Abstract
Objective
The bidirectional nature of mother-child interaction is widely acknowledged during infancy and childhood. Prevailing models during pregnancy focus on unidirectional influences exerted by the pregnant woman on the developing fetus. Prior work has indicated that the fetus also affects the pregnant woman. Our objective was to determine whether a maternal psychophysiological response to stimulation of the fetus could be isolated.
Methods
Using a longitudinal design, an airborne auditory stimulus was used to elicit a fetal heart rate and motor response at 24 (n = 47) and 36 weeks (n = 45) gestation. Women were blind to condition (stimulus versus sham). Maternal parameters included cardiac (heart rate) and electrodermal (skin conductance) responses. Multilevel modeling of repeated measures with 5 data points per second was used to examine fetal and maternal responses.
Results
As expected, compared to a sham condition, the stimulus generated a fetal motor response at both gestational ages, consistent with a mild fetal startle. Fetal stimulation was associated with significant, transient slowing of maternal heart rate coupled with increased skin conductance within 10 s of the stimulus at both gestational ages. Nulliparous women showed greater electrodermal responsiveness. The magnitude of the fetal motor response significantly corresponded to the maternal skin conductance response at 5, 10, 15, and 30 s following stimulation.
Conclusion
Elicited fetal movement exerts an independent influence on the maternal autonomic nervous system. This finding contributes to current models of the dyadic relationship during pregnancy between fetus and pregnant woman.
Keywords: pregnancy, psychophysiology, fetal heart rate, fetal movement, maternal-infant interaction
Initial developmental research into the maternal-child relationship was guided by the historical philosophical view of the child as a tabula rasa and children were primarily regarded as vessels upon which the environment, and most notably their parents, acted. A seminal paper by Bell [1] challenged the existing view of parent-child interaction as a unidirectional phenomenon leading to the now accepted view of the maternal-child relationship as dynamic and transactional. Temporally based associations between parent and offspring have been variously termed synchrony, mutual responsiveness, or attunement [2–5], and are core to understanding the development of regulatory processes within each partner. This research has identified such relations in behavioral domains that include affective and attentional processes, and have also been identified at the psychophysiological level.
There is currently great interest in the manner in which the prenatal period sets the stage for later life [6–9]. Prevailing models focus on downstream effects of psychological, environmental, and physiological influences that flow from the pregnant woman to the developing fetus [9–12]. Baseline levels of maternal psychological characteristics related to stress and anxiety [13–16] and products of the hypothalamic-pituitary-adrenal axis [17–19] have been associated with fetal neurobehavior. Perhaps the most convincing evidence of a link between maternal psychological functioning and fetal neurobehavior has been generated from experimental designs in which maternal state is experimentally manipulated and effects on the fetus are observed. Both induced maternal stress [20–25] and relaxation [26, 27] have been shown to affect fetal heart rate patterns and/or motor activity.
While such findings are supportive of the role of the maternal context in affecting prenatal development, generally unacknowledged is the potential upstream effects, from fetus to pregnant woman, which may serve as potential regulators of subsequent maternal adaptation to pregnancy and child-rearing. The same neurobehavioral constructs that are central to infant and early childhood have been traditionally applied to fetal development [28, 29], although measurement methodology is necessarily different. The prenatal and postnatal environments share little similarity, but the underlying capacities and neurological organization of the developing organism are essentially the same as the fetus adapts from intrauterine function to extrauterine life. It is also clear that the fetus, through its own behavior, plays an active role in epigenesis and ontogeny [30, 31] as it interfaces with characteristics of the intrauterine environment.
Previously, in a sample of 137 maternal-fetal pairs assessed longitudinally during the second half of pregnancy, we demonstrated that spontaneous fetal motor activity transiently stimulates maternal sympathetic arousal [32] even though women perceive only a small proportion of fetal movements [33]. This association, which showed little change between the 20th and 38th week of gestation, was replicated in an additional sample of women (n = 195) from different sociodemographic and ethnic background [34]. These findings were based on second by second time series analyses of contemporaneous maternal-fetal recordings during undisturbed, baseline periods that were 50 minutes long. In both reports, spontaneous fetal movements were observed to generate an increase in maternal heart rate and electrodermal activity within 2–3 seconds at each of the six gestational periods studied. Initially, these analyses were not focused on detecting an effect from fetus to mother. Rather, dual data streams were empirically analyzed +/− 100 s of origin without respect to originating axis thereby allowing effects to emerge in either direction. From this process, we identified change in the fetus (i.e., in motor activity) to be the impetus for change in the pregnant woman.
