogen observed in aging may lead to a prolonged half-life of this protein.[55] While the subsequent effects are not well investigated, a longer half-life may enhance the likelihood of glycation, given that protein half-life is one of the key determinants of glycation at moderate glucose concentrations.[58] Curiously, fibrinolytic activity of plasmin is impaired due to glycation of fibrinogen.[59] Collectively, these results suggest that the decreased rate of degradation observed in aging leads to a prolonged half-life of fibrinogen, heightening the susceptibility to glycation, which may further limit fibrinolytic activity. The role of these age-related changes in pathogenesis of chronic disease is not certain yet, although irrespective of the mechanism, it is clear that increased fibrinogen concentration has a direct and significant effect on hemorheology.
In addition to the prolonged half-life of plasma fibrinogen in older individuals,[55] fibrinogen synthesis is increased due to tissue injury, inflammation and infection in certain individuals, since this protein is an acute phase reactant. Given that aging is associated with a subclinical pro-inflammatory state in healthy older adults, even when controlled for classic cardiovascular risk factors, the associated low-level inflammation may increase plasma fibrinogen concentration.[60],[61]
4.3. The effect of aging on hematocrit
It is not immediately clear whether aging significantly alters hematocrit. The NHANES II cohort of > 15,000 individuals indicated that hematocrit was similar for all ages > 20 years.[62] More recent cross-sectional studies tend to report a decrease in hematocrit by approximately 8–10 L/L between the youngest (i.e., age 20–30 yrs) and oldest (i.e., age ∼90 years) participants.[63] On the other hand, a longitudinal study by Carallo, et al.,[45] observed no significant change in hematocrit over an 11.6 years period. Possible explanations for these dissimilar findings include the shorter time period observed by Carallo, et al.,[45] that may not have been long enough to observe the gradual decline in hematocrit predicted over the human lifespan. Daily variations in hematocrit may also diminish the likelihood of detecting associations given that simple postural changes and/or performing relatively mild exercise, such as walking, significantly influence hematocrit.[64] Collectively, these findings suggest that aging has a limited effect on hematocrit, and may not be responsible for altered hemorheology in aging.
While the well-documented increase in plasma fibrinogen concentration clearly increases the fluid phase viscosity, the possible reduction or even stable (i.e., unchanged) hematocrit seemingly introduces a paradox: if hematocrit–one of the primary determinants of blood viscosity–does not increase during human aging, what other factors might explain the age-related increase in blood viscosity over-and- above that induced by elevated plasma viscosity?
4.4. Human aging, RBC lifespan, and functional consequences
It is possible that human aging may decrease the RBC lifespan. While it is difficult to systematically determine cell age in vivo, the increase in cell density that occurs throughout the 120 day lifespan of the human RBC allows for indirect assessment of cell age (i.e., older cells are more dense). Human aging skews the distribution of RBC density, whereby older humans present with a larger fraction of low-density RBC and circulating reticulocytes, and a smaller fraction of higher-density RBC, when compared with younger individuals.[65],[66] This shift towards lower-density RBC is generally considered to reflect a reduced lifespan of RBC in older adults.
The functional consequences of altered cell density distribution with aging appear to be varied and have a direct effect on hemorheological properties. Yaari[67] measured RBC electrophoretic mobility, which indicates cell surface charge density, of cells that had been separated using a density gradient technique. An inverse relationship was observed between electrophoretic mobility and cell density; those RBC which were most dense exhibited the lowest mobility, whereas younger cells were significantly more mobile. This apparent loss of electronegative surface charge in older RBC has been attributed to decreased sialic acid content, which is believed to be the primary source of the RBC negative charge.[68] Throughout the RBC lifespan, sialic acid may be lost from the cell membrane, leading to a reduction of negative surface charge by ∼30% for older RBC.[69] In the first instance, these findings imply that older humans that have an increased fraction of younger RBC and thus a greater number of RBC with preserved negative surface charge. However, Mazzanti, et al.,[70] reported decreased sialic acid content in RBC membranes from older individuals compared with younger controls. It appears that human aging accelerates the rate of sialic acid loss from RBC membranes, possibly contributing to the reduced RBC lifespan in older adults via enhanced destruction by macrophages.[69] The reduction in cell surface charge has direct implications for RBC aggregation, as discussed later.
4.5. Mechanisms for age-related decrease in RBC deformability
Most reports dealing with the increase of blood viscosity with donor age have utilized moderate-to-high shear rate viscometry methods and hence, are suggestive of decreased RBC deformability. More specifically, Ward, et al.,[71] investigated the elasticity modulus of RBC membranes in smokers and non-smokers aged 20–60 years, and found that while cigarette smoking had no effect on the dependent variables, age was positively associated with an increased RBC membrane elasticity modulus (i.e., decreased deformability).
