Abstract
Objective
The association between maternal psychological state during pregnancy and birth outcomes is well established. The focus of previous studies has been on the potentially detrimental consequences of maternal stress on pregnancy and birth outcomes, particularly shortened gestation and increased risk of preterm birth. Despite a growing literature linking positive affect with favorable health outcomes this construct has received little attention in the context of pregnancy. Therefore, in the current study, we tested the hypothesis that maternal positive affect during pregnancy is associated with beneficial consequences in terms of increased length of gestation and reduced risk of preterm birth above that of the absence of stress.
Methods
In 169 pregnant women maternal positive affect and perceived stress were serially assessed at 15.2 ± 0.9 weeks (T1; mean ± SD), 19.7 ± 0.9 weeks (T2) and 30.7 ± 0.7 weeks (T3) gestation. Pregnancy and birth outcomes were abstracted from the medical record.
Results
Higher maternal positive affect and a steeper increase in maternal positive affect over pregnancy were positively associated with length of gestation (p < .05) and reduced risk of preterm delivery (p < .01), whereas maternal perceived stress was not significantly associated with shorter length of gestation (p > .10).
Conclusions
These findings suggest that maternal positive affect may be beneficial for outcomes related to the length gestation, and that this effect cannot be accounted for by the lower stress levels associated with higher positive affect. Interventions to increase maternal positive affect may be beneficial for fetal development.
Keywords: length of gestation, positive affect, positive psychology, preterm birth, psychosocial stress, resilience
1. Introduction
The belief that a mother’s emotional state during pregnancy may influence the development of her fetus has persisted across time and culture. This has stimulated research on maternal psychological state during pregnancy and various pregnancy and birth outcomes. One of the most consistent findings in this literature is the observed association between higher levels of maternal psychological stress during pregnancy and shortened length of gestation and increased risk of preterm birth [1–14]. Although a growing body of literature has examined and demonstrated that positive affect is independently associated with more favorable health outcomes [15] this question has received relatively little attention in the context of pregnancy and birth outcomes.
Several studies, for example, have shown associations between positive affect and improved cardiovascular function, with positive affect being related to accelerated recovery from cardiovascular reactivity [15–17], decreased blood pressure in ambulatory assessments [18, 19], and elevated parasympathetic activation [20]. Positive affect also has been linked to lower cortisol concentrations over the course of the day [21, 22] and to higher antibody responses to hepatitis B vaccination [23].
One of the few studies on positive maternal affect during pregnancy found that women with stronger personal resources (mastery, self-esteem, optimism) had higher birth weight babies, even after controlling for the effects of gestational age at birth, psychosocial stress, and other variables [4]. Another study reported that maternal dispositional optimism was related to higher infant birth weight [24]. A more recent study described associations of positive state of mind and emotional stability in the immediate post-partum period with having experienced a normal delivery, however, positive affect was assessed in the immediate post-partum period and the positive delivery experience may have caused the higher positive affect in these women and not vice versa [25]. Thus, there is some preliminary evidence suggesting that maternal positive affect may be beneficial in the context of pregnancy and birth outcomes.
The objective of the present study was to assess the relationship between positive affect and length of gestation. We hypothesized that high maternal positive affect would be associated with longer length of gestation, and that this association would be significant even after controlling for the effects of maternal stress levels.
2. Method
2.1 Participants
Data for the present analysis were collected in the context of a longitudinal pregnancy and birth outcomes study conducted by the University of California, Irvine, Development, Health and Disease Research Program. All study procedures were approved by the institutional review board and all participants provided written, informed consent.
The study population comprised a population-based cohort of 169 pregnant women assessed serially over the course of gestation (at 15.2 ± 0.9 weeks (T1; mean ± SD), 19.7 ± 0.9 weeks (T2) and 30.7 ± 0.7 weeks (T3)) and followed through birth. Women who participated in at least two study visits during pregnancy were included in the current analyses, which allowed assessing rate of change in positive affect over the course of gestation. Subjects were English-speaking adult women with singleton, intrauterine pregnancies. Exclusion criteria included tobacco, alcohol, or other drug use in pregnancy, use of in vitro fertilization/reproductive technology, and uterine or cervical abnormalities.
Furthermore, women who had an elective cesarean section (n = 66) and women who had missing information about mode of delivery (n = 25) were excluded from the present analyses. The final sample included 169 women. Socio-demographic characteristics of the sample are displayed in Table 1.