However, measurement under baseline conditions does not control for potential joint stimulation of both members of the dyad by uncontrolled sources. Here we use an experimental model in an effort to isolate a maternal response to an elicited fetal movement. Fetuses can detect and respond to sounds external to the uterine environment [35, 36], thereby affording us the chance to stimulate the fetus independently of the woman and record any maternal response. The fetal response to brief application of vibroacoustic or auditory stimulation has been well documented in academic literature as early as the 1930’s [37]. Examples of vibroacoustic stimuli applied directly to the maternal abdomen have included those designed for obstetric purposes to stimulate a dormant fetus [38–41], an electronic artificial larynx [42, 43], and other devices that emit vibrations [44, 45]. In general, fetuses respond to initial applications of vibroacoustic stimuli with transient increases in fetal motor activity and heart rate, consistent with a startle response.
The magnitude of the fetal response tends to be commensurate with the intensity of the stimulus. Thus, airborne auditory signals delivered above and not touching the maternal abdomen tend to elicit a less intense response, but are necessary to a design in which pregnant women must be blind to stimulus presentation. Airborne stimuli shown to elicit a fetal response include electronically generated signals of varying intensity and frequency [46–49], speech sounds [50–52], and music [37, 53].
We hypothesized that, as observed with spontaneous movements, elicited fetal movements would generate a transient maternal autonomic response as measured by heart rate, which includes both parasympathetic and sympathetic influences, and electrodermal activity, which is singly innervated by sympathetic processes [54]. Fetal sex and maternal parity were evaluated as potential moderators of either the fetal or maternal response based on existing data indicating differential responsiveness of male fetuses to stimulation [40] and higher background electrodermal activity in nulliparous as compared to multiparous women [55].
Method
Participants
Participants were 50 volunteer, non-smoking pregnant women with normally progressing, singleton pregnancies and full-term deliveries. All 50 participants attended the first visit; 47 returned for the second visit. Accurate dating of the pregnancy, based on early first trimester pregnancy testing or examination with early ultrasound confirmation was required (M gestational age at pregnancy detection = 5.0 weeks; sd = 1.9). This was the first pregnancy for 52% of participants (n = 26). The sample represents a relatively stable population of well-educated (M years education = 16.8 years, sd = 1.7, mature (M age = 32.0, sd = 4.1), married (90%) women. Most were non-Hispanic white (78%); the remainder was African-American (6%), Hispanic or Asian (18%). Nearly half of the fetuses (42%) were female. Infants had normal birth outcomes outcomes (M birth weight = 3482.1 g, M gestational age = 39.5 weeks, M 5 minute Apgar score = 8.9).
Procedure
The experimental procedure was implemented at 24 and 36 weeks gestation. This followed a 50 minute undisturbed period of maternal-fetal data collection, which is not part of this report. Participants were in a semi-recumbent position. The protocol involved two sets of 3 minute baseline periods that were followed by either a stimulus or sham condition, with order randomly determined (Figure 1). The stimulus was composed of particulates (i.e., un-popped popcorn kernels) enclosed in a cardboard tube (17.5 cm long × 6 cm diameter) with metal end plates shaken sharply three times and positioned 4 inches above the maternal abdomen near the fetal head. This created a series of sharp, percussive retorts of 0.52 s duration each (total stimulus duration = 1.6 s). The peak sound level intensity generated by each of the 3 shakes was 89 dB. Pilot data collected previously on 40 maternal-fetal pairs (unpublished) in our laboratory indicated that this device was effective at generating a fetal motor response at 24 and 36 weeks. During the sham condition, the investigator stood in the same location and shook an empty tube in the same manner and at the same distance from the uterus. Women wore gelled eye masks to prevent them from observing the approach of the investigator, and headphones designed to cancel room noise (MSA Headband Earmuff; EN352) coupled with an inputted stream of classical music for further acoustic masking. The protocol was modified if high levels of fetal motor activity were present, suggestive of an active awake fetal state (4F) [56] such that the stimulus or sham was delayed for 3 additional minutes.