The age-related decrease of RBC deformability appears to be due to the cumulative effect of various intra- and extra-cellular factors. Perhaps the most investigated mechanism for reduced RBC deformability is based on the oxidant theory of aging. Reactive oxygen species have been consistently demonstrated to impair the mechanical properties of RBC,[71]–[74] with distinct mechanisms depending on whether the radicals were generated inside or outside of the cell.[72] Several methods for inducing reactive oxygen species within the RBC have been shown to decrease RBC deformability due to cross-linking of membrane proteins.[74],[75] Although the interaction of various proteins favor the high deformability of RBC while maintaining membrane stability,[76] the spectrin-based network is of particular significance given its central role in these functions. Treatment of RBC with hydrogen peroxide, thereby producing intracellular superoxide, promoted formation of a hemoglobin-spectrin complex which was associated with increased cell membrane rigidity and decreased RBC deformability.[77] Superoxide produced outside the cell, similar to what may occur in vivo due to systemic oxidative stress, also decreases RBC deformability via lipid peroxidation.[78] Ultimately, to connect the decreased RBC deformability observed in aging with in vitro results for increased oxidative stress, there must be evidence for an imbalance between oxidant production and antioxidant defense in older humans. While it is outside the scope of the present review, there is accumulating evidence that the oxidative stress theory of aging, while not exhaustive, forms an important component of current understanding of the aging process, and that there is indeed an imbalance between oxidant production and clearance in older adults.[79] Importantly, age-related oxidative stress may be observed specifically in RBC.[80]
Other age-related factors that appear to decrease RBC deformability include altered sialic acid content and the Na+-K+ ATPase activity of the cell membrane. While not indicating causality, an interesting association between sialic acid content and cell membrane rigidity has been observed, whereby the membrane bending modulus increased linearly with reduced negative cell surface charge.[69] Altered Na+-K+ ATPase activity has also been observed in the RBC of aged animals,[81] and older women.[82] Given the central role of this protein in regulating RBC volume and intracellular viscosity, it is not surprising that cell membrane fluidity is decreased due to impaired Na+-K+ ATPase activity.[82],[83]
4.6. Mechanisms for age-related increase in RBC aggregation
As indicated above, the relationship between plasma fibrinogen concentration, plasma viscosity and RBC aggregation with respect to aging is well documented.[41],[53],[54]. Plasma fibrinogen strongly promotes RBC aggregate formation, and hence, as plasma fibrinogen concentration increases, RBC aggregation also increases. The rate of RBC aggregate formation is also significantly increased with increasing plasma fibrinogen concentration (Figure 3). The expected positive correlation between plasma viscosity and RBC aggregation has not always been detected,[50] and even an inverse relationship between plasma viscosity and RBC aggregation was reported.[43] This puzzling finding probably reflects the contribution of cellular factors to RBC aggregation in addition to plasma fibrinogen concentration.[12] However, most studies assessed RBC aggregation in autologous plasma and therefore their data cannot be used to interpret the cellular contribution to aggregate formation (i.e., RBC aggregability). Christy, et al.,[54] measured RBC aggregation both in autologous plasma and in a 3% solution of dextran (MW = 70 kDa) in isotonic buffer in order to estimate the contribution of cellular factors to the observed alterations with age. They reported that RBC aggregation in plasma was significantly higher in the age group of 50–59 years compared to the group of subjects at 20–29 years of age; no significant difference in aggregation measured in the standard medium was observed,[54] indicating that the cellular factors that determine RBC aggregation were not altered due to aging.
Aging is associated with several factors that decrease the repulsive forces between RBC and thus promote RBC aggregation. As discussed earlier, the primary source of the negative cell surface charge of RBC is attributed to the sialic acid content of the membrane. Consequently, the reduced sialic acid content of RBC associated with human aging reduces the negative surface charge of RBC, enhancing the likelihood of RBC rouleaux formation. Collectively, these findings suggest that age-related increased RBC aggregation is the result of altered cellular (i.e., decreased cell surface charge) and plasma (i.e., increased plasma fibrinogen concentration) factors, the consequence of which is increased low-shear blood viscosity and elevated risk for various chronic diseases.