Table 1.
Participant characteristics of the study sample (N = 169)
| Variable | Total sample (n = 169) | Low positive affect (n = 85) | High positive affect (n = 84) |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Maternal age a | 28.6 ± 5.6 yrs | 27.2 ± 5.3 yrs | 30.0 ± 5.5 yrs* |
| Race/ethnicity b | |||
| Non-Hispanic White | 70 (41.4%) | 35 (41.2%) | 35 (41.7%) |
| Hispanic White | 58 (34.3%) | 33 (38.8%) | 25 (29.8%) |
| Other | 41 (24.3%) | 17 (20.0%) | 24 (28.5%) |
| Annual family income b | |||
| Under $20,000 | 28 (16.6%) | 17 (22.1%) | 11 (13.8%)* |
| Between $20,000 and $50,000 | 43 (25.5%) | 29 (37.6%) | 14 (17.5%)* |
| Between $50,000 and $80,000 | 35 (20.7%) | 16 (20.8%) | 19 (23.7%)* |
| Over $80,000 | 51 (30.2%) | 15 (19.5%) | 36 (45.0%)* |
| Marital status b | |||
| Separated/divorced from or not living with baby’s father | 15 (8.9%) | 13 (17.1%) | 2 (2.5%)* |
| Pregnancy-related characteristics | |||
| Obstetric risk b | 48 (28.4%) | 27 (31.8%) | 21 (25.0%) |
| Parity b (≥1) | 93 (55.0%) | 46 (54.1%) | 47 (56.0%) |
Note. A median split was performed to create high and low positive affect groups
values represent mean ± SD;
values represent frequency N (% of total sample or group)
difference between high and low positive affect group significant at p < .05
2.2 Study Protocol
Study participants were assessed serially at least two and up to three times over the course of gestation. Gestational age was determined by best obstetric estimate with a combination of last menstrual period and early uterine size, and was confirmed by obstetric ultrasonographic biometry using standard clinical criteria [26]. Pregnancy and birth outcomes were abstracted from medical charts. Sociodemographic information (i.e., marital status, family income and maternal age at delivery) was assessed by interview.
2.3 Measures
2.3.1 Maternal Positive affect
Positive affect was assessed using a questionnaire on attitudes towards pregnancy, adapted from prior research in pregnancy [27, 28]. This self-report questionnaire consists of 7 positive and 6 negative feelings towards pregnancy. Participants read statements such as “In the last week, I often felt happy about being pregnant” and responded with answers ranging from 1 (never), 2 (rarely), 3 (sometimes), 4 (often) to 5 (always). At each assessment a sum score for positive attitudes toward pregnancy, termed positive affect, was computed from the 13 items, for which purpose the negative items were reverse coded. Thus, scores could range from 13 to 65 with higher scores reflecting higher positive affect. Average scores at each of the three assessments are depicted in Table 2.
Table 2.
Overview of positive affect and perceived stress scores over the course of gestation
| Variable | T1 (15.2 ± 0.9 a) | T2 (19.7 ± 0.9 a) | T3 (30.7 ± 0.7 a) |
|---|---|---|---|
| Positive Affect (Mean ± SD) | 52.4 ± 8.0 | 53.9 ± 7.6 | 54.2 ± 8.2 |
| Perceived Stress (Mean ± SD) | 2.2 ± 0.6 | 2.2 ± 0.7 | 2.2 ± 0.7 |
average weeks gestation at assessment ± SD
2.3.2 Maternal perceived stress
At each study visit, current levels of perceived stress were measured with the Perceived Stress Scale [29]. The PSS consists of 12 items that are designed to measure how uncontrollable, unpredictable and overloaded participants find their lives. Responses are given on a 5-point Likert scale from 0 to 4. For each participant, an average score was computed over all time points of assessment and used as a covariate in the analyses.
2.3.3 Obstetric conditions and birth outcomes
Length of gestation was abstracted from medical charts after delivery and assessed as a continuous variable by completed weeks gestation. Obstetric risk was defined as the presence of major medical complications in the index pregnancy, i.e., gestational diabetes, vaginal bleeding, placenta abruptio, pregnancy-induced hypertension, preeclampsia, or infection. Information on presence of any of these conditions was retrieved by medical interviews with the pregnant women at each of the three pregnancy visits and by medical chart abstraction. Obstetric risk was then coded as a dichotomous variable as previously described [14].
2.4. Data analysis
Previous studies have reported that both average levels of psychological state as well as its change over the course of pregnancy can have an impact on pregnancy and birth outcomes [30, 31]. Therefore, we assessed whether mean positive affect scores changed over gestation and determined the associations between level as well as rate of change of positive affect over gestation with birth outcomes.
Gestational age at the time of study visit was centered at the gestational week of the first study visit (T1), i.e. time zero in the centered variable is equivalent to the mean gestational age at T1 (15.18 weeks). A linear regression model was fitted using the positive affect scores as outcomes and the centered gestational age as predictor. For each participant, the intercept of the regression line was used as level of positive affect at the first study visit and the slope of the regression line as a measure of change in maternal positive affect. Both variables were used as predictors for gestational length in a linear regression model.
The demonstration of associations between positive affective states and biological parameters may simply reflect the absence of negative affect, leading to ambiguous results. Therefore, Steptoe (2005) suggests controlling for negative affect in order to investigate the impact of positive affect on psychobiological outcomes [32]. We therefore controlled for perceived stress as an indicator of negative affect in all analyses. Furthermore, to account for the potential confounding influence of other factors that could be associated with gestational length and/or positive affect, the following variables were included as covariates: parity, maternal age, race/ethnicity, family income, marital status, and presence of obstetric risk.
Additionally, a logistic regression was conducted with the same predictors and covariates and preterm birth (< 37 completed weeks of gestation) as the outcome of interest. The assumptions for logistic regression were met. Non-multicollinearity of the predictors was indicated by tolerance values of the predictors >.04 and variance inflation factor (VIF) <3 [33, 34].
All analyses were performed using IBM SPSS Statistics version 20.
3. Results
In our sample, the mean length of gestation at birth was 38.9 ± 2.1 weeks (± SD), and ranged from 26.3 to 42.0 weeks. 20 of these deliveries (11.8%) were preterm (≤ 37 completed weeks gestation). The average levels of positive affect and perceived stress at each pregnancy assessments are depicted in Table 2. Mean positive affect significantly increased over gestation (F(1.7;249.8) = 5.63; p = .006), specifically values at the first assessment were significantly lower than at the second and third assessment. Perceived stress did not change over gestation (p > .10).
As expected, maternal mean positive affect and mean perceived stress were inversely correlated (r = −0.644, p <.001).
3.1 Association between positive affect and length of gestation
Table 3 shows the results for the regression model predicting gestational length by the intercept and slope of positive affect, with parity, maternal age, race/ethnicity, family income, marital status, obstetric risk and mean perceived stress score as covariates. Both the positive affect score at mean gestational age at T1 (intercept) and the rate of change over the course of gestation (slope) significantly and positively predicted length of gestation, indicating that more positive attitudes toward pregnancy in the early second trimester as well as a steeper increase in positive affect over gestation are associated with longer duration of pregnancy. Specifically, a 1SD (7.97 point) increase in the intercept (representing positive affect levels in the early second trimester) is associated with a 4.57 day increase in length of gestation. Furthermore, a 1SD (0.76 point) increase in the rate of change in positive affect over gestation is associated with 3.50 days increase in pregnancy duration. These effects are independent of negative affect, as measured by the perceived stress scale, and are significant even after controlling for the effects of several potential confounders. The only other significant predictor of gestational length was presence of any obstetric risk condition, which - if present - decreased gestational length by approximately 1 week.
Table 3.