Figure 1.
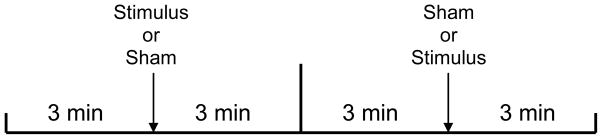
Schematic of experimental design involving airborne stimulus and sham conditions. Order of presentation was randomly determined.
Maternal-fetal data collection
Women were instrumented with a 3-lead electrocardiogram (with thigh ground) to detect R-waves and dual silver-silver chloride electrodes with gelled transducers affixed to the fingertips to measure electrodermal activity. Continuous physiological signals were amplified and digitized at 1000 Hz. Skin conductance was measured by administering a constant 0.5V root-mean-square 30Hz AC excitation signal and detecting the current flow. Data quantification proceeded off-line using the PHY General Physiology System and IBI Analysis Systems (James Long Company, Caroga Lake NY). ECG data underwent R-wave detection and manual editing for artifact. Inter-beat intervals were timed (ms) and subsequently converted to heart rate (MHR) in beats per minute (bpm) to be consistent with the fetal heart rate metric. Skin conductance units were scaled from 0 to 25 microsiemens and detrended by applying a high-pass filter to the raw signal and amplifying the resultant values [(i.e., ± 2.5 μS full scale; skin conductance response (SCR)].
Fetal data were collected using a Toitu (MT320) fetal actocardiograph. This monitor detects fetal movement and fetal heart rate through the use of a single wide array transabdominal Doppler transducer. Doppler detects and quantifies motions of the fetal heart using autocorrelation techniques and is the standard method for antepartum fetal heart rate recording. The actograph detects fetal movements by preserving the remaining signal after bandpassing frequency components of the Doppler signal that are associated with fetal heart rate and maternal somatic activity. Reliability studies comparing actograph based versus ultrasound visualized fetal movements have found this monitor to be highly accurate in detecting both fetal motor activity and quiescence [57]. Digitized fetal data were resampled at 5 Hz for analysis. Fetal heart rate underwent error rejection procedures based on moving averages of acceptable values as needed using customized software; fetal movement data represent raw voltage values generated from the actograph that are scaled in arbitrary units (GESTATE; James Long Company).
Data analysis
Equipment or signal detection failure resulted in unusable data for 3/50 cases at 24 weeks and 1/47 cases at 36 weeks. One fetus remained too active to participate in the protocol even after 6 minutes of baseline at 36 weeks; resulting in final samples of n = 47 (24 weeks) and n = 45 (36 weeks). All fetuses evaluated at 36 weeks also provided data at 24 weeks.
Variable values were examined for signal distortion and outliers, resulting in slightly different ns per data stream in each analysis. Baseline means of the fetal and maternal measures were derived by averaging baseline data prior to the presentation of the stimulus and the sham conditions. Change in baseline from 24 to 36 weeks for each measure was evaluated via paired t-tests. The focus of the data analysis centered on multilevel modeling with random effects (SAS PROC MIXED) of repeated measures data with 5 data points per second. The MIXED procedure is a robust tool for modeling repeated measures data. In addition to examining between subject variation through fixed effects, specification of random effects accounts for correlations among measurements on the same subject over time. Unlike analysis of variance, it does not exclude cases with missing data. This strategy was used to examine maternal and fetal responsiveness to presentation of the airborne stimulus as compared to the sham condition, modeling sequential (i.e., repeated) maternal physiological parameters by condition (i.e., stimulus versus sham).