5. Physical activity improves hemorheological health
Given the clear deterioration of hemorheological health in aging, prevention and management of such impairments are of clinical interest. Regular physical activity has been demonstrated to be one of the most effective methods for preventing impaired hemorheology, although a single period of exercise is usually associated with increased plasma viscosity, blood viscosity and decreased RBC deformability.[82],[84]–[87] It is intriguing therefore, that regular exercise training is associated with improved hemorheological health.
Cross-sectional studies indicate that plasma viscosity is decreased in habitually active individuals. This finding may be explained, in part, by the volume expansion of plasma that occurs as a result of regular exercise.[88] Plasma fibrinogen concentration is also lower among more active individuals when compared with sedentary controls.[89] Stratton, et al.,[90] demonstrated that six months of regular exercise training that improved aerobic capacity significantly decreased plasma fibrinogen concentration by 13% in older men. These authors observed a shift towards increased fibrinolytic activity: both an increased activity of plasminogen activators and a decreased activity of plasminogen inhibitors were observed.[90] It is also plausible that the so-called “anti-oxidant effects” of aerobic exercise[91] may diminish the age- and pro-inflammatory-related increase of plasma fibrinogen concentration.
The reduction in plasma fibrinogen that occurs following exercise training is supported by studies reporting decreased RBC aggregation. Three months of resistance training by younger adults significantly decreased RBC aggregation,[82],[92] whereas eight weeks of aerobic training did not alter RBC aggregation in overweight and middle-aged individuals.[93] There have been limited studies investigating the effects of exercise training on RBC aggregation in older individuals, although Simmonds, et al.,[94] demonstrated that 12 weeks of regular walking significantly decreased RBC aggregation in women with Type 2 diabetes, aged 65–74 years. The mechanisms responsible for decreased RBC aggregation following exercise training are not well understood, yet it seems quite logical that decreased plasma fibrinogen concentration may explain, in part, these findings. Simmonds, et al.,[94] provided evidence that intrinsic factors, such as increased cell surface charge, may also improve due to exercise training; this suggestion is based on their report of decreased “aggregability” of RBC in a 3% solution of dextran (MW = 70 kDa) in isotonic buffer. Collectively, these findings suggest that exercise training may decrease RBC aggregation due to both intrinsic and extrinsic factors, although further studies are warranted to evaluate these findings in healthy older individuals.
There is also very limited evidence supporting the concept that regular exercise improves RBC deformability in healthy individuals. While cross-sectional studies indicate higher RBC deformability for elite athletes when compared with sedentary controls,[95] few studies have investigated the effects of regular exercise training on RBC deformability in normal healthy individuals. The exercise training-induced improvements of hemorheological profiles among patients with age-related pathologies has been recently reviewed elsewhere.[96] Nevertheless, it is unclear as to what extent aging, per se, contributes to the altered hemorheology in these extreme cases. Ernst, et al.,[88] performed one of the few relevant studies in healthy younger individuals and reported that three months of exercise training increased RBC deformability in healthy sedentary young males. It is interesting to speculate that the improved anti-oxidant defense systems observed in the trained state may limit the adverse effects of oxidative stress, and thereby might improve RBC membrane fluidity. Clearly, further observational and mechanistic studies are required to investigate the effects of regular exercise on RBC deformability.
In summary, exercise training appears to be a multifaceted “golden bullet” for improved hemorheological health. There is strong evidence that plasma viscosity and whole blood viscosity are improved following regular exercise as a consequence of decreased plasma fibrinogen concentration. There is also indirect evidence that the adverse effects of aging on the intrinsic properties of the RBC may be diminished due to regular exercise. Well-designed observational and mechanistic studies are required, however, to determine the effects of regular exercise on hemorheological parameters in otherwise healthy mid-age and older individuals.
Acknowledgments
This paper is dedicated to our dear colleague Oguz, who passed unexpectedly while the manuscript was in production. This work was supported by the Turkish Academy of Sciences, NIH research Grants HL015722 and HL090511.
References
- 1.Whittaker SRF, Winton FR. The apparent viscosity of blood flowing in the isolated hindlimb of the dog and its variation with corpuscular concentration. J Physiol Lond. 1933;78:339–368. doi: 10.1113/jphysiol.1933.sp003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baskurt OK, Yalcin O, Meiselman HJ. Hemorheology and vascular control mechanisms. Clin Hemorheol Microcirc. 2004;30:169–178. [PubMed] [Google Scholar]
- 3.Yalcin O, Uyuklu M, Armstrong JK, et al. et al. Graded alterations of RBC aggregation influence in vivo blood flow resistance. Am J Physiol-Heart Circ Physiol. 2004;287:H2644–H2650. doi: 10.1152/ajpheart.00534.2004. [DOI] [PubMed] [Google Scholar]
- 4.Baskurt OK, Levi E, Caglayan S, et al. et al. The role of hemorheological factors in the coronary circulation. Clin Hemorheol. 1991;11:121–127. [Google Scholar]
- 5.Meiselman HJ. Hemorheologic alterations in hypertension: chicken or egg? Clin Hemorheol Microcirc. 1999;21:195–200. [PubMed] [Google Scholar]
- 6.Cokelet G. Hemorheology and Hemodynamics. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. 2011;3:1–140. [Google Scholar]
- 7.Cokelet GR, Meiselman HJ. Macro- and micro-rheological properties of blood. In: Baskurt OK, Hardeman MR, Rampling MW, Meiselman HJ, editors. Handbook of Hemorheology and Hemodynamics. Amsterdam, Berlin, Oxford, Tokyo, Washington, DC, USA: IOS Press; 2007. pp. 45–71. [Google Scholar]
- 8.Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003;29:435–450. doi: 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- 9.Chien S. Red cell deformability and its relevance to blood flow. Ann Rev Physiol. 1987;49:177–192. doi: 10.1146/annurev.ph.49.030187.001141. [DOI] [PubMed] [Google Scholar]
- 10.Baskurt OK, Meiselman HJ. Lessons from comparative hemorheology studies. Clin Hemorheol Microcirc. 2010;45:101–108. doi: 10.3233/CH-2010-1287. [DOI] [PubMed] [Google Scholar]
- 11.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rampling MW, Meiselman HJ, Neu B, et al. et al. Influence of cell-specific factors on red blood cell aggregation. Biorheology. 2004;41:91–112. [PubMed] [Google Scholar]
- 13.Baskurt OK, Meiselman HJ. Red blood cell aggregability. Clin Hemorheol Microcirc. 2009;43:353–354. doi: 10.3233/CH-2009-1255. [DOI] [PubMed] [Google Scholar]
- 14.Baskurt OK, Meiselman HJ. In vivo hemorheology. In: Baskurt OK, Hardeman MR, Rampling MW, Meiselman HJ, editors. Handbook of Hemorheology and Hemodynamics. Amsterdam, Berlin, Oxford, Tokyo, Washington, DC, USA: IOS Press; 2007. pp. 322–338. [Google Scholar]
- 15.Baskurt OK. In vivo correlates of altered blood rheology. Biorheology. 2008;45:629–638. [PubMed] [Google Scholar]
- 16.Baskurt OK, Meiselman HJ. Erythrocyte aggregation: basic aspects and clinical importance. Clin Hemorheol Microcirc. 2012;53:23–37. doi: 10.3233/CH-2012-1573. [DOI] [PubMed] [Google Scholar]
- 17.Chien S, Dormandy J, Ernst E, et al. et al. Dordrecht/Boston/Lancaster: Martinus Nijhoff Publ; 1987. Clinical Hemorheology. [Google Scholar]
- 18.Isbister JP. Hyperviscosity: Clinical disorders. In: Baskurt OK, Hardeman MR, Rampling MW, et al., editors. Handbook of Hemorheology and Hemodynamics. Amsterdam, the Netherlands: IOS Press; 2007. pp. 371–391. [Google Scholar]
- 19.Lowe GDO. Boca Raton, FL, USA: CRC Press; 1988. Clinical Blood Rheology. [Google Scholar]
- 20.Toth K, Kesmarky G. Clinical significance of hemorheological alterations. In: Baskurt OK, Hardeman MR, Rampling MW, Meiselman HJ, editors. Hand book of hemorheology and hemodynamics. Amsterdam, Berlin, Oxford, Tokyo, Washington DC: IOS Press; 2007. pp. 392–432. [Google Scholar]
- 21.Kwaan HC, Bongu A. The hyperviscosity syndromes. Semin Thromb Hemostas. 1999;25:199–208. doi: 10.1055/s-2007-994921. [DOI] [PubMed] [Google Scholar]
- 22.Kwaan H, Wang J. Hyperviscosity in polycythemia vera and other red cell abnormalities. Semin Thromb Hemost. 2003;29:451–458. doi: 10.1055/s-2003-44552. [DOI] [PubMed] [Google Scholar]
- 23.Baskurt OK, Meiselman HJ. Iiatrogenic hyperviscosity and thromboss. Semin Thromb Hemost. 2012;38:854–864. doi: 10.1055/s-0032-1325616. [DOI] [PubMed] [Google Scholar]
- 24.Whicher JT. Cytokines and acute phase proteins. Rev Port Hemorreol. 1990;5:49–56. [Google Scholar]
- 25.Kwaan HC. Role of plasma proteins in whole blood viscosity: A brief clinical review. Clin Hemorheol Microcirc. 2010;44:167–176. doi: 10.3233/CH-2010-1271. [DOI] [PubMed] [Google Scholar]
- 26.Rampling M. Hyperviscosity as a Complication in a Variety of Disorders. Semin Thromb Hemost. 2003;29:459–466. doi: 10.1055/s-2003-44553. [DOI] [PubMed] [Google Scholar]
- 27.Alexy T, Sangkatumvong S, Connes P, et al. et al. Sickle cell disease: Selected aspects of pathophysiology. Clin Hemorheol Microcirc. 2010;44:155–166. doi: 10.3233/CH-2010-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beutler E. Hemoglobinopathies associated with unstable hemoglobin. In: Beutler E, Lichtman MA, Coller BS, editors. Williams Hematology. McGraw Hill; 2001. pp. 607–610. [Google Scholar]
- 29.Baskurt OK. Mechanisms of blood rheology alterations. In: Baskurt OK, Hardeman MR, Rampling MW, Meiselman HJ, editors. Handbook of Hemorheology and Hemodynamics. IOS Press: Amsterdam, Berlin, Oxford, Tokyo, Washington, DC, USA; 2007. pp. 170–190. [Google Scholar]
- 30.An XU, Mohandas N. Disorders of red cell membrane. Br J Haematol. 2008;141:367–375. doi: 10.1111/j.1365-2141.2008.07091.x. [DOI] [PubMed] [Google Scholar]
- 31.George A, Pushkaran S, Li LN, et al. et al. Altered phosphorylation of cytoskeleton proteins in sickle red blood cells: The role of protein kinase C, Rac GTPases, and reactive oxygen species. Blood Cells Mol Dis. 2010;45:41–45. doi: 10.1016/j.bcmd.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muravyov AV, Tikhomirova IA. Role of molecular signaling pathways in changes of red blood cell deformability. Clin Hemorheol Microcirc. 2013;53:45–59. doi: 10.3233/CH-2012-1575. [DOI] [PubMed] [Google Scholar]
- 33.Rampling MW. Red cell aggregation and yield stress. In: Lowe GDO, editor. Clinical Blood Rheology. Florida: CRC Press, Inc.; 1988. pp. 45–64. [Google Scholar]
- 34.Raphael SS. Philadelphia: W.B. Saunders Co; 1983. Lynch's Medical Laboratory Technology. [Google Scholar]
- 35.Berliner S, Rogowski O, Aharonov S, et al. et al. Erythrocyte adhesiveness/aggregation; A novel biomarker for the detection of low-grade internal inflammation in individuals with atherothrombotic risk factors and proven vascular disease. Am Heart J. 2005;149:260–267. doi: 10.1016/j.ahj.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 36.Baskurt OK, Neu B, Meiselman HJ. Boca Raton, Florida: CRC Press; 2012. Red blood cell aggregation. [Google Scholar]
- 37.Baskurt OK, Meiselman HJ. Erythrocyte aggregation: basic aspects and clinical importance. Clin Hemorheol Microcirc. 2013;53:23–37. doi: 10.3233/CH-2012-1573. [DOI] [PubMed] [Google Scholar]
- 38.Neumann FJ, Katus HA, Hoberg E, et al. et al. Increased plasma viscosity and erythrocyte aggregation: indicators of an unfavourable clinical outcome in patients with unstable angina pectoris. Br Heart J. 1991;66:425–430. doi: 10.1136/hrt.66.6.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baskurt OK, Bor-Kucukatay M, Yalcin O, et al. et al. Standard aggregating media to test the “aggregability” of rat red blood cells. Clin Hemorheol Microcirc. 2000;22:161–166. [PubMed] [Google Scholar]
- 40.Ajmani RS, Rifkind JM. Hemorheological Changes during Human Aging. Gerontology. 1998;44:111–120. doi: 10.1159/000021993. [DOI] [PubMed] [Google Scholar]
- 41.Feher G, Koltai K, Kesmarky G, et al. et al. Hemorheological parameters and aging. Clin Hemorheol Microcirc. 2006;35:89–98. [PubMed] [Google Scholar]
- 42.Feher G, Koltai K, Toth K. Are hemorheological parameters independent of aging? Clin Hemorheol Microcirc. 2007;36:181–182. [PubMed] [Google Scholar]
- 43.Manetta J, Aloulou I, Varlet-Marie E, et al. et al. Partially opposite hemorheological effects of aging and training at middle age. Clin Hemorheol Microcirc. 2006;35:239–244. [PubMed] [Google Scholar]
- 44.Desimone G, Devereux RB, Chien S, et al. et al. Relation of blood-viscosity to demographic and physiologic variables and to cardiovascular risk-factors in apparently normal adults. Circulation. 1990;81:107–117. doi: 10.1161/01.cir.81.1.107. [DOI] [PubMed] [Google Scholar]
- 45.Carallo C, Irace C, De Franceschi MS, et al. et al. The effect of aging on blood plasma viscosity. An 11.6 years follow-up study. Clin Hemorheol Microcirc. 2011;47:67–74. doi: 10.3233/CH-2010-1367. [DOI] [PubMed] [Google Scholar]
- 46.Galduroz JCF, Antunes HK, Santos RF. Gender- and age-related variations in blood viscosity in normal volunteers: A study of the effects of extract of Allium sativum and Ginkgo biloba. Phytomedicine. 2007;14:447–451. doi: 10.1016/j.phymed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Cavestri R, Radice L, Ferrarini F, et al. et al. Influence of erythrocyte aggregability and plasma fibrinogen concentration on CBF with aging. Acta Neurol Scand. 1992;85:292–298. doi: 10.1111/j.1600-0404.1992.tb04046.x. [DOI] [PubMed] [Google Scholar]
- 48.Coppola L, Caserta F, De Lucia D, et al. et al. Blood viscosity and aging. Arch Gerontol Geriatr. 2000;31:35–42. doi: 10.1016/s0167-4943(00)00063-7. [DOI] [PubMed] [Google Scholar]
- 49.Hager K, Felicetti M, Seefried G. Fibrinogen and Aging. Aging (Milano) 1994;6:133–138. doi: 10.1007/BF03324226. [DOI] [PubMed] [Google Scholar]
- 50.Kovacs A, Szikszai Z, Varady E, et al. et al. Study on the hemorheological parameters of oldest-old residents in the East- Hungarian city, Debrecen. Clin Hemorheol Microcirc. 2006;35:83–88. [PubMed] [Google Scholar]
- 51.Laharragne PF, Cambus JP, Fillola G, et al. et al. Plasma fibrinogen and physiological aging. Aging-Clin Exp Res. 1993;5:445–449. doi: 10.1007/BF03324200. [DOI] [PubMed] [Google Scholar]
- 52.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of literature. Ann Intern Med. 1993;118:956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 53.Avellone G, Garbo D, Panno AV, et al. et al. Haemorheological components in the pre-geriatric and geriatric age range in a randomly selected western Sicily population sample (Casteldaccia Study) Clin Hemorheol. 1993;13:83–92. [Google Scholar]
- 54.Christy RM, Baskurt OK, Gass GC, et al. et al. Erythrocyte aggregation and neutrophil function in an aging population. Gerontology. 2010;56:175–180. doi: 10.1159/000242461. [DOI] [PubMed] [Google Scholar]
- 55.Fu AZ, Nair SK. Age effect on fibrinogen and albumin synthesis in humans. Am J Physiol-Endocrinol Metabol. 1998;275:E1023–E1030. doi: 10.1152/ajpendo.1998.275.6.E1023. [DOI] [PubMed] [Google Scholar]
- 56.Ward WF. The relentless effects of the aging process on protein turnover. Biogerontology. 2000;1:195–199. doi: 10.1023/a:1010076818119. [DOI] [PubMed] [Google Scholar]
- 57.Toyama BH, Hetzer MW. Protein homeostasis: live long, won't prosper. Nature Rev Mol Cell Biol. 2013;14:55–61. doi: 10.1038/nrm3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Austin GE, Mullins RH, Morin LG. Non-Enzymic Glycation of Individual Plasma-Proteins in Normoglycemic and Hyperglycemic Patients. Clin Chem. 1987;33:2220–2224. [PubMed] [Google Scholar]
- 59.Brownlee M, Vlassara H, Cerami A. Non-Enzymatic Glycosylation Reduces the Susceptibility of Fibrin to Degradation by Plasmin. Diabetes. 1983;32:680–684. doi: 10.2337/diab.32.7.680. [DOI] [PubMed] [Google Scholar]
- 60.Ferrucci L, Corsi A, Lauretani F, et al. et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anuurad E, Enkhmaa B, Gungor Z, et al. et al. Age as a Modulator of Inflammatory Cardiovascular Risk Factors. Arterioscler Thromb Vasc Biol. 2011;31:2151–2156. doi: 10.1161/ATVBAHA.111.232348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yip R, Johnson C, Dallman PR. Age-Related-Changes in Laboratory Values Used in the Diagnosis of Anemia and Iron-Deficiency. Am J Clin Nutr. 1984;39:427–436. doi: 10.1093/ajcn/39.3.427. [DOI] [PubMed] [Google Scholar]
- 63.Ujie H, Kawasaki Y, Suzuki Y, et al. et al. Influence of age and hematocrit on the coagulation of blood. Biorheology. 2009;23:111–114. [Google Scholar]
- 64.Mayer GA. Diurnal, postural and postprandial variations of hematocrit. Can Med Assoc J. 1965;93:1006–1008. [PMC free article] [PubMed] [Google Scholar]
- 65.Glass GA, Gershon D, Gershon H. Some characteristics of the human-erythrocyte as a function of donor and cell age. Exp Hematol. 1985;13:1122–1126. [PubMed] [Google Scholar]
- 66.Pinkofsky HB. The effect of donor age on human erythrocyte density distribution. Mech Age Dev. 1997;97:73–79. doi: 10.1016/s0047-6374(97)01885-x. [DOI] [PubMed] [Google Scholar]
- 67.Yaari A. Mobility of human red blood cells of different age groups in an electric field. Blood. 1969;33:159–163. [PubMed] [Google Scholar]
- 68.Eylar EH, Madoff MA, Brody OV, et al. et al. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem. 1961;237:1992–2000. [PubMed] [Google Scholar]
- 69.Huang YX, Wu ZJ, Mehrishi J, et al. et al. Human red blood cell aging: correlative changes in surface charge and cell properties. J Cell Mol Med. 2011;15:2634–2642. doi: 10.1111/j.1582-4934.2011.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mazzanti L, Rabini RA, Salvolini E, et al. et al. Sialic acid, diabetes, and aging: A study on the erythrocyte membrane. Met-Clin Exp. 1997;46:59–61. doi: 10.1016/s0026-0495(97)90168-2. [DOI] [PubMed] [Google Scholar]
- 71.Ward KA, Baker C, Roebuck L, et al. et al. Red-Blood-Cell Deformability - Effect of Age and Smoking. Age. 1991;14:73–77. [Google Scholar]
- 72.Baskurt OK, Temiz A, Meiselman HJ. Effect of superoxide anions on red blood cell rheologic properties. Free Radic Biol Med. 1998;24:102–110. doi: 10.1016/s0891-5849(97)00169-x. [DOI] [PubMed] [Google Scholar]
- 73.Hebbel RP, Leung A, Mohandas N. Oxidation-induced changes in microrheologic properties of the red blood cell membrane. Blood. 1990;76:1015–1020. [PubMed] [Google Scholar]
- 74.Uyesaka N, Hasegawa S, Ishioka N, et al. et al. Effect of superoxide anions on red cell deformability and membrane proteins. Biorheology. 1992;29:217–229. doi: 10.3233/bir-1992-292-303. [DOI] [PubMed] [Google Scholar]
- 75.Baskurt OK. Activated granulocyte induced alterations in red blood cells and protection by antioxidant enzymes. Clin Hemorheol. 1996;16:49–56. [Google Scholar]
- 76.Chasis JA, Mohandas N. Erythrocyte membrane deformability and stability: two distinct membrane properties that are independently regulated by skeletal protein associations. J Cell Biol. 1986;103:343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Snyder LM, Fortier N, Trainor J, et al. et al. Effect of hydrogen peroxide exposure on normal human erythrocyte deformability, morphology, surface characteristics, and spectrin-hemoglobin cross-linking. J Clin Invest. 1985;76:1971–1977. doi: 10.1172/JCI112196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simmonds MJ, Meiselman HJ, Marshall-Gradisnik SM, et al. et al. Assessment of oxidant susceptibility of red blood cells in various species based on cell deformability. Biorheology. 2011;48:293–304. doi: 10.3233/BIR-2012-0599. [DOI] [PubMed] [Google Scholar]
- 79.Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Rad Biol Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 80.Goi G, Cazzola R, Tringali C, et al. et al. Erythrocyte membrane alterations during ageing affect beta-D-glucuronidase and neutral sialidase in elderly healthy subjects. Exp Gerontol. 2005;40:219–225. doi: 10.1016/j.exger.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 81.Amano M, Imataka K, Suzuki K, et al. et al. Age-Related Reduction in the Number of Rabbit Erythrocyte Na, K-Atpase. Tohoku J Exp Med. 1989;159:131–137. doi: 10.1620/tjem.159.131. [DOI] [PubMed] [Google Scholar]
- 82.Simat BM, Morley JE, From AH, et al. et al. Variables affecting measurement of human red cell Na+,K+APTase activity: technical factors, feeding, aging. Am J Clin Nutr. 1984;40:339–345. doi: 10.1093/ajcn/40.2.339. [DOI] [PubMed] [Google Scholar]
- 83.Tsuda K, Nishio I, Masuyama Y. The role of sodium-potassium adenosine triphosphatase in the regulation of membrane fluidity of erythrocytes in spontaneously hypertensive rats: an electron paramagnetic resonance investigation. Am J Hypertens. 1997;10:1411–1414. doi: 10.1016/s0895-7061(97)00365-8. [DOI] [PubMed] [Google Scholar]
- 84.Ajmani RS, Fleg JL, Demehin AA, et al. et al. Oxidative stress and hemorheological changes induced by acute treadmill exercise. Clin Hemorheol Microcirc. 2003;28:29–40. [PubMed] [Google Scholar]
- 85.Oostenbrug GS, Mensink RP, Hardeman MR, et al. et al. Exercise performance, red blood cell deformability, and lipid peroxidation: effects of fish oil and vitamin E. J Appl Physiol. 1997;83:746–752. doi: 10.1152/jappl.1997.83.3.746. [DOI] [PubMed] [Google Scholar]
- 86.Yalcin O, Erman A, Muratli S, et al. et al. Time course of hemorheological alterations after heavy anaerobic exercise in untrained human subjects. J Appl Physiol. 2003;94:997–1002. doi: 10.1152/japplphysiol.00368.2002. [DOI] [PubMed] [Google Scholar]
- 87.Novosadova J. The changes in hematocrit, hemoglobin, plasma volume and proteins during and after different types of exercise. Eur J Appl Physiol Occup Physiol. 1977;36:223–230. doi: 10.1007/BF00421753. [DOI] [PubMed] [Google Scholar]
- 88.Ernst E. Influence of Regular Physical-Activity on Blood Rheology. Eur Heart J. 1987;8:59–62. doi: 10.1093/eurheartj/8.suppl_g.59. [DOI] [PubMed] [Google Scholar]
- 89.Rosengren A, Wilhelmsen L, Welin L, et al. et al. Social influences and cardiovascular risk-factors as determinants of plasma-fibrinogen concentration in a general-population sample of middle-aged men. Br Med J. 1990;300:634–638. doi: 10.1136/bmj.300.6725.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stratton JR, Chandler WL, Schwartz RS, et al. et al. Effects of Physical Conditioning on Fibrinolytic Variables and Fibrinogen in Young and Old Healthy-Adults. Circulation. 1991;83:1692–1697. doi: 10.1161/01.cir.83.5.1692. [DOI] [PubMed] [Google Scholar]
- 91.Gleeson M, Bishop NC, Stensel DJ, et al. et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nature Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 92.Kilic-Toprak E, Ardic F, Erken G, et al. et al. Hemorheological responses to progressive resistance exercise training in healthy young males. Med Sci Mon. 2012;18:CR351–CR360. doi: 10.12659/MSM.882878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aloulou I, Varlet-Marie E, Mercier J, et al. et al. Hemorheologic effects of low intensity endurance training in sedentary patients suffering from the metabolic syndrome. Clin Hemorheol Microcirc. 2006;35:333–339. [PubMed] [Google Scholar]
- 94.Simmonds MJ, Minahan CL, Serre KR, et al. et al. Preliminary findings in the heart rate variability and haemorheology response to varied frequency and duration of walking in women 65–74 yr with type 2 diabetes. Clin Hemorheol Microcirc. 2012;51:87–99. doi: 10.3233/CH-2011-1514. [DOI] [PubMed] [Google Scholar]
- 95.Smith JA, Martin DT, Telford RD, et al. et al. Greater erythrocyte deformability in world-class endurance athletes. Am J Physiol-Heart Circ Physiol. 1999;276:H2188–H2193. doi: 10.1152/ajpheart.1999.276.6.H2188. [DOI] [PubMed] [Google Scholar]
- 96.Connes P, Simmonds MJ, Brun JF, et al. et al. Exercise hemorheology: Classical data, recent findings and unresolved issues. Clin Hemorheol Microcirc. 2013;53:187–199. doi: 10.3233/CH-2012-1643. [DOI] [PubMed] [Google Scholar]