Results of the linear regression model showing the association between positive affect and the covariates included in the model and length of gestation
| Variable | Coefficient Estimate | SE a | t-value | p-value |
|---|---|---|---|---|
| Positive Affect Intercept | 0.082 | 0.029 | 2.81 | 0.006* |
| Positive Affect Slope | 0.658 | 0.267 | 2.46 | 0.015* |
| Perceived Stress | −0.076 | 0.393 | −0.19 | 0.848 |
| Parity | 0.015 | 0.182 | 0.09 | 0.933 |
| Maternal Age | −0.027 | 0.038 | −0.70 | 0.482 |
| Non-Hispanic White | −0.209 | 0.428 | −0.49 | 0.626 |
| Hispanic White | 0.075 | 0.467 | 0.16 | 0.873 |
| Obstetric Risk | −1.020 | 0.376 | −2.71 | 0.008* |
| Family Income | 0.083 | 0.061 | 1.36 | 0.175 |
| Marital Status | −1.059 | 0.589 | −1.80 | 0.074 |
SE = Standard error of the mean
p < 0.05
3.2 Association between positive affect and preterm birth
Table 4 shows the results for the logistic regression model predicting preterm delivery by the intercept and slope of positive affect, with parity, maternal age, race/ethnicity, family income, obstetric risk, marital status, and perceived stress included as covariates. Positive affect at T1 (intercept) was significantly and inversely related to preterm delivery (OR = 0.843; [95% CI = 0.757 – 0.939]; p = .002). This means that 1 point increase in positive affect intercept is associated with a 18.6% reduced risk of delivering preterm (for OR < 1: % = [(OR/1)−1]*100). However, positive affect slope was not a significant predictor of preterm delivery (OR = 0.551; [95% CI = 0.210 – 1.445]; p = .226), indicating that the change in positive affect over pregnancy did not differ in mothers who delivered preterm as compared to mothers who delivered at term.
Table 4.
Results of the logistic regression model showing the association between positive affect and the covariates included in the model and preterm delivery (< 37 weeks gestation)
| Variable | Wald | p-value | ORa | 95% CIb for ORa |
|---|---|---|---|---|
| Positive Affect Intercept | 9.66 | 0.002* | 0.843 | 0.76 – 0.94 |
| Positive Affect Slope | 1.47 | 0.226 | 0.551 | 0.21 – 1.45 |
| Perceived Stress | 0.33 | 0.568 | 0.647 | 0.15 – 2.88 |
| Parity | 0.00 | 1.000 | 1.000 | 0.53 – 1.90 |
| Maternal Age | 2.88 | 0.090 | 1.133 | 0.98 – 1.31 |
| Non-Hispanic White | 1.06 | 0.304 | 2.258 | 0.48 – 10.68 |
| Hispanic White | 0.51 | 0.477 | 0.508 | 0.08 – 3.29 |
| Obstetric Risk | 9.02 | 0.003* | 10.588 | 2.27 – 49.39 |
| Marital Status | 4.81 | 0.028* | 18.574 | 1.37 – 252.77 |
| Family Income | 5.92 | 0.015* | 0.711 | 0.54 – 0.94 |
Note.
OR = Odd’s Ratio;
CI = Confidence interval;
p < .05. Nagelkerke R2N = .365; Model χ2(10) = 31.92, p < .01
Presence of an obstetric condition was associated with increased risk for preterm birth, family income was negatively associated with preterm birth and being married to or living with the baby’s father was positively associated with preterm birth risk, which was probably due to the fact that the group of women living with their babies’ father was disproportionately larger than the group of women not cohabiting with the father. Perceived stress and the other covariates were not significantly related to preterm delivery (see Table 4 for details). In Figure 1, positive affect at each time point in gestation is depicted for women who delivered at term versus preterm.
Fig. 1.
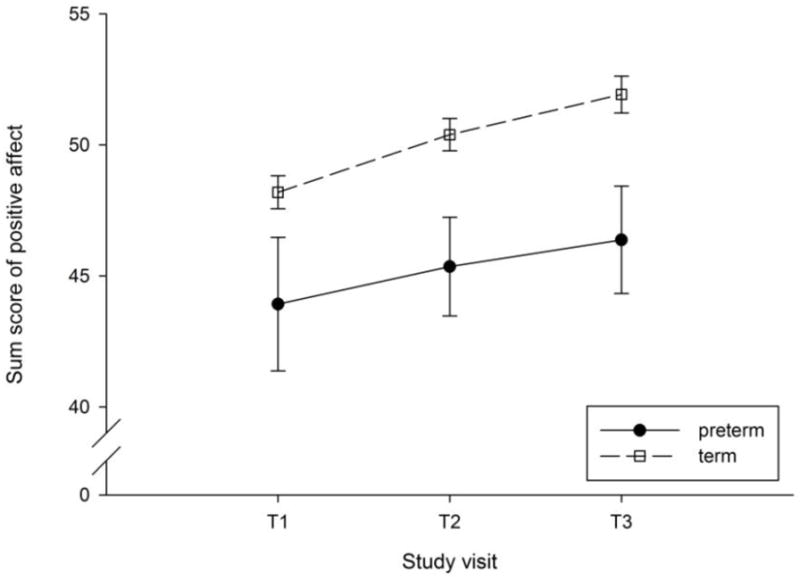
Positive affect at 15.2 ± 0.9 weeks (T1), 19.7 ± 0.9 weeks (T2) and 30.7 ± 0.7 weeks (T3) gestation is depicted for women who delivered at term versus preterm. Values represent sum scores (± standard error of the mean) for positive affect residualized by race/ethnicity, parity, maternal age, obstetric risk, family income, marital status, and perceived stress. Higher maternal positive affect decreased risk of preterm delivery.
4. Discussion
Results from the present study suggest that higher levels of positive affect in pregnancy are associated with longer length of gestation and with a reduced risk of delivering preterm. The level of positive affect in the early second trimester of pregnancy as well as the rate of increase in positive affect over the course of pregnancy were positively associated with a longer length of gestation. The magnitude of this effect was such that every point increase in positive affect in the early second trimester was associated with a 18.6% reduced risk of delivering preterm. Moreover, the association between maternal positive affect and length of gestation was independent of maternal perceived stress, suggesting that it is not solely the absence of maternal stress that accounts for the beneficial effect of maternal positive affect on length of gestation.
Several empirical studies have identified high maternal psychosocial stress as a risk factor for adverse pregnancy and birth outcomes [1, 2, 7, 35]. We and others have reported that after accounting for the influence of established obstetric and socio-demographic risk factors, maternal psychosocial stress is significantly and independently associated with an increased risk of preterm birth and restricted fetal growth [3–6]. Alterations in stress-related maternal-placental-fetal endocrine and immune physiology have been suggested as pathways that may underlie this association [1]. In support of this suggested biological transmission pathway, elevated levels of maternal cortisol, pro-inflammatory cytokines and placental corticotrophin-releasing hormone (CRH) have been shown to be associated with shorter length of gestation [9, 12, 14, 36–39] and reduced fetal growth [6, 14, 40].
It is possible that maternal positive affect may exert its beneficial effects on length of gestation by also impacting maternal-placental-fetal endocrine and immune physiology. In non-pregnant individuals associations have been reported between positive affect or optimism and lower cortisol concentrations, attenuated cortisol awakening responses, lower stress reactivity, and a lower inflammatory milieu [16, 22, 41–43]. Also, in the very small number of studies that addressed the association between maternal positive affect or positive life events and stress in pregnancy, preliminary evidence suggests that positive affect may buffer the effect of stress. A study by Nierop and colleagues reported that a higher number of psychosocial resources was associated with dampened stress reactivity in pregnant women [44]. After a mental stress test, higher resources and daily uplifts were associated with a blunted alpha-amylase reactivity and lower psychological stress. Higher resources predicted lower cortisol stress reactivity of borderline significance [44]. Furthermore, a recent study on 60 pregnant women demonstrated that positive life events predicted significantly lower third-trimester maternal morning cortisol levels across the cortisol awakening response [45]. These findings suggest that positive affect dampens endocrine and psychological responses to stress in pregnancy, which may be protective for the developing fetus.
Theoretical perspectives on resilience and positive affect suggest that the experience of positive emotions may be important in helping resilient individuals recover quickly from stress. According to the broaden-and-build theory of positive emotions [46, 47], negative emotions narrow momentary thoughts and actions to produce autonomic nervous system activation that prepares the body for specific action. Positive emotions are believed to play a homeostatic role by inhibiting autonomic arousal and by returning to a cardiovascular equilibrium.
Our report has some limitations. Because our assessment of positive affect was based on a self-report questionnaire, and was not assessed on a daily basis, it is possible that participants were inaccurate in their recall of positive affect over the past week. We suggest that future studies should use ecological momentary assessment of positive affect. Another limitation is the absence of additional possible confounding or mediating variables that were not assessed such as social support and health behaviors related to diet and physical activity. A meta-analysis of studies of positive affect and mortality has found the resilient effects of positive affect persist after accounting for the effects of behavioral factors [48].
Despite these limitations, to the best of our knowledge, this is the first study to show in a prospective, longitudinal design that positive affect is associated with length of gestation and decreased risk of preterm delivery. In this study population maternal positive affect in pregnancy was a better predictor of gestational length than perceived stress. Therefore, our data support the premise that interventions aimed to increase maternal positive affect may be beneficial for fetal development. More empirical research is needed to examine the role of maternal positive affect as a resilience factor and its effect on pregnancy physiology, and maternal health promoting behavior and fetal development.
Acknowledgments
This study was supported by US PHS National Institutes of Health (NIH) grants R01 MH-091351 to CB, R01 HD-065825 to SE, and R01 HD-060628, R01 HD-33506 and R01 HD-041696 to PDW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wadhwa PD, Entringer S, Buss C, Lu MC. The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol. 2011;38:351–84. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alder J, Fink N, Bitzer J, Hosli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Matern Fetal Neonatal Med. 2007;20:189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- 3.Hobel CJ, Goldstein A, Barrett ES. Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol. 2008;51:333–48. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
- 4.Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol. 1999;18:333–45. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- 5.Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–42. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- 6.Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol. 1993;169:858–65. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- 7.Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatr Perinat Epidemiol. 2008;22:438–66. doi: 10.1111/j.1365-3016.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 8.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62:531–58. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 9.Entringer S, Buss C, Andersen J, Chicz-DeMet A, Wadhwa PD. Ecological momentary assessment of maternal cortisol profiles over a multiple-day period predicts the length of human gestation. Psychosom Med. 2011;73:469–74. doi: 10.1097/PSY.0b013e31821fbf9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Porto M, Sandman CA. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosom Med. 1996;58:432–46. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180:S257–63. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 13.Erickson K, Thorsen P, Chrousos G, Grigoriadis DE, Khongsaly O, McGregor J, et al. Preterm birth: associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol Metab. 2001;86:2544–52. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- 14.Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191:1063–9. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 15.Dockray S, Steptoe A. Positive affect and psychobiological processes. Neurosci Biobehav Rev. 2010;35:69–75. doi: 10.1016/j.neubiorev.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bostock S, Hamer M, Wawrzyniak AJ, Mitchell ES, Steptoe A. Positive emotional style and subjective, cardiovascular and cortisol responses to acute laboratory stress. Psychoneuroendocrinology. 2011;36:1175–83. doi: 10.1016/j.psyneuen.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Tugade MM, Fredrickson BL, Barrett LF. Psychological resilience and positive emotional granularity: examining the benefits of positive emotions on coping and health. J Pers. 2004;72:1161–90. doi: 10.1111/j.1467-6494.2004.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong AD, Allaire JC. Cardiovascular intraindividual variability in later life: the influence of social connectedness and positive emotions. Psychol Aging. 2005;20:476–85. doi: 10.1037/0882-7974.20.3.476. [DOI] [PubMed] [Google Scholar]
- 19.Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci U S A. 2005;102:6508–12. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharyya MR, Whitehead DL, Rakhit R, Steptoe A. Depressed mood, positive affect, and heart rate variability in patients with suspected coronary artery disease. Psychosom Med. 2008;70:1020–7. doi: 10.1097/PSY.0b013e318189afcc. [DOI] [PubMed] [Google Scholar]