Because an immediate response was expected, fetal responses to the stimulus versus the sham were modeled within +/− 5 s of the origin. Maternal responses were modeled within 10 s following onset of fetal stimulation or sham. Linear and quadratic patterns were examined to address rate of change and curvature of the slope, respectively. The influence of order (stimulus applied first or second), sex, and parity were tested independently in these models.
Following this, change scores (deltas) were computed to examine response to the stimulus condition alone. Delta values were created by subtracting the average baseline level from each data point 30 s post stimulus application, in accord with methods employed elsewhere to discern prenatal responsiveness [40]. Pearson correlation coefficients of the averaged delta values were used to evaluate correspondence between the overall magnitude of fetal and maternal changes to stimulus. Multilevel modeling of delta values from between 1 to 30 s post stimulus application was used to compare the trajectories of the fetal and maternal responses between 24 and 36 weeks gestation.
Results
Per design protocol, presentation of the stimulus/sham condition had to be delayed for an additional 3 minute period in 8 instances due to excessive fetal movement at 36 weeks; no cases required delayed protocol onset at 24 weeks.
Fetal response to stimulation
Mean pre-condition baseline values for fetal measures at each gestational age are presented in Table 1. Although mean values were not used in the data analyses, these are included to provide information on level and variation over gestation. No order effects (i.e., stimulus or sham first) were detected for either fetal motor activity (24 weeks: t = −1.61, p = .11; 36 weeks: t = −0.48, p = .64) or fetal heart rate response to stimulation (24 weeks: t = −0.64, p = .53; 36 weeks: t = −0.23, p = .82). Similarly, there were no differences in fetal motor activity (24 weeks: t = −1.23, p = .23; 36 weeks: t = −0.71, p = .48) or heart rate response (24 weeks: t = −0.36, p = .72; 36 weeks: t = 0.82, p = .42) as a result of fetal sex. Order and fetal sex were not included in further analytic models.
Table 1.
Mean fetal and maternal 3-minute pre-condition baseline values by gestational age
| 24 weeks (n = 47) | 36 weeks (n = 45) | ||||
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | na | Mean (SD) | na | t | |
| Fetal measures | |||||
| Heart rate (bpm) | 146.68 (5.33) | 47 | 140.01 (8.76)) | 45 | −4.63* |
| Motor activity (aus) | 4.46 (1.11) | 47 | 4.75 (2.01) | 44 | 1.36 |
| Maternal measures | |||||
| Heart rate (bpm) | 78.19 (9.11) | 45 | 81.07 (9.74) | 44 | 3.47* |
| Skin conductance (μS) | 5.46 (2.29) | 44 | 6.47 (3.14) | 42 | 0.93 |
| Skin conductance response (μS) | −0.045 (0.04) | 44 | −0.049 (0.05) | 42 | 0.49 |
p < .01
Note. Minor variation in ns reflects variable-specific deletion of outliers or problematic signal.
The airborne stimulus was effective at generating the requisite fetal response. Fetuses reacted to the stimulus within 5 seconds after application, evidenced by a significant interaction effect for time by condition (i.e., stimulus v sham) predicting fetal motor activity. As a group, fetuses exhibited a transient increase in movement at both 24 weeks (β = .10, SE = .03, t = 3.02, p < .01) and 36 weeks gestation (β = .12, SE = .03, t = 4.19, p < .01) to the stimulus but not to the sham. Fetuses also reacted with an acceleratory heart rate response to the stimulus in comparison to sham (β = .21, SE = .06, t = 3.54, p < .01) at 36 weeks but not at 24 weeks (β = −.04, SE = .04, t = −1.05, p = .29).
Maternal response to fetal stimulation
Mean pre-condition baseline values for maternal measures at each visit are presented in Table 1. Note that skin conductance level is provided for contextual information; the de-trended skin conductance response variable was used for remaining analyses. There were no order effects for presentation at either gestational age for either maternal heart rate (24 weeks: t = −0.50, p = .63; 36 weeks: t = −0.76, p = .45) or skin conductance response (24 weeks: t = 0.76, p = .45; 36 weeks: t = −1.21, p = .23) to the stimulus condition. Fetal sex was also unrelated to maternal heart rate (24 weeks: t = 1.03, p = .31; 36 weeks: t = 0.02, p = .98) and skin conductance (24 weeks: t = 0.23, p = .82; 36 weeks: t = −1.30, p = .20). Order and fetal sex were not included in further analytic models.
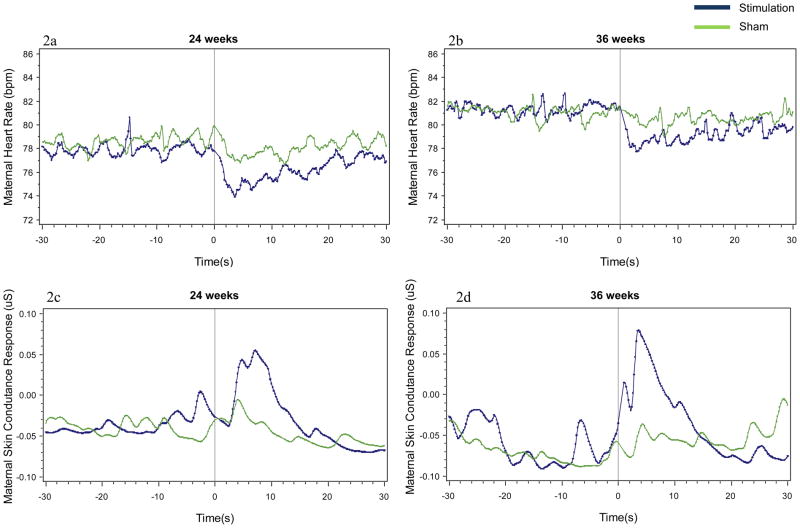
The maternal cardiac and electrodermal responses following fetal stimulus and sham conditions are presented in Figures 2a – 2d. Note that the figures provide data in seconds for clarity of viewing; analyses are based on 5 points per second. Maternal data were optimally modeled by quadratic change over time which captures the maternal reaction and recovery response. In contrast to the sham condition, the fetal stimulus condition induced significant suppression of maternal heart rate within 5 s after onset at 24 weeks gestation (Figure 2a, β = .24, SE = .12, t = 2.01, p < .05) and 36 weeks gestation (Figure 2b, β = .27, SE = .12, t = 2.34, p < .05). This was accompanied by a significant increase in maternal skin conductance response within 10 s at 24 weeks (Figure 2c, β = −0.001, SE = .001, t = −2.17, p < .05) and 36 weeks (Figure 2d, β = −0.002, SE = .001, t = −2.07, p < .05) compared to the sham.
Figure 2.
Mean maternal heart rate and maternal skin conductance response 30 seconds before and 30 seconds following stimulus and sham conditions at 24 weeks (a, c) and 36 (b, d) weeks.
Correlation coefficients computed on mean delta values for each maternal and fetal parameter did not detect any significant maternal-fetal associations at 24 weeks. At 36 weeks, greater fetal motor responses were associated with larger maternal skin conductance responses at 5, 10, 15, and 30 seconds after the stimulus (rs (42) = 0.48, 0.39, 0.36, and 0.42, respectively; ps range from p < .05 to p < .001). Significant or trend correlations were detected between the magnitude of the heart rate change in both mothers and fetuses (rs (44) = 0.29, 0.31, 0.31, and 0.26, respectively; ps range from p < .10 to p < .05). No significant associations between fetal movement and maternal heart rate responses, or fetal heart rate and maternal skin conductance responses were detected.
Parity (1st child vs all others) was related to maternal responsiveness. Nulliparous women exhibited greater skin conductance responsiveness than multiparous women in response to the fetal stimulation compared to sham condition at both gestational ages, indicated by a significant interaction between parity and condition (24 weeks, β = .04, SE = .01, t = 4.32, p < .05, 25 nullipara, 22 multipara; 36 weeks, β = .16, SE = .01, t = 11.23, p < .05, 24 nullipara, 21 multipara). At 24 weeks, nulliparous women also showed greater heart rate suppression to fetal stimulation (β = −1.33, SE = .31, t = −4.27, p < .05). In contrast, at 36 weeks, multipara showed greