Abstract
By using the gap overlap task, we investigated disengagement from faces and objects in children (9-17 years old) with and without autism spectrum disorder (ASD) and its neurophysiological correlates. In typically developing (TD) children, faces elicited larger gap effect, an index of attentional engagement, and larger saccade-related event-related potentials (ERPs), compared to objects. In children with ASD, by contrast, neither gap effect nor ERPs differ between faces and objects. Follow-up experiments demonstrated that instructed fixation on the eyes induces larger gap effect for faces in children with ASD, whereas instructed fixation on the mouth can disrupt larger gap effect in TD children. These results suggest a critical role of eye fixation on attentional engagement to faces in both groups.
Keywords: autism spectrum disorder, face, disengagement, saccade-related ERPs, gap overlap task
Introduction
The face conveys critical information in human social interaction and communication, such as identity, age, gender, ethnicity, facial expression and gaze direction (Bruce, 1988). Not surprisingly, humans are biased to attend to faces rather than non-face objects. Recent studies that adopted a visual search paradigm support the claim that the face captures attention in typically developing (TD) adults (e.g., Langton, Law, Burton, & Schweinberger, 2008). In their visual search task, TD individuals took longer to detect a target (a butterfly image) from an array of objects when a face appeared as a distracting item than they did when the face did not appear. Note that this effect was not found in the target-absent array, and the authors did not attribute it to disengagement difficulty. Furthermore, several studies have adopted the change blindness paradigm (see Simons & Rensink, 2005, for reviews), which involves the detection of a change in a part of the stimulus display, to test whether attention is captured by faces (Humphreys, Hodsoll, & Campbell, 2005; Ro, Russell, & Lavie, 2001, but see Palermo & Rhodes, 2003 for contrary results). For example, TD individuals detected changes to upright faces more rapidly and accurately than they detected changes to objects (Ro, Russell, & Lavie, 2001). Another study used inhibition of return (IOR), which only occurs after attention is reflexively moved to that location, and revealed that saccade latencies towards the previous location of a face were longer than those towards the previous location of an object (Theeuwes & van der Stigchel, 2006). However, these data needs to be treated with caution as VanRullen (2006) raised a possibility that these “face pop-out effect” could be based on uncontrolled low-level perceptual differences between face stimuli and non-face stimuli used in these studies.
Once being attended to, faces can retain the observer’s attention. For example, Bindemann, Burton, Hooge, Jenkins, and de Hann (2005) demonstrated that in TD adults, an upright face had a stronger hold of attention than an inverted face or an object. In their experiments, participants had to focus on a central go/no-go signal before responding whether a vertical line target appearing at a peripheral location was to the left or right of a fixation point. In a go trial (designed by a green dot), attentional disengagement from the central signal and attentional shift to the peripheral target were required. The go/no-go signal was superimposed on an upright face, inverted face, meaningful non-face object (an image of a fruit) or blank background. The RT in the upright face condition was longer than that in the other conditions, suggesting an increased attentional dwell time on and delayed disengagement from the upright face as compared to other stimuli. In another study that used a visual search paradigm, the faster responses to face targets and slower responses to other targets with faces as distracters suggest that faces produce a larger magnitude of attentional dwell than do objects (Ro, Friggel, & Lavie, 2007).
Individuals with autism spectrum disorders (ASD) could have impairments in focusing attention on faces, in addition to the difficulty in face and gaze processing (Itier & Batty, 2009; Jemel, Mottron, & Dawson, 2006; Nation & Penny, 2008; Sasson, 2006). They suffer from atypical development of social interaction and communication, accompanied by restricted, repetitive and stereotyped patterns of behaviour, interest and activity (American Psychiatric Association, 2000). Several studies using behavioural observation techniques have found that infants or young children with ASD orient less to others’ faces (e.g., Osterling, Dawson, & Munson, 2002; Swettenham et al., 1998). Other studies using eye-tracking techniques have found mixed results. Some found that individuals with ASD fixate less on the eyes of faces than do TD individuals (Boraston, Corden, Miles, Skuse, & Blakemore, 2008; Jones, Carr, & Klin, 2008; Klin, Jones, Schultz, Volkmar, & Cohen, 2002; Pelphrey et al., 2002; Norbury, et al., 2009; Sterling et al, 2008), but others did not find differences in fixation on the eyes in children (Dapretto et al., 2006; van der Geest, Kemner, Verbaten, & van Engeland, 2002) or adults (Rutherford & Towns, 2008) with ASD. Interestingly, a recent study demonstrated that the fixation on inner facial features declined between 2 and 4 years old in children with ASD (Chawarska & Shic, 2009), which may suggest atypical developmental trajectory of eye fixation behaviour in ASD. Some have argued that atypical fixation on the eyes found in some ASD studies could be due to gaze avoidance (e.g. Dalton et al., 2005; Kylliäinen & Hietanen, 2006), but a recent thorough review failed to confirm such a claim and concluded that atypical pattern of eye contact in ASD would be best explained by the failure to detect social and communicative salience of others’ eyes, rather than its active avoidance (Senju & Johnson, 2009a).
In addition, Chawarska, Klin, and Volkmar (2003) reported that young children with ASD showed shorter saccade latencies towards peripheral targets when a face was presented at the centre of the fixation point, but not when a scrambled face was presented. A recent follow-up study by the same group (Chawarska, Volkmar & Klin, 2010) reported that such shorter saccade latencies in ASD manifests only when active disengagement from the central face is required. However, the lack of adequate control, such as the comparison between gap and overlap conditions (see below), precludes us from making a strong conclusion as to whether children with ASD have atypical attentional disengagement from faces. Moreover, no study has examined neurophysiological correlates of disengagement from faces in ASD.
The current study uses the gap overlap task (Figure 1d) to examine disengagement from faces and non-face objects (houses) in children with ASD. Participants are instructed to fixate on a central fixation stimulus and make a saccade to a peripheral target stimulus as soon as it appears. Previous studies have consistently found that when a temporal gap is introduced between the disappearance of a central fixation stimulus and the appearance of a peripheral target stimulus (i.e., gap condition), saccadic reaction time (SRT), the delay between the onset of target stimulus and the onset of saccade, becomes shorter than the baseline condition in which the peripheral stimulus appears immediately after the disappearance of central stimuli, or the overlap condition in which the central fixation stimuli remain present (gap effect; Saslow, 1967). The gap effect is argued to be based on the difference in the time required for attentional disengagement (Fischer & Weber, 1993). In the gap condition, in which an initial fixation point disappears before a target appears, attention to the fixation point is disengaged exogenously, whereas in the overlap condition, in which the fixation point remains after the target appears, attention to the fixation point needs to be disengaged endogenously to shift attention to the peripheral target stimulus. Thus, SRT is longer in the overlap condition than in the gap condition.
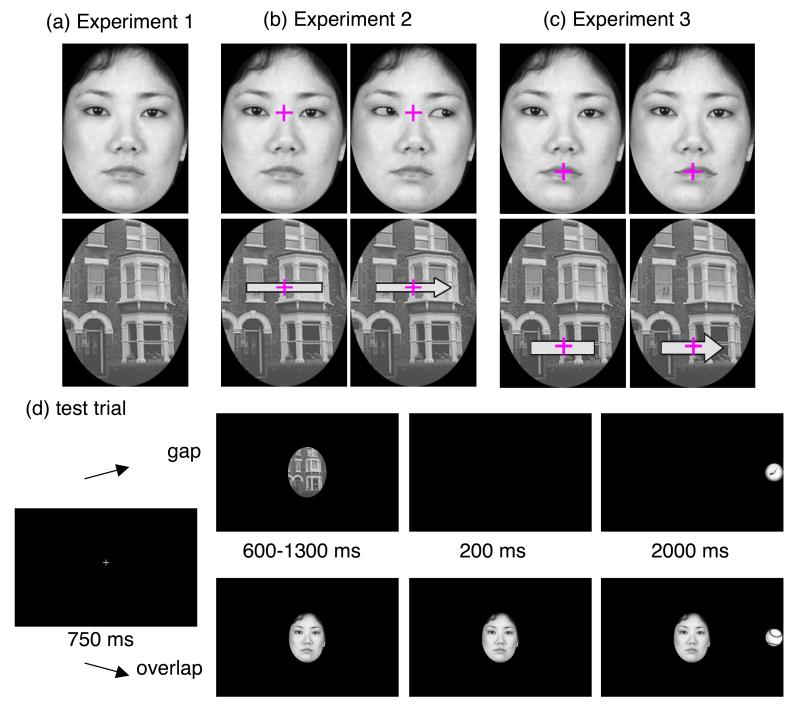
Figure 1.
(a) Example of the central face (top) and object (bottom) stimuli in Experiment 1; (b) Examples of the central face stimulus (top left), the averted gaze stimulus in the face catch trial (top right), the central object stimulus (bottom left) and the arrow stimulus in the object catch trial (bottom right) in Experiment 2; (c) Examples of the central face stimulus (top left), the averted gaze stimulus in the face catch trial (top right), the central object stimulus (bottom left) and the arrow stimulus in the object catch trial (bottom right) in Experiment 3; (d) Examples of stimulus sequence in an object and gap condition (top) and a face and overlap condition (bottom) in Experiment 1; (e) Examples of stimulus sequence in a averted gaze catch trial (top) and an arrowhead catch trial (bottom) in Experiment 2.
Several studies have adopted the gap overlap task to assess disengagement in ASD by using non-face stimuli. Some reported a larger gap effect in young children with ASD (Landry & Bryson, 2004) and adults with ASD (Kawakubo et al., 2007), whereas others did not find differences in the gap effect in children (van der Geest, Kemner, Camfferman, Verbaten, & van Engeland, 2001), adolescents (Goldberg et al., 2002) or adults (Kawakubo, Maekawa, Itoh, Hashimoto, & Iwanami, 2004) with ASD. These inconsistencies could be due to the task demand. For example, when participants had to distinguish one central stimulus from another in the gap overlap task (Kawakubo et al., 2007), individuals with ASD showed a larger gap effect than TD individuals.
In addition to SRT, we measured event-related potentials (ERPs) to assess the neurophysiological correlates of attentional disengagement in ASD. Previous studies have reported two components in saccade-related ERPs. One is the spike potential (SP), which shows a sharp peak at approximately 8–20 ms prior to the saccade, and the other is the pre-saccadic positivity (PSP), which forms a more prolonged peak at approximately 100–60 ms before the saccade execution (e.g., Kurtzberg & Vaughan, 1982; Balaban & Weinstein, 1985). Like the SP, the PSP peaks in the centro-parietal region. The sources of the SP and PSP are thought to be identical (Csibra Johnson & Tucker, 1997). Csibra et al. (1997) investigated saccade-locked ERPs in TD adults during the gap overlap task. As Posner, Walker, Friedrich, and Rafal (1984) found neuropsychological evidence that the parietal cortex is critical for the disengagement process, Csibra et al. predicted additional parietal activity between the target and saccade execution in the overlap trials, and found that the PSP in Cz and Pz is significantly prolonged, and the SP amplitude is higher, in a window from 60 ms to 0 ms prior to the saccade onset, in the overlap trials than in the gap trials. Similarly, Gómez, Atienza, and Gómez and Vázquez (1996) investigated saccade-locked ERPs during the gap/non-gap task in TD adults. Unlike Csibra et al., they found no statistically significant differences between the gap and non-gap conditions in Pz, and argued that differences between the gap and non-gap conditions have a frontal origin (Dias and Bruce, 1994). This pre-activation of frontal circuits for generating eye movements would allow the faster processing of a visuomotor task in the gap condition. Finally, Kawakubo et al. (2007) examined ERPs in the gap overlap task in adults with ASD and found higher PSP amplitude in the ASD group than in the control group in the overlap condition. Their results demonstrate electrophysiological abnormalities of disengagement in adults with ASD. However, to the best of our knowledge, no previous study has directly examined the electrooculogram (EOG) and ERPs elicited by attentional disengagement from faces in children with and without ASD.
Another aim of the current study is to assess the impact of instructed fixation on attentional engagement to faces in children with and without ASD. In previous fMRI studies, it was reported that instructed fixation on the eyes could enhance activation of fusiform gyrus in ASD (Hadjikhani et al., 2004) but not the extended face processing network (Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2007). More strikingly, Morris, Pelphrey, & McCarthy (2007) reported that instructed “atypical” scanpaths (with fewer fixations on the eyes) distorts typical activation of fusiform gyrus in TD population. Thus, we tested whether instructed fixation on the eyes and on the mouth could modulate attentional engagement to the face in children with ASD as well as TD children.
Three experiments were conducted by controlling fixation. In Experiment 1, participants were instructed to fixate on the face, but they were free to fixate to any part of the face. We predicted delay disengagement from faces rather than non-face objects in TD children, as in TD adults (Bindemann, Burton, Hooge, Jenkins, & de Hann, 2005). We also predicted that faces would not delay disengagement in children with ASD, which would result in similar SRT between face and object conditions. In Experiment 2 and Experiment 3, we controlled participants’ fixation, and restricted it to the eyes (Experiment 2) or to the mouth (Experiment 3). If atypical attention to faces is based on atypical eye fixation in ASD, instructed fixation on the eyes, but not on the mouth, should delay disengagement from faces in ASD. It was also predicted that instructed fixation on the mouth could distort delayed disengagement from faces in TD children.
Experiment 1
Using the gap overlap task, we investigated attentional disengagement from faces and non-face objects in children with and without ASD. The EOG and electroencephalogram (EEG) were measured to assess SRT and ERP components such as the SP and PSP. We predicted that TD children should show longer SRT when fixated on faces rather than objects in the overlap condition, but not in the gap condition, like TD adults in the go/no-go task (Bindemann, Burton, Hooge, Jenkins, & de Hann, 2005). We also predicted higher SP and PSP amplitudes in response to face fixation rather than object fixation, but only in the overlap condition. By contrast, disengagement from faces should not differ from disengagement from objects in children with ASD, that is, we predicted no difference in the face and object conditions in SRT and ERPs. We made no specific prediction regarding overall group differences as previous studies have reported conflicting results (Goldberg et al., 2002; Kawakubo, Maekawa, Itoh, Hashimoto, & Iwanami, 2004, Kawakubo et al., 2007; Landry & Bryson, 2004; van der Geest, Kemner, Camfferman, Verbaten, & van Engeland, 2001). Engaged visual attention tends to inhibit the express saccade, which is defined by its extremely short SRT (approximately 100 ms), and disengagement of attention leads to the express saccade (Fischer & Weber, 1993). We analysed the express saccade because it is a useful measure of the state of attention (Kawakubo et al., 2004).
We also assessed the N170 for the central face or object stimuli, even though it was not the main focus of our study. An earlier peak of occipito-temporal negativity, the N170 has been assumed to reflect face-sensitive activities in TD adults (Bentin, Allison, Puce, Perez, & McCarthy, 1996; Eimer, 1998; George Evans, Fiori, Davidoff, & Renault, 1996) and children (e.g., Taylor, Batty, & Itier, 2004). As there were inconsistencies in the reports of the N170 recordings in ASD (see Itier & Batty, 2009; Jemel, Mottron, & Dawson, 2006 for reviews) and there is a need for more data to resolve these inconsistencies, we provided N170 data in the current population.
Method
Participants
The participants consisted of 15 children with ASD (13 males and 2 females—9.5–16.1 years old, average age: 12.8 years, SD: 2.4) and 15 TD children (8 males and 7 females; —9.4–15.3 years old, average age: 11.8 years, SD: 2.0). All the children were students of or had graduated from a primary school for children both with and without ASD. The children with ASD had been diagnosed by at least one child psychiatrist when they joined the school. In addition, after parental interviews and clinical observations, experienced clinical psychologists (YT and KY) confirmed the diagnoses according to DSM-IV criteria (American Psychiatric Association, 1994). The participants’ parents completed the Japanese version of the Autism Screening Questionnaire (ASQ-J; Berument, Rutter, Lord, Pickles, & Bailey, 1999; Dairoku, Senju, Hayashi, Tojo, & Ichikawa, 2004) to confirm their clinical manifestation (children with ASD—average score: 22.1, SD: 5.90, 14 children with ASD above the cut-off point (13); TD children—average score: 1.47, SD: 1.55). Note that the main results remained the same when only children with ASD above cut-off point were included in the analyses. In this paper we present full data to maximise statistical power. An abbreviated version of the Japanese WISC-III (Wechsler, 1992; Japanese WISC-III Publication Committee, 1998) was also administered to measure the children’s IQ (children with ASD—average score: 100.6, SD: 17.7; TD children—average score: 105.8, SD: 10.1). Written informed consent was obtained from the children and their parents. The study was approved by the Research Ethics Committee of University of Tokyo.
Apparatus
The experiment was conducted on a 17-inch CRT monitor with a black background by using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA). The participants were seated at a distance of approximately 70 cm from the monitor.
Stimuli
The central stimuli (5.7° × 7.6°) were 8 greyscale images of faces (4 males and 4 females) from JACNeuF (Matsumoto & Ekman, 1988), 8 greyscale images of houses (Wojciulik, Kanwisher, & Driver, 1998) and 1 greyscale image of a sunflower (Figure 1a). The peripheral stimuli (1.4° × 1.4°) were 5 illustrations (ball, clock, orange, tambourine and sunflower), which were presented at approximately 13° to the left or right of the central stimulus.
Procedure
The participants initiated each trial by clicking a mouse, following which a fixation cross was presented in the centre of the monitor (Figure 1d). After 750 ms, a central stimulus replaced the fixation cross. A peripheral stimulus was presented to the left or right of the central stimulus for 2000 ms. To minimize the possibility of participants anticipating the peripheral stimulus onset, it was randomized between 600 and 1500 ms after the onset of the central stimulus. To keep children engaged in the task, we also introduced catch trials: Children were instructed to click the mouse when a target stimulus, a sunflower, appeared in either the centre or periphery of the fixation point. In some trials, the target stimulus was presented in both the centre and the peripheral visual field. Thus, even if the target was presented in the centre, saccades were required. Participants had to move their eyes to the peripheral stimulus in order to distinguish the target stimulus from other stimuli. In the gap condition, the central stimulus disappeared 200 ms before peripheral stimulus onset, whereas in the overlap condition, the central stimulus remained until the children’s response. Each participant received 12 practice trials to ensure that the instructions had been understood.
Design
There were 128 test trials, that is, 32 trials for each of the 4 conditions ([face or object] × [gap or overlap]). In addition, 16 catch trials were inserted between the test trials, in which a sunflower appeared at the centre and/or periphery of the fixation point. The test trials and catch trials were presented in a random order in 4 blocks of 36 trials each. The probability of the appearance of a catch trial during recording was 11.1 % (16/144). The experimental design consisted of one between-participants factor of group (children with ASD or TD children) and two within-participants factors of stimulus (face or object) and disengagement (gap or overlap).
Recordings
The scalp EEG was recorded using Ag/AgCl electrode caps (Neuroscan, Inc., Charlotte, NC) from 16 electrode sites (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4, O1, Oz, O2, T7, T8, P7, P8), according to the international 10-20 system. Recordings were referenced to the electrode located between Cz and Cpz and the re-referenced offline to the average of the left and right earlobes (Kawakubo et al., 2007). The horizontal EOG was recorded at the outer canthi of both eyes and the vertical EOG was recorded from the electrodes placed below and above the left eye. Electrode impedance was kept below 10 kΩ. The bandpass filter was set at 0.1–30 Hz and the sampling rate was 500 Hz. The EEGs and EOGs were analysed using SCAN software with SynAmps2 (Neuroscan, Inc.).
Data analysis of N170
Based on a visual inspection of individual ERPs as well as previous studies with the same age range (e.g., Taylor, Batty, & Itier, 2004), peak amplitudes and latencies of the N170 were measured between 150 and 280 ms at P7 and P8. Epochs from 100 ms pre-central stimulus to 300 ms post-central stimulus were extracted from the continuous data, and the baseline was corrected using the data for 100 ms prior to the central stimulus onset based on previous studies (e.g. Taylor, Batty, & Itier, 2004). Trials with EEG range exceeding 75 μV at any EOG electrode were automatically rejected as artifacts.
Data analyses of SRT and saccade-related potentials
Saccades were identified manually as a monotonic slope in either direction lasting at least 20 ms, and with a slope of more than 1 μV/ms (Csibra, Tucker, & Johnson, 1998; Csibra, Tucker, Volein, & Johnson, 2000). The first sampling points of these slopes were taken as the saccade onset, and the latency of this time point from the peripheral stimulus onset was taken as the SRT. The left and right eye movements were averaged together. This method, developed by Kurtzberg and Vaughan (1982), eliminates the contamination of the EEG signal by the corneo-retinal potential, and preserves the cortical potentials associated with eye movements (Kurtzberg & Vaughan, 1982; Brooks-Eidelberg & Adler, 1992). The trials were excluded from further analysis if (1) the eyes moved before the peripheral stimulus onset, (2) the saccade occurred in the incorrect direction, (3) SRTs were less than 80 ms, which suggests an anticipatory saccade, (4) SRTs were longer than 1000 ms, or (5) blinks and noise visually exceeding approximately 75 μV at any EOG electrode for the 500 ms prior to the saccade. In addition, the recordings in the catch trials were not used for analysis. An express saccade was defined as the saccade occurring between 80 and 130 ms after the presentation of the peripheral stimulus (Fischer and Weber, 1993). We used the 100 ms period starting 500 ms before the saccade onset as the baseline. Only ERPs in the overlap task were analysed because the main question of this study was whether there was a difference between disengagement from faces and disengagement from objects, and because the gap condition could elicit anticipation for the peripheral target onset, which of these could recruit frontal attention network and contaminate the response to face/object stimuli (Gómez, Atienza, Gómez, & Vázquez, 1996). The minimum of accepted sweeps was 17, which was comparable with previous studies (8 in Csibra et al., 2000 and 20 in Kawakubo et al., 2007). The PSP was defined as a slowly developing positivity as the mean amplitude in a window between 100 and 40 ms before the saccade onset, and the SP was manually detected as a sharp positivity peaking immediately before the saccade onset. The PSP and SP was analysed at 9 electrodes (C3, Cz, C4, P3, Pz, P4, O1, Oz, O2).
Results
There were no significant group differences in the IQ score (t = 1.0, p > .3) or chronological age (t = 1.25, p > .2).
N170
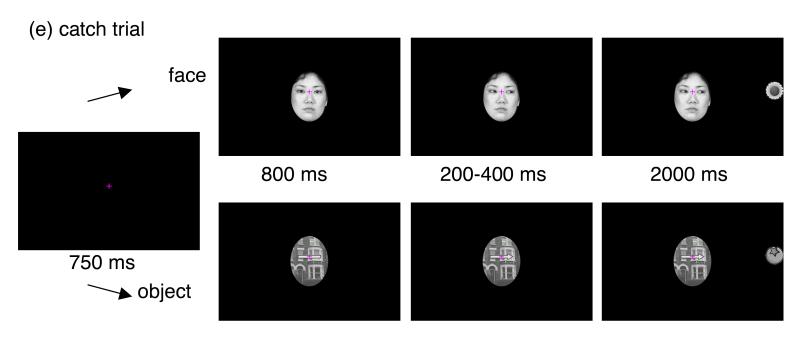
ERPs in response to the central face or object stimuli were averaged for each participant and compared to the averages of the central object stimuli (Figure 2a). The mean number of N170 accepted sweeps per group and stimulus (ASD: face = 48.8, object = 48.9; TD: face = 56.5, object = 56.8) significantly differ between groups (F (1, 28) = 7.79, p < .01, ). A follow-up inspection of rejected data revealed that the difference in rejection rate between groups were mainly due to the difference in the numbers of trials in which children blinked in response to the presentation of the central face or object stimulus, which happened more frequently in ASD group than in TD group (F (1, 28) = 7.92, p < .01, ). The N170 was analysed with the mixed three-way ANOVA with group (ASD or TD) as the between-participants factor and electrodes (P7 or P8) and stimulus (face or object) as the within-participants factors. The N170 peak amplitudes were significantly larger to faces than to objects (F (1, 28) = 30.2, p < .01, ). No other main effects or interactions reached significance (F < 2.29, p > .1). These results suggest that higher N170 amplitudes to faces were common in both groups. No main effects or interactions were significant for N170 latencies (F < 2.14, p > .1).
Figure 2.
(a) N170 for faces and objects at P7 and P8; (b) SRT, (c) Gap Effect for faces and objects; (d) Waveforms at Pz and ERP map series for faces and objects in children with ASD and TD children in Experiment 1. ASD: autism spectrum disorder, TD: typically developing.
SRT
The mean number of artifact-free trials used for the analyses of SRT did not differ between groups (F (1, 28) = .000, p > .9). Figure 2(b) shows the SRT for faces and objects and the gap and overlap conditions in each group. The mixed three-way ANOVA was carried out on the SRT with group (ASD or TD) as the between-participants factor and stimulus (face or object) and disengagement (gap or overlap) as the within-participant factors. There was a significant main effect of disengagement (F (1, 28) = 36.8, p < .01, ), which was modulated by a significant stimulus × disengagement interaction (F (1, 28) = 6.89, p < .05, ) and significant three-way interaction between the group, stimulus and disengagement (F (1, 28) = 5.45, p < .05, ). The group × stimulus interaction was also significant (F (1, 28) = 5.74, p < .05, ). Follow-up simple effect analyses revealed that in the overlap condition, face fixation elicited longer SRT in TD children than in children with ASD (F (1, 112) = 4.12, p < .05, ); however, SRT did not differ between groups for object fixations (F (1, 112) = .001, p > .9). Moreover, SRT for faces was longer than that for objects in TD children (F (1, 56) = 17.2, p < .01, ), but not in children with ASD (F (1, 56) = .30, p > .5). In the gap condition, there was no group difference in the face condition (F (1, 112) = .028, p > .8) or the objects condition (F (1, 112) = .223, p > .6), and SRT for faces and objects was not different in children with ASD (F (1, 56) = .632, p > .4) or TD children (F (1, 56) = .007, p > .9). No other main effect or interactions were significant (F (1, 28) < 3.07, p > .09).
Gap effect
The gap effect was defined as the difference in SRT between the overlap and gap conditions. Figure 2(c) shows the gap effect for the face and object conditions for each group. The mixed two-way (group × stimulus) ANOVA was carried out. There was a marginally significant main effect of group (F (1, 28) = 3.07, p = .09, ) and significant main effect of stimulus (F (1, 28) = 6.89, p < .05, ). The interaction between group and stimulus was also significant (F (1, 28) = 5.45, p < .05, ). Simple effect analyses revealed that the gap effect for faces was significantly different between children with ASD and TD children (F (1, 56) = 6.66, p < .01, ). By contrast, the gap effect for objects was not significantly different between groups (F (1, 56) = .348, p > .5). Moreover, within TD children, the gap effect for faces was larger than that for objects (F (1, 28) = 12.3, p < .01, ), whereas within children with ASD, the gap effect for faces and that for objects were not different (F (1, 28) = .042, p > .8).
Express saccade
The frequency of the express saccade did not differ between groups in the gap condition (children with ASD—face: 9.99 %, object: 12.1 %; TD children—face: 11.8 %, object: 13.2 %) or overlap condition (children with ASD—face: 9.62 %, object: 8.20 %; TD children—face: 12.8 %, object: 13.9 %; all F (1, 28) < .515, p > .4).
Pre-saccadic positivity (PSP)
The PSP increased between 100 ms and 40 ms prior to the saccade onset (Figure 2d). The mean number of accepted sweeps used for the analyses of saccade-related potentials (ASD: face = 23.7, object = 22.1; TD: face = 26.1, object = 24.6) did not significantly differ between groups (F (1, 28) = 3.85, p = .06). For the mean PSP amplitude in a window between 100 and 40 ms prior to the saccade onset, the mixed three-way (group × stimulus × electrode) ANOVA showed no significant main effects and interaction were found (all F (1, 28) < 3.37, p > .08).
Pre-saccadic spike potential (SP)
The Pre-saccadic spike potential (SP) that peaked at approximately 8-10 ms prior to the saccade onset was observed at broad centro-parietal sites in all groups and conditions (Figure 2d). For the SP amplitude, the mixed three-way (group × stimulus × electrode) ANOVA showed significant main effect of group (F (1, 28) = 4.46, p < .05, ), stimulus (F (1, 28) = 5.20, p < .05, ) and electrode (F (8, 224) = 13.3, p < .01, ), and significant stimulus × electrode interaction (F (8, 224) = 1.99, p < .05, ). Although group × stimulus interaction did not reach significance (F (1, 28) = 1.90, p > .1), we conducted exploratory analyses to compare the SP for faces and objects in each group due to its theoretical importance. In the face condition, the SP of TD children was higher than that of children with ASD (F (1, 56) = 6.29, p < .05, ) whereas in the object condition, the SP did not differ between groups (F (1, 56) = 1.55, p > .2). Moreover, SP amplitude was higher in the face condition compared to the object condition in TD children (F (1, 28) = 6.69, p < .05, ), whereas in children with ASD, the SP of the face condition was not different from that of the object condition (F (1, 28) = .406, p > .5). The overall SP of the face condition was higher than that of the object condition at P3, Pz, O1, Oz and O2 (all F (1, 252) > 3.95, p < .05, ).
Potential gender-specific effect
We also examined whether there are any gender-specific effect in the measurements used in the current study. Results revealed that none of the main measurements were significantly different between males and females in TD children.
Discussion
This is the first study to investigate disengagement from faces and non-face objects measuring EOG and EEG in children with and without ASD by using the gap overlap task. As predicted, TD children showed longer SRT for faces than for objects in the overlap condition, and the gap effect for faces was greater than that for objects. These results suggest longer attentional dwell-time on and delayed disengagement from faces than objects. The current results are consistent with the study on TD adults in that upright faces retain attention as compared to objects in the go/no-go task (Bindemann, Burton, Hooge, Jenkins, & de Hann, 2005). By contrast, for children with ASD, neither SRT in the overlap condition nor the gap effect differed between faces and objects. These results suggest that unlike TD children, children with ASD do not show longer attentional dwelling on others’ faces.
Although in children with ASD, SRT for faces in the overlap condition was shorter and the gap effect for faces was smaller than in TD children, the two groups did not differ in SRT for objects in the overlap condition or in the gap effect for objects. Thus, our study differed from previous studies, which reported that SRT for non-face stimuli in the overlap condition was longer in the ASD group than in the control group (Kawakubo et al., 2007; Landry & Bryson, 2004). It is not clear why our findings did not replicate theirs, but it could be due to some methodological differences such as the saliency of the stimuli, which were more varying and salient in previous studies than in this one. For example, these studies found delayed disengagement in ASD group often used numbers of interesting coloured cartoon animations to “keep children’s interest”, whereas our study used static images of faces and objects. Actually, a recent study using static images reported similar results as ours, in which children with ASD did not show delayed disengagement from static images of blurred faces (Chawarska, Volkmar, & Klin, 2010).
The saccade-related ERPs mirrored behavioural results in that disengagement from faces elicited larger SP in TD children than in children with ASD, whereas disengagement from objects did not elicit different SP amplitudes between groups. Similarly, in TD children, the SP showed higher amplitudes to faces than to objects, which is consistent with the SRT. Thus, the current results suggest that in TD children, disengagement from faces requires a higher level of cortical activation than disengagement from objects. By contrast, SP amplitudes did not differ between faces and objects in children with ASD. These results were consistent with the SRT, and suggest that disengagement from faces might not recruit additional cortical engagement in children with ASD. As with SRT, children with ASD did not elicit higher PSP than TD children. This result is not consistent with that of a previous study (Kawakubo et al., 2007), possibly because of task differences.
The N170 amplitude was larger to faces than to objects in children with and without ASD. These results are consistent with previous studies (McPartland, Dawson, Webb, Panagiotides, & Carver, 2004; O’Connor, Hamm, & Kirk, 2007). By contrast, the N170 peak latency did not differ between groups, which fails to replicate McPartland et al. (2004) and O’Connor et al. (2007), possibly due to differences in the age range of participants. For example, one study found delayed N170 latencies in adults with Asperger’s syndrome (AS) but not in AS children (O’Connor, Hamm, & Kirk, 2005). Another possibility is the difference in the references used in EEG recording and analyses. We followed Kawakubo et al. (2007) to use earlobes reference so that we could reliably measure the PSP and SP, the reference which may not be optimal for the recording of the N170. It was impossible for us to use average reference in the current study due to smaller number of electrodes than required for the calculation of average references (e.g. Junghöfer, Elbert, Tucker, & Rockstroh, 2000).
How come faces retain attention in TD children but not in children with ASD? There are at least two possibilities. The first is that children with ASD lack the specialized cortical network for selectively attending to faces. The second is the atypical pattern of face fixation, especially reduced fixation on the eyes (for a review, see Senju & Johnson, 2009a), in children with ASD. In the current study, participants were instructed to attend to faces (and objects), but they could freely choose which part of the face to attend to. Thus, it is possible that children with ASD fixated less on the eyes, which would have affected the results. Other neuroimaging studies also report that instructed fixation on the eyes recruit additional activation of the fusiform gyrus (FG) (Hadjikhani et al., 2004; Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2007), and higher spontaneous fixation on the eyes correlates with increased activation of the FG and amygdala (Dalton et al., 2005) in individuals with ASD. Moreover, in TD adults, typical scanpaths (fixating 70% on the eyes and 20% on the mouth) evoked more activity within the ventral occipitotemporal cortex, which contains the FG, than atypical scanpaths (fixating only 12% on the eyes or mouth) (Morris, Pelphrey, & McCarthy, 2007). Although these two hypotheses are not necessary mutually exclusive, we conducted another experiment to test whether fixation on the eyes recruits increased attentional dwelling on and delayed disengagement from others’ faces in individuals with ASD.
Experiment 2
In Experiment 2, we investigated disengagement from faces and non-face objects in children with and without ASD when instructed to fixate on the eyes. If reduced fixation on the eyes is the sole cause of atypical disengagement in children with ASD, instructed fixation on the eyes should result in increased dwell time on faces, which should be manifest in both SRTs and ERPs.
Method
Participants
The participants consisted of 14 children with ASD (12 males and 2 females—9.5–16.8 years old, average age: 12.9 years, SD: 2.3) and 14 TD children (7 males and 7 females—10.8–14.8 years old, average age: 12.4 years, SD: 1.5). The participants’ parents completed the ASQ-J to confirm their clinical manifestation (children with ASD—average score: 18.9, SD: 5.57, 12 children with ASD above the cut-off (13); TD children—average score: 1.0, SD: 1.5). As in Experiment 1, the main results remained the same when only children with ASD above cut-off point were included in the analyses. In this paper we present full data to maximise statistical power. An abbreviated version of the Japanese WISC-III was also administered to measure the children’s IQ (children with ASD—average score: 100.4, SD: 18.5; TD children—average score: 104.7, SD: 15.1). Written informed consent was obtained from the children and their parents. The study was approved by the Research Ethics Committee of the University of Tokyo. Some participants overlapped with those in Experiment 1 (9 children with ASD and 7 TD children). Experiment 2 was conducted one or two years after Experiment 1.
Apparatus and Stimuli
In order to ensure that the participants fixate on the eyes, a magenta-coloured fixation cross was presented in between the eyes on the central face stimuli. On the central object stimuli, a fixation cross and bar were presented (Figure 1b). Instead of the sunflowers, face stimuli with averted gaze to the left or right and object stimuli with arrows to the left or right were prepared as central stimuli in the catch trials in order to further encourage participants’ attention to and fixations on the eyes. Sunflowers were used as the peripheral target stimuli in the catch trials, as in Experiment 1, and all the other stimuli and apparatuses were identical to those in Experiment 1.
Procedure
The procedure was the same as in Experiment 1 except for the following changes so as to ensure fixation on the eyes. Firstly, participants were explicitly instructed to fixate on the fixation cross. Secondly, there are two kinds of catch trials. In one of the catch trials (Figure 1e), in which the central face stimulus was replaced with a face with averted gaze, participants were instructed to move their eyes rapidly in the direction of the gaze because the peripheral target was always presented in that direction; for the object stimuli, the bar was replaced with an arrow, and participants were instructed to move their eyes rapidly in the direction of the arrow. In another kind of catch trials, participants clicked the mouse when they detected the target (sunflower) presented on the periphery as in Experiment 1.
Design
Experiment 2 consisted of two face blocks and two object blocks, comprising 32 test trials and 12 catch trials for each block. Six children with ASD and 8 TD children observed the face blocks first, and the other observed the object blocks first. Within each block, trials were presented in a random order. There were 128 test trials, which includes 32 trials for each of the 4 conditions, 48 catch trials (16 with averted gaze, 16 with an arrow, that is, 8 trials for each of the 4 conditions ([gaze or arrow] × [right or left] and 16 with peripheral target). The probability of the appearance of the catch trials was 27.3 (48/176) %. The experimental design consisted of one between-participants factor of group (children with ASD or TD children) and two within-participants factors of stimulus (face or object) and disengagement (gap or overlap). The recordings and data analysis were the same as in Experiment 1.
Results
There was no significant group difference in the IQ score (t = .67, p > .5) or chronological age (t = .70, p > .4).
N170
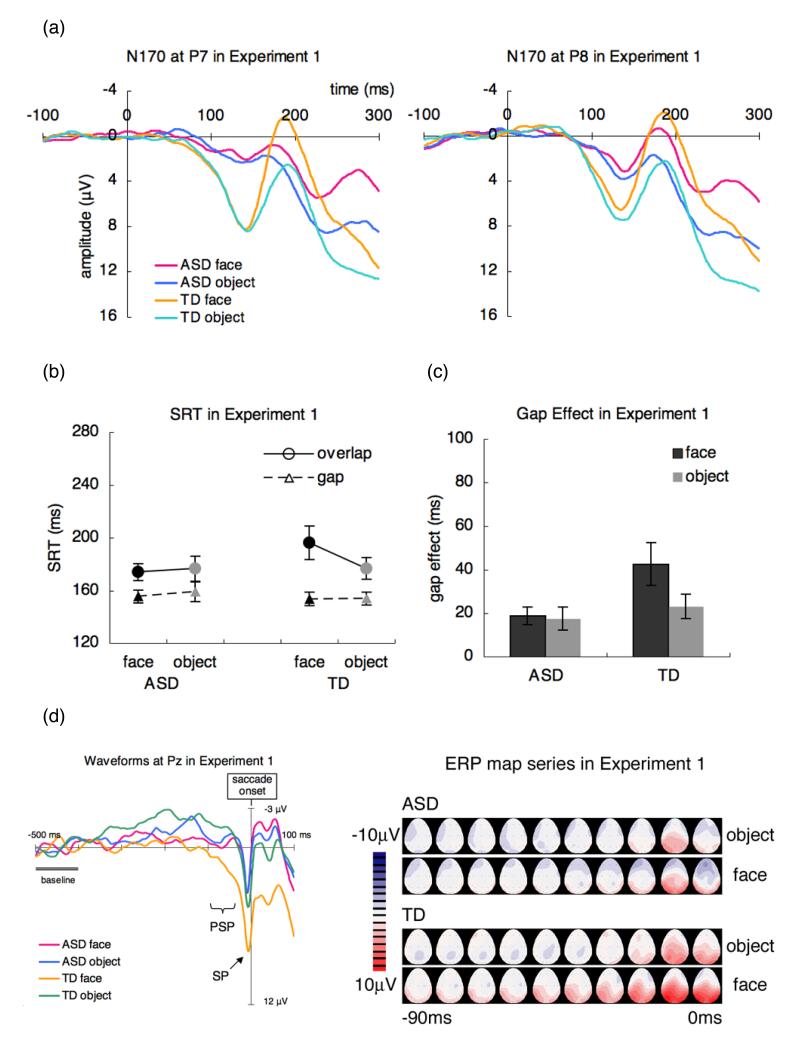
The mean number of accepted sweeps used for the analyses of N170 (maximum of 80 trials; 64 test trials and 16 catch trials) for group and stimulus (ASD: face = 58.1, object = 56.1; TD: face = 67.6, object = 63.9) did not significantly differ between groups (F (1, 26) = 2.81, p > .1). The N170 (Figure 3a) was analysed with the mixed three-way (group × electrodes × stimulus) ANOVA. The N170 amplitude showed a significant main effect of stimulus (F (1, 26) = 21.8, p < .01, ) This means that the N170 amplitude to faces was larger than that to objects. No other main effects or interactions reached significance (F < 4.22, p > .05). For N170 latencies, no main effects or interactions were significant for N170 latencies (F < 1.79, p > .1).
Figure 3.
(a) N170 for faces and objects at P7 and P8; (b) SRT, (c) Gap Effect for faces and objects; (d) Waveforms at Pz and ERP map series for faces and objects in children with ASD and TD children in Experiment 2. ASD: autism spectrum disorder, TD: typically developing.
SRT
The mean number of artifact-free trials used for the analyses of SRT did not differ between groups (F (1, 26) = .149, p > .7). Figure 3(b) shows the SRT for faces and objects and the gap and overlap conditions in each group. The mixed three-way (group × stimulus × disengagement) ANOVA was carried out on the SRT. There was a significant main effect of stimulus (F (1, 26) = 4.43 p < .05, ) and disengagement (F (1, 26) = 63.9, p < .01, ), which was modulated by a significant interaction between stimulus and disengagement (F (1, 26) = 12.5, p < .01, ). Simple effect analyses revealed that SRT for faces was longer than that for objects in the overlap condition (F (1, 52) = 14.4, p < .01, ) but not in the gap condition (F (1, 52) = .167, p > .6). There was a marginal significant main effect of group (F (1, 26) = 4.08, p = .05, ), suggesting shorter SRT in the ASD group than in the TD group, but no other interactions with group were significant (F (1, 26) < 1.78, p > .1).
Gap effect
Figure 3(c) shows the gap effect for the face and object conditions for each group in Experiment 2. The mixed two-way (group × stimulus) ANOVA was carried out on the gap effect. There was a significant main effect of stimulus (F (1, 26) = 12.5, p < .01, ). This means that both groups responded slower to the face than to the object. No other main effects or interactions were significant (F (1, 26) < 1.53, p > .2).
Express saccade
The express saccade occurred more frequently in the gap condition (children with ASD—face: 5.5 %, object: 4.3 %; TD children—face: 2.8 %, object: 4.7 %) than in the overlap condition (children with ASD—face: 2.0 %, object: 2.1 %; TD children—face: 1.9 %, object: 1.4 %), suggesting increased attentional load in the overlap condition (F (1, 26) = 10.6, p < .01, ).
PSP
The mean number of accepted sweeps used for the analyses of saccade-related potentials (ASD: face = 29.0, object = 26.0; TD: face = 27.8, object = 27.1) did not significantly differ between groups (F (1, 28) = .003, p > .9). For the mean PSP amplitude (Figure 3d), the three-way (group × stimulus × electrode) ANOVA showed a main effect of electrode (F (8, 208) = 4.24, p < .01, ) and significant stimulus × electrode interaction (F (8, 208) = 2.05, p < .05, ). The effect of electrode was significant in the face condition (F (8, 416) = 5.44, p < .01, ) but not in the object condition (F (8, 416) = 1.02, p > .4). The overall PSP of the face condition was higher than that of the object condition at Pz and O1 (all F (1, 234) > 2.79, p < .1, ).
SP
For the SP amplitude (Figure 3d), the mixed three-way (group × stimulus × electrode) ANOVA showed significant main effect of electrode (F (8, 208) = 11.1, p < .01, ) and marginally significant group × stimulus (F (1, 26) = 3.72, p = .06, ), stimulus × electrode (F (8, 208) = 1.98, p = .05, ), and significant group × electrode (F (8, 208) = 2.08, p < .05, ) interaction. Simple effect analyses revealed that in the face condition, the SP of TD children was higher than that of children with ASD (F (1, 52) = 4.01, p = .05, ), whereas in the object condition, the SP did not differ between groups (F (1, 52) = .02, p > .8). Moreover, SP amplitude was higher in the face condition compared to the object condition in TD children (F (1, 26) = 4.41, p < .05, ), whereas in children with ASD, the SP of the face condition was not different from that of the object condition (F (1, 26) = .396, p > .5). The overall SP of TD children was higher than that of children with ASD at P3, Pz and P4 (all F (1, 234) > 4.12, p < .05, ). The overall SP of the face condition was higher than that of the object condition at O1 (F (1, 234) = 5.62, p < .01, ).
Potential gender-specific effect
We also examined whether there are any gender-specific effect in the measurements used in the current study. Results revealed that none of the main measurements were significantly different between males and females in TD children.
Discussion
We replicated Experiment 1 in Experiment 2, in that TD children showed longer SRT for faces than for objects in the overlap condition, and the gap effect for faces was larger than that for objects. Interestingly, in Experiment 2, faces delayed disengagement more than objects in children with ASD, and no group difference was found in the gap effect. These results contrast with those of Experiment 1, in which the gap effect did not differ between faces and objects in children with ASD. It was suggested that in Experiment 1, reduced spontaneous fixation on the eyes (Senju & Johnson, 2009a) may have contributed to the atypical disengagement from faces in children with ASD; instructed fixation on the eyes elicits increased attentional dwell-time on and delayed disengagement from faces in children with ASD. Interestingly, the occurrence of the express saccade was significantly less frequent in Experiment 2 than to Experiment 1 (F (1, 54) = 9.61, p < .01, ). It is consistent with the difference in task and instruction and suggests that participants engaged more to the central fixation in Experiment 2. In addition, one could argue that the difference in the task design might have contributed to the discrepancies between Experiment 1 and 2: In Experiment 2, faces and objects were presented in different blocks so that we could minimize the task difficulty caused by two kinds of catch trials (i.e. gaze discrimination and arrowhead discrimination). Thus it is possible that the task design further helped children with ASD to attend to the face, which helped revealed their tendency to engage more to the face than to the objects once they successfully attend to them.
However, we still find clear group differences in saccade-related ERPs, especially in the SP amplitudes. As in Experiment 1, the SP amplitude to faces was larger than that to objects in TD children, but not in children with ASD. The apparent dissociation between SRT and SP might suggest a different neural basis of delayed disengagement from faces in individuals with ASD from that in TD individuals. The current results are consistent with Hadjikhani, Joseph, Snyder, and Tager-Flusberg (2007), who reported that individuals with ASD showed FG activation when instructed to fixate on the eyes, but eye fixation did not elicit activation in the wider face-processing network like in the amygdala, superior temporal sulcus or inferior frontal cortex. In the current experiment, it is difficult to identify the neural mechanism underlying delayed disengagement from faces in ASD as we did not find any ERPs that mirrored SRT. This might even suggest the involvement of subcortical structures such as the superior colliculus (SC) and/or amygdala. For example, Dalton et al. (2005) demonstrated that the amount of fixation on the eyes correlates with amygdala activation in ASD. It is also known that the SC is involved in saccade inhibition (Munoz & Wurtz, 1992; see also Munoz & Everling, 2004, for review). In the General Discussion, we will revisit the involvement of these subcortical structures in face processing, which has been hypothesized elsewhere (e.g., Johnson, 2005; Senju & Johnson, 2009b). Further study will be required on the functioning of such subcortical face-processing routes in individuals with ASD.
The N170 amplitude was larger to faces than to objects in children with and without ASD. This result was found in Experiment 1 and is consistent with those of previous studies (McPartland, Dawson, Webb, Panagiotides, & Carver, 2004, O’Connor, Hamm, & Kirk, 2007). The N170 peak latency did not differ between ASD and TD groups as in Experiment 1.
Experiment 3
In Experiment 2, faces delayed SRT compared to objects in children with ASD, as well as TD children. It was consistent with the argument that instructed fixation on the eyes elicits increased attentional dwell-time on and delayed disengagement from faces in children with ASD. However, as we discussed above, there is still another possibility: Participants might have engaged more to the central fixation in Experiment 2 simply because of the altered task structure, such as the introduction of fixation cross and additional emphases on the fixation, which has nothing to do with the point of fixation. Thus, in Experiment 3, we tested whether delayed disengagement from faces would occur when participants were instructed to fixate on the other region of the face; the mouth region. If delayed disengagement occurred because of the fixation on the eyes, instructed fixation on the mouth should not result in increased dwell time on faces compared to objects, in either of children with ASD and TD children.
Method
Participants
The participants consisted of 12 children with ASD (9 males and 3 females—10.8–17.8 years old, average age: 13.5 years, SD: 2.3) and 12 TD children (8 males and 4 females—10.8–17.3 years old, average age: 13.1 years, SD: 1.8). The participants’ parents completed the ASQ-J to confirm their clinical manifestation (children with ASD—average score: 19.7, SD: 6.18, 10 children with ASD above the cut-off (13); TD children—average score: 1.25, SD: 1.71). As in Experiment 1 and 2, the main results remained the same when only children with ASD above cut-off point were included in the analyses. In this paper we present full data to maximise statistical power. An abbreviated version of the Japanese WISC-III was also administered to measure the children’s IQ (children with ASD—average score: 101.8, SD: 17.4; TD children—average score: 109.8, SD: 11.7). Written informed consent was obtained from the children and their parents. The study was approved by the Research Ethics Committee of the University of Tokyo. Some participants overlapped with those in Experiment 1 (8 children with ASD and 5 TD children) and Experiment 2 (11 children with ASD and 12 TD children). Experiment 3 was conducted one year after Experiment 2.
Apparatus, Stimuli and Procedure
In order to ensure that the participants fixate on the mouth, a magenta-coloured fixation cross were presented in the mouth region (Figure 1c). In the catch trial, face stimuli with disarranged mouth to the left or right were prepared as central stimuli in order to further encourage participants’ attention to, and fixations on, the mouth. On the central object stimuli, a fixation cross and bar were presented on the level of the mouth, instead of the level of eyes. All the other stimuli and apparatuses were identical to those in Experiment 2. The procedure was also the same as in Experiment 2 except that children were instructed to fixate on the mouth. Seven children with ASD and 5 TD children observed the face blocks first, and the other observed the object blocks first.
Results
There was no significant group difference in the IQ score (t = 1.32, p > .2) or chronological age (t = .55, p > .5).
N170
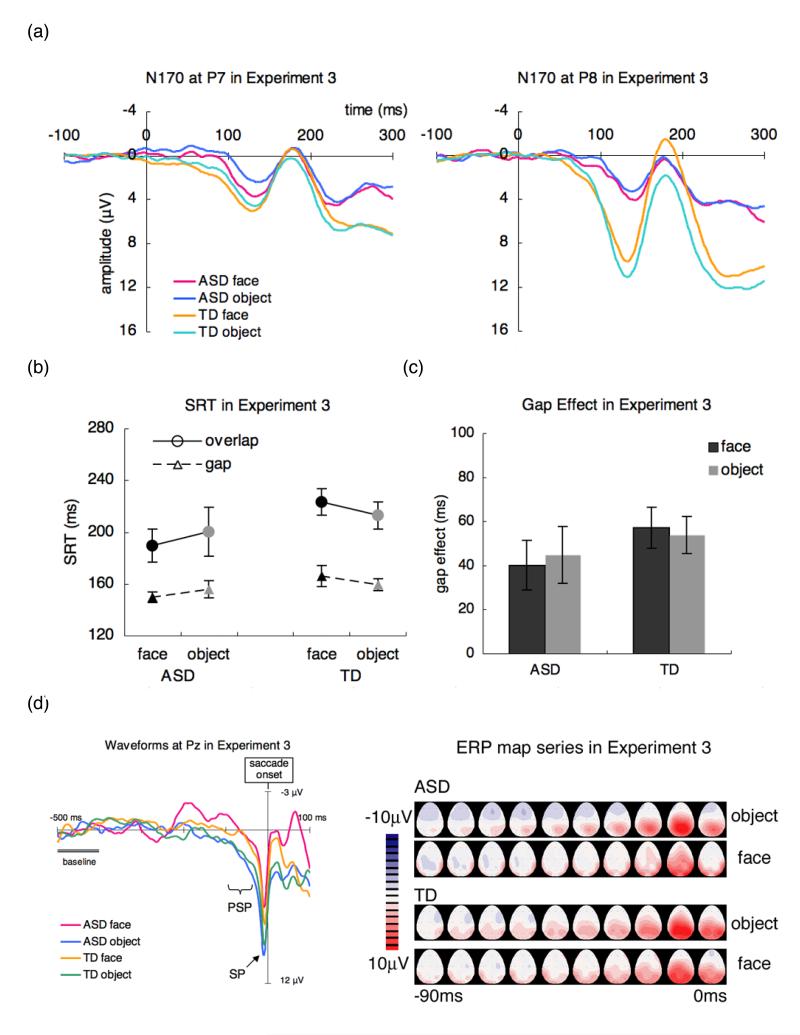
The mean number of accepted sweeps for the analyses of N170 (maximum of 80 trials; 64 test trials and 16 catch trials) for group and stimulus (ASD: face = 57.1, object = 55.5; TD: face = 70.8, object = 67.8) significantly differ between groups (F (1, 22) = 4.86, p < .05, ). The N170 (Figure 4a) was analysed with the mixed three-way (group × stimulus × electrode) ANOVA. The N170 amplitude and latency showed no significant main effect or interaction (F (1, 22) < 2.65, p > .1).
Figure 4.
(a) N170 for faces and objects at P7 and P8; (b) SRT, (c) Gap Effect for faces and objects; (d) Waveforms at Pz and ERP map series for faces and objects in children with ASD and TD children in Experiment 3. ASD: autism spectrum disorder, TD: typically developing.
SRT
The mean number of artifact-free trials used for the analyses of SRT did not differ between groups (F (1, 22) = .820, p > .3). Figure 4(b) shows the SRT for faces and objects and the gap and overlap conditions in each group. The mixed three-way (group × stimulus × disengagement) ANOVA was carried out on the SRT. There were no significant main effect or interaction (F (1, 22) < 2.32, p > .1) except a significant main effect of disengagement (F (1, 22) = 52.2, p < .01, ).
Gap effect
Figure 4(c) shows the gap effect for the face and object conditions for each group in Experiment 3. The mixed two-way (group × stimulus) ANOVA was carried out on the gap effect. There were no significant main effect or interaction (F (1, 22) < .463, p > .5).
Express saccade
The express saccade occurred more frequently in children with ASD relative to TD children (F (1, 22) = 5.03, p < .05, ). Moreover, the express saccade occurred more frequently in the gap condition (children with ASD—face: 4.9 %, object: 6.3 %; TD children—face: 3.6 %, object: 3.1 %) than in the overlap condition (children with ASD—face: 5.4 %, object: 2.9 %; TD children—face: 1.6 %, object: 2.0 %), suggesting increased attentional load in the overlap condition (F (1, 22) = 4.12, p = .05, ).
PSP
The mean number of accepted sweeps for the analyses of saccade-related potentials (ASD: face = 25.5, object = 25.2; TD: face = 27.2, object = 25.6) did not differ significantly between groups (F (1, 22) = .447, p > .5). For the mean PSP amplitude (Figure 4d), the three-way (group × stimulus × electrode) ANOVA showed a main effect of electrode (F (8, 176) = 4.20, p < .01, ). The overall PSP was higher at parietal and occipital region. No other main effect or interaction was significant (all F < 1.33, p > .2).
SP
For the SP amplitude (Figure 4d), the mixed three-way (group × stimulus × electrode) ANOVA showed significant main effect of electrode (F (8, 176) = 11.0, p < .01, ) and significant stimulus × electrode interaction (F (8, 176) = 2.47, p < .05, ). The overall SP of the object condition was higher than that of the face condition at Pz and P4 (all F (1, 198) > 5.30, p < .05, ). No other significant main effect or interaction was significant (F (1, 22) < 1.33, p > .2).
Potential gender-specific effect
We also examined whether there are any gender-specific effect in the measurements used in the current study. Results revealed that none of the main measurements were significantly different between males and females in TD children.
Discussion
No differences were found in the SRT or in the gap effect between faces and objects in children with ASD or TD children. The results suggest that delayed disengagement for faces did not occur even in TD group when the participants were instructed to fixate on the mouth. Thus, the current result rules out the possibility that altered task structure between Experiments 1 and 2 facilitated delayed disengagement from face in ASD group, and support the claim that the fixation on the eye is necessary for increased dwell time on faces in both children with ASD and TD children. In addition, to test the possibility that detecting gaze shift (Experiment 2) was harder than detecting the direction of mouth displacement (Experiment 3), but did not find significant difference in the SRTs between Experiment 2 and Experiment 3 (F (1, 96) = 2.11, p > .1). Thus, it is unlikely that the nature of the behavioural tasks in catch trials can fully explain the results.
There was no group difference in the saccade-related ERPs. However, the SP amplitude of the object was higher than that of the face in a couple of channels. It is not clear why the higher SP amplitude for objects was found and this was not consistent with SRT and the gap effect. Unlike Experiment 1 and Experiment 2, the SP amplitude of object condition was exceeding 20 μV in two of the participants (one ASD, one TD). The stimulus × electrode interaction (F (8, 160) = 1.99, p > .05) as well as the main effect of stimulus (F (8, 160) =.440, p > .5) did not reach significance. Note that all the other main results remained the same when these two “outliers” were removed from analyses. At least, it is consistent with the argument that fixation on the eyes contributes to the increased SP amplitudes in TD children.
The N170 amplitude for faces and objects was not different in both children with ASD and TD children when they were instructed to fixate on the mouth. It is consistent with a previous research that the N170 amplitude of the lip was smaller than that of the eyes (Taylor, Itier, Allison, & Edmonds, 2001) and suggest that the relative increase in N170 amplitude for faces than for objects can be modulated by the region of fixation.
General Discussion
This is the first study to investigate attentional disengagement from faces and non-face objects measuring EOG and EEG in children with and without ASD by using the gap overlap task. As predicted, TD children showed increased attentional dwelling on faces as compared to objects, which was not the case with children with ASD in the free viewing condition. These results were consistent in both behavioural (i.e., SRT) and ERP (i.e., SP amplitudes). By contrast, when children were instructed to fixate on the eyes, children with ASD showed increased SRT for faces as compared to objects, just as the TD children did. However, such increased attentional dwell time on the face was not reflected in saccade-related ERPs, as the SP amplitude did not differ between face and object fixations in the ASD group. This results contrasts with those of TD group, in which the stronger SP amplitude for face fixation was present. Moreover, when children were instructed to fixate on the mouth, delayed disengagement from faces diminished in both TD and ASD groups, both in saccade latency and in ERPs. Thus, it was suggested that fixation on the eyes could be necessary to manifest the delayed disengagement from faces in children with ASD and in TD children. In addition, the discrepancy between SRT and the SP in Experiment 2 suggests that while instructed fixation on the eyes enhances engagement to the face in children with ASD, the neural mechanism underlying this engagement differs from that in TD individuals.
The current results are consistent with those of previous studies, which demonstrated that instructed fixation on the eyes elicits cortical responses to faces (Hadjikhani et al., 2004), but the effect is limited to the FG and does not extend to the wider area of the social brain network (Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2007). Although it is not clear which neural structure is responsible for delayed disengagement from faces in the ASD group when fixation is on the eyes, the lack of ERP correlates might suggest the involvement of subcortical structures such as the SC and/or amygdala. It is consistent with the reports that the SC is involved in saccade inhibition (Munoz & Wurtz, 1992; see also Munoz & Everling, 2004 for review), and fixation on the eyes correlates with the functional activation (Dalton et al., 2005) and structural volume (Nacewicz et al., 2006) of the amygdala in ASD. This is a very interesting possibility because these subcortical structures are hypothesized to play a critical role in the development of cortical specialization for social processing (Johnson, 2005; Senju & Johnson, 2009b). In typical development, they are hypothesized to detect face and/or eye contact and modulate input to the cortical structures, which helps these structures develop the specialization for social stimuli such as faces. Thus, the current results are consistent with the hypothesis that atypical development of the social brain in ASD originates in the lack of influence from the subcortical face and eye contact detection route, which is hypothesized to guide the emergent specialization of cortical structures during development. Further studies, ideally with additional neuroimaging techniques capable of assessing subcortical structures (i.e., PET, fMRI), would be required to test whether (and how) these subcortical structures are involved in the engagement to faces in ASD.
Another, and possibly more conservative, interpretation would be that there is an overall difference between TD and ASD in the attentional network which modulate SP, but additional atypical strategies in ASD mediated face-sensitive behavioural effect in Experiment 2. Although this model does not explain the neural mechanism underlying this “atypical strategy”, it is consistent with some of the current findings, such as the overall group difference in SP amplitudes in Experiment 1. Again, further studies using additional neuroimaging techniques will be necessary to find out the neural basis of atypical disengagement from face in individuals with ASD.
Consistent with previous studies (McPartland Dawson, Webb, Panagiotides, & Carver, 2004, O’Connor, Hamm, & Kirk, 2007), the N170 peak amplitude was larger to faces than to objects in both children with ASD and TD children in Experiment 1 and 2. With respect to N170 peak latency, it is consistent with some previous studies (e.g., Kemner, Schuller, & van Engeland, 2006), but not with other studies such as McPartland et al. (2004) or O’Connor et al. (2007), who reported that individuals with ASD (15–42 years old) or adults with Asperger’s syndrome exhibited longer N170 latencies to faces than TD individuals, but comparable latencies to objects. However, small sample size in the current study as well as the inconsistencies in the previous literatures (see Itier & Batty, 2009; Jemel, Mottron, & Dawson, 2006 for reviews) prevents us from drawing any firm conclusion. In addition, the use of earlobes reference in the current study has made it slightly difficult to directly compare the current results with previous data, which often uses different references. Further studies will be required to test the N170 for face processing in ASD, and how the task structures and participants’ ages would affect the results.
Several limitations of the current study should be noted. First, the relatively small sample size may have reduced the statistical power to detect smaller effects such as those in PSP amplitudes. Second, the use of ERP measurements makes it extremely difficult to detect any subcortical activities, which are too far from surface electrodes. Third, the limited age range (9–17 years old) does not allow for analyses of the developmental trajectory in earlier and later age range. At last, due to the lack of concurrent eye-tracking, we do not have direct data about the amount of fixations on the eyes during EEG recording. Although this does not undermine the main findings of the study, further studies with a larger sample size and wider age range of participants, and hopefully, with other neuroimaging techniques such as fMRI, will be beneficial to fully understand the mechanism underlying atypical attentional engagement to faces in ASD. It would be particularly critical to test infants and younger children with ASD to better understand the developmental course of attention to faces in ASD. For example, the data presented in Chawarska, Klin, & Volkmar (2003) suggests that 2-year-old children with ASD also show atypical engagement to the face, which was replicated in their recent study (Chawarska, Volkmar, & Klin, 2010). Thus, it is possible that modifying children’s fixation could be effective in engaging younger children with ASD to faces. As the gap overlap task is commonly used in infants at a high risk for ASD (Elsabbagh et al., 2009; Zwaigenbaum et al., 2005) and infant siblings of children with autism showed longer disengagement in the overlap condition as well as less facilitation in the gap condition for non-social stimuli relative to the control (Elsabbagh et al., 2009), the current paradigm is plausible and would provide critical insight on how individuals with ASD develop atypical face processing in early infancy.
To summarize, the current study investigated attentional disengagement from faces or objects in children with and without ASD by using the gap overlap task. In TD children, faces rather than objects retain attention on the behavioural level, and this effect was observed in saccade-related ERPs, that is, the higher SP around the parietal region, which is critical for the disengagement process (Posner, Walker, Friedrich, & Rafal, 1984). Children with ASD did not show attentional dwell time on faces when allowed to freely view the faces, but did so when instructed to fixate on the eyes. However, such delayed disengagement from faces in ASD when fixating on the eyes did not modulate the saccade-related ERP.
Acknowledgments
We would like to acknowledge all the children, their parents and the teachers of Musashino Higashi Gakuen. I thank all of the staffs for their assistance in data collection and thank Monika Kiss for providing house stimuli, Katarzyna Chawarska and Gergely Csibra for comments on earlier version of drafts, Rie Fukumoto and all other members of Hasegawa Lab for their supports in testing. This study was supported by the Japan Society for the Promotion of Science (JSPS): Grant-in-Aid for JSPS Fellows (199041), JSPS: the 21st Century COE Program J05 “Center for Evolutionary Cognitive Sciences at the University of Tokyo” and JSPS: Grant-in-Aid for Scientific Research (B; 21330166) and (B; 19330210). AS was also supported by the ESRC Research Fellowship (RES-063-27-0207).
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: 1994. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) 4th edn American Psychiatric Publishing, Inc.; 2000. [Google Scholar]
- Balaban CD, Weinstein JM. The human pre-saccadic spike potential: influences of a visual target, saccade direction, electrode laterality and instructions to perform saccades. Brain Research. 1985;347:49–57. doi: 10.1016/0006-8993(85)90888-1. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthey G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neurosicence. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bindemann M, Burton AM, Hooge IT, Jenkins R, de Haan EHF. Faces retain attention. Psychonomic Bulletin & Review. 2005;12:1048–1053. doi: 10.3758/bf03206442. [DOI] [PubMed] [Google Scholar]
- Boraston ZL, Corden B, Miles LK, Skuse DH, Blakemore S. Brief report: Perception of genuine and posed smiles by individuals with autism. Journal of Autism and Developmental Disorders. 2008;38:574–580. doi: 10.1007/s10803-007-0421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Eidelberg BA, Adler G. A frontal cortical potential associated with saccades in humans. Experimental Brain Research. 1992;89:441–446. doi: 10.1007/BF00228260. [DOI] [PubMed] [Google Scholar]
- Bruce V. Recognising faces. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Chawarska K, Klin A, Volkmar F. Automatic attention cueing through eye movement in 2-year old children with autism. Child Development. 2003;74:1108–1122. doi: 10.1111/1467-8624.00595. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Volkmar F, Klin A. Not a captive audience: attentional abnormalities in response to novel faces in toddlers with ASD. Archives of General Psychiatry. 2010;67:178–185. doi: 10.1001/archgenpsychiatry.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibra G, Johnson MH, Tucker LA. Attention and oculomotor control: a high-density ERP study of the gap effect. Neuropsychologia. 1997;35:855–865. doi: 10.1016/s0028-3932(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Csibra G, Tucker LA, Johnson MH. Neural correlates of saccade planning in infants: a high-density ERP study. International Journal of Psychophysiology. 1998;29:201–215. doi: 10.1016/s0167-8760(98)00016-6. [DOI] [PubMed] [Google Scholar]
- Csibra G, Tucker LA, Volein Á, Johnson MH. Cortical development and saccade planning: the ontogeny of the spike potential. Neuro Report. 2001;5:1069–1073. doi: 10.1097/00001756-200004070-00033. [DOI] [PubMed] [Google Scholar]
- Dairoku H, Senju A, Hayashi E, Tojo Y, Ichikawa H. Development of Japanese version of autism screening questionnaire. Kokuritsu Tokushu Kyoiku Sougou Kenkyusho Bunshitsu Ippan Kenkyu Houkokusho. 2004;B-184:19–34. [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies M, Pfeifer JH, Scott AA, Sigman M, Bookhimer SY, et al. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias EC, Bruce CJ. Physiological correlate of fixation disengagement in the primate’s frontal eye field. Journal of Neurophysiology. 1994;72:2532–2537. doi: 10.1152/jn.1994.72.5.2532. [DOI] [PubMed] [Google Scholar]
- Eimer M. Does the face-specific N170 component reflect the activity of a specialized eye processor? Neuro Report. 1998;9:2945–2948. doi: 10.1097/00001756-199809140-00005. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S, et al. Visual orienting in the early broader autism phenotype: disengagement and facilitation. Journal of Child Psychology and Psychiatry. 2009;50:637–642. doi: 10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Weber H. Express saccades and visual attention. Behavioral and Brain Sciences. 1993;16:553–610. [Google Scholar]
- George N, Evans J, Fiori N, Davidoff J, Renault B. Brain events related to normal and moderately scrambled faces. Cognitive Brain Research. 1996;4:65–76. doi: 10.1016/0926-6410(95)00045-3. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Tien A, Landa RJ. Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia. 2002;40:2039–2049. doi: 10.1016/s0028-3932(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Gómez C, Atienza M, Gómez GJ, Vázquez M. Response latencies and event-related potentials during the gap paradigm using saccadic responses in human subjects. International Journal of Psychophysiology. 1996;23:91–99. doi: 10.1016/0167-8760(96)00034-7. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Josephe RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Human Brain Mapping. 2007;28:441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GW, Hodsoll J, Campbell C. Attending but not seeing: The “other race” effect in face and person perception studied through change blindness. Visual Cognition. 2005;12:249–262. [Google Scholar]
- Japanese WISC-III Publication Committee . Nihonban WISC-III chinou kensahou (Japanese Wechsler Intelligence Scale for Children Third Edition) Nihon Bunka Kagakusha; Tokyo: 1998. [Google Scholar]
- Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives of General Psychiatry. 2008;65:946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- Junchöfer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artefacts in sense array EEG/MEG studies. Psychophysiology. 2000;37:523–532. [PubMed] [Google Scholar]
- Itier R, Batty M. Neural bases of eye and gaze processing: The core of social cognition. Neuroscience and Biobehavioral Reviews. 2009;33:843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemel B, Mottorn L, Dawson M. Impaired face processing in autism: face or artifact? Journal of Autism and Developmental Disorders. 2006;36:91–106. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Neuroscience. 2005;6:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Kasai K, Okazaki S, Hosokawa-Kakurai M, Watanabe K, Kuwabara H, et al. Electrophysiological abnormalities of spatial attention in adults with autism during the gap overlap task. Clinical Neurophysiology. 2007;118:1464–1471. doi: 10.1016/j.clinph.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Maekawa H, Itoh K, Hashimoto O, Iwanami A. Spatial attention in individuals with pervasive developmental disorders using the gap overlap task. Psychiatry Research. 2004;125:269–275. doi: 10.1016/j.psychres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Kemner C, Schuller A-M, van Engeland H. Electrocortical reflections of face and gaze processing in children with pervasive developmental disorder. Journal of Child Psychology and Psychiatry. 2006;47:1063–1072. doi: 10.1111/j.1469-7610.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D, Vaughan HG., Jr. Topographic analysis of human cortical potentials preceding self-initiated and visually triggered saccades. Brain Research. 1982;243:1–9. doi: 10.1016/0006-8993(82)91115-5. [DOI] [PubMed] [Google Scholar]
- Kylliäinen A, Hietanen JK. Skin conductance responses to another person’s gaze in children with autism. Journal of Autism and Developmental Disorders. 2006;36:517–525. doi: 10.1007/s10803-006-0091-4. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry. 2004;45:1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Langton SRH, Law AS, Burton AM, Schweinberger SR. Attention capture by faces. Cognition. 2008;107:330–342. doi: 10.1016/j.cognition.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. Japanese and Caucasian Neutral Faces (JACNeuF) [CD] San Francisco State University; San Francisco: 1988. [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45:1235–1245. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Controlled scanpath variation alters fusiform face activation. Social Cognitive and Affective Neuroscience. 2007;2:31–38. doi: 10.1093/scan/nsl023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everlaing S. Look away: the anti-saccade task and the voluntary control of eye movement. Nature Neurosicence. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Role of the rostal superior colliculus in active visual fixation and execution of express saccades. Journal of Neurophysiology. 1992;63:1417–1428. doi: 10.1152/jn.1992.67.4.1000. [DOI] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, et al. Amygdala volume and nonverbal social impairment in adoloscent and adult males with autism. Archives of General Psychiatry. 2006;59:809–816. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation K, Penny S. Sensitivity to eye gaze in autism: Is it normal? Is it automatic? Is it social? Development and Psychopathology. 2008;20:79–97. doi: 10.1017/S0954579408000047. [DOI] [PubMed] [Google Scholar]
- Norbury CF, Brock J, Cragg L, Einav S, Griffiths H, Nation K. Eye-movement patterns are associated with communicative competence in autistic spectrum disorders. Journal of Child Psychology and Psychiatry. 2009;50:834–842. doi: 10.1111/j.1469-7610.2009.02073.x. [DOI] [PubMed] [Google Scholar]
- O’Connor K, Hamm JP, Kirk IJ. The neurophysiological correlates of face processing in adults and children with Asperger’s syndrome. Brain and Cognition. 2005;59:82–95. doi: 10.1016/j.bandc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- O’Connor K, Hamm JP, Kirk IJ. Neurophysiological responses to face, facial regions, and objects in adults with Asperger’s syndrome: an ERP investigateion. International Journal of Psychophysiology. 2007;63:283–293. doi: 10.1016/j.ijpsycho.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Palermo R, Rhodes G. Change detection in the flicker paradigm: Do faces have an advantage? Visual Cognition. 2003;10:683–713. [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. Journal of Autism and Developmental Disorders. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. The Journal of Neuroscience. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro T, Russell C, Lavie N. Changing faces: A detection advantage in the flicker paradigm. Psychological Science. 2001;12:94–99. doi: 10.1111/1467-9280.00317. [DOI] [PubMed] [Google Scholar]
- Ro T, Friggel A, Lavie N. Attentional biases for faces and body parts. Visual Cognition. 2007;15:322–348. [Google Scholar]
- Rutherford MD, Towns AM. Scan path differences and similarities during emotion perception in those with and without autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:1371–1381. doi: 10.1007/s10803-007-0525-7. [DOI] [PubMed] [Google Scholar]
- Saslow MG. Effects of components of displacement step stimuli upon latency for saccadic eye movements. Journal of Optical Society of America. 1967;57:1024–1029. doi: 10.1364/josa.57.001024. [DOI] [PubMed] [Google Scholar]
- Sasson NJ. The development of face processing in autism. Journal of Autism and Developmental Disorders. 2006;36:381–394. doi: 10.1007/s10803-006-0076-3. [DOI] [PubMed] [Google Scholar]
- Senju A, Johnson MH. Atypical eye contact in autism: Models, mechanisms and development. Neuroscience and Biobehavioral Reviews. 2009a;33:1204–1214. doi: 10.1016/j.neubiorev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Senju A, Johnson MH. The eye contact effect: mechanisms and development. Trends in Cognitive Sciences. 2009b;13:127–134. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Rensink RA. Change blindness: past, present, and future. Trends in cognitive Sciences. 2005;9:16–20. doi: 10.1016/j.tics.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Sterling L, Dawson G, Webb S, Murias M, Munson J, Panagiotides H, et al. The role of face familiarity in eye tracking of faces by individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:1666–1675. doi: 10.1007/s10803-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, et al. The frequency and distribution of spontaneous attention shifts between social and non social stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. Journal of Child Psychology and Psychiatry. 1998;39:747–753. [PubMed] [Google Scholar]
- Taylor MJ, Batty M, Itier RJ. The faces of development: a review of early face processing over childhood. Journal of Cognitive Neuroscience. 2004;16:1426–1442. doi: 10.1162/0898929042304732. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Van der Stigchel S. Faces capture attention: Evidence from inhibition of return. Visual Cognition. 2006;13:657–665. [Google Scholar]
- Van der Geest JN, Kemner C, Camfferman G, Verbaten MN, van Engeland H. Eye movements, visual attention and autism: a saccadic reaction time study using the gap and overlap paradigm. Biological Psychiatry. 2001;50:614–619. doi: 10.1016/s0006-3223(01)01070-8. [DOI] [PubMed] [Google Scholar]
- Van der Geest JN, Kemner C, Verbaten MN, van Engeland H. Gaze behavior of children with pervasive developmental disorder toward human faces: a fixation time study. Journal of Child Psychology and Psychiatry. 2002;43:449–478. doi: 10.1111/1469-7610.00055. [DOI] [PubMed] [Google Scholar]
- VanRullen R. On second glance: Still no high-level pop-out effect for faces. Vision Research. 2006;46:3017–3027. doi: 10.1016/j.visres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed Psychological Corporation; London: 1992. [Google Scholar]
- Wojciulik E, Kanwisher N, Driver J. Covert visual attention modulates face-specific activity in the human fusiform gyrus: fMRI study. Journal of Neurophysiology. 1998;79:1574–1578. doi: 10.1152/jn.1998.79.3.1574. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]