- 21.Brummett BH, Boyle SH, Kuhn CM, Siegler IC, Williams RB. Positive affect is associated with cardiovascular reactivity, norepinephrine level, and morning rise in salivary cortisol. Psychophysiology. 2009;46:862–9. doi: 10.1111/j.1469-8986.2009.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steptoe A, Gibson EL, Hamer M, Wardle J. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology. 2007;32:56–64. doi: 10.1016/j.psyneuen.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Marsland AL, Cohen S, Rabin BS, Manuck SB. Trait positive affect and antibody response to hepatitis B vaccination. Brain Behav Immun. 2006;20:261–9. doi: 10.1016/j.bbi.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Lobel M, DeVincent CJ, Kaminer A, Meyer BA. The impact of prenatal maternal stress and optimistic disposition on birth outcomes in medically high-risk women. Health Psychol. 2000;19:544–53. doi: 10.1037//0278-6133.19.6.544. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Martinez C, Val VA, Murphy M, Busquets PC, Sans JC. Relation between positive and negative maternal emotional states and obstetrical outcomes. Women Health. 2011;51:124–35. doi: 10.1080/03630242.2010.550991. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien GD, Queenan JT, Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139:540–5. doi: 10.1016/0002-9378(81)90514-7. [DOI] [PubMed] [Google Scholar]
- 27.Mancuso RA, Schetter CD, Rini CM, Roesch SC, Hobel CJ. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosom Med. 2004;66:762–9. doi: 10.1097/01.psy.0000138284.70670.d5. [DOI] [PubMed] [Google Scholar]
- 28.Gurung RAR, Dunkel-Schetter C, Collins N, Rini CM, Hobel CJ. Psychosocial predictors of prenatal anxiety. J Soc Clin Psychol. 2005;24:497–519. [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 30.Glynn LM, Schetter CD, Wadhwa PD, Sandman CA. Pregnancy affects appraisal of negative life events. J Psychosom Res. 2004;56:47–52. doi: 10.1016/S0022-3999(03)00133-8. [DOI] [PubMed] [Google Scholar]
- 31.Glynn LM, Schetter CD, Hobel CJ, Sandman CA. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychol. 2008;27:43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- 32.Steptoe A, Wardle J. Positive affect and biological function in everyday life. Neurobiol Aging. 2005;26 (Suppl 1):108–12. doi: 10.1016/j.neurobiolaging.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Meyers R. Classical and modern regression with applications. 2. Boston: Duxbury; 1990. [Google Scholar]
- 34.Menard S. Applied logistic regression analysis. Thousand Oaks: Sage; 1995. [Google Scholar]
- 35.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–35. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 36.Buss C, Entringer S, Reyes JF, Chicz-DeMet A, Sandman CA, Waffarn F, et al. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol. 2009;201:398, e1–8. doi: 10.1016/j.ajog.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 37.Santhanam U, Avila C, Romero R, Viguet H, Ida N, Sakurai S, et al. Cytokines in normal and abnormal parturition: elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine. 1991;3:155–63. doi: 10.1016/1043-4666(91)90037-e. [DOI] [PubMed] [Google Scholar]
- 38.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murtha AP, Sinclair T, Hauser ER, Swamy GK, Herbert WN, Heine RP. Maternal serum cytokines in preterm premature rupture of membranes. Obstet Gynecol. 2007;109:121–7. doi: 10.1097/01.AOG.0000250474.35369.12. [DOI] [PubMed] [Google Scholar]
- 40.Bolten MI, Wurmser H, Buske-Kirschbaum A, Papousek M, Pirke KM, Hellhammer D. Cortisol levels in pregnancy as a psychobiological predictor for birth weight. Arch Womens Ment Health. 2011;14:33–41. doi: 10.1007/s00737-010-0183-1. [DOI] [PubMed] [Google Scholar]
- 41.Steptoe A, O’Donnell K, Badrick E, Kumari M, Marmot M. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: the Whitehall II study. Am J Epidemiol. 2008;167:96–102. doi: 10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- 42.Brydon L, Walker C, Wawrzyniak AJ, Chart H, Steptoe A. Dispositional optimism and stress-induced changes in immunity and negative mood. Brain Behav Immun. 2009;23:810–6. doi: 10.1016/j.bbi.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polk DE, Cohen S, Doyle WJ, Skoner DP, Kirschbaum C. State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology. 2005;30:261–72. doi: 10.1016/j.psyneuen.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Nierop A, Wirtz PH, Bratsikas A, Zimmermann R, Ehlert U. Stress-buffering effects of psychosocial resources on physiological and psychological stress response in pregnant women. Biol Psychol. 2008;78:261–8. doi: 10.1016/j.biopsycho.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Pluess M, Wurmser H, Buske-Kirschbaum A, Papousek M, Pirke KM, Hellhammer D, et al. Positive life events predict salivary cortisol in pregnant women. Psychoneuroendocrinology. 2012;37:1336–40. doi: 10.1016/j.psyneuen.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol. 2001;56:218–26. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fredrickson BL, Levenson RW. Positive Emotions Speed Recovery from the Cardiovascular Sequelae of Negative Emotions. Cogn Emot. 1998;12:191–220. doi: 10.1080/026999398379718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chida Y, Steptoe A. Positive psychological well-being and mortality: a quantitative review of prospective observational studies. Psychosom Med. 2008;70:741–56. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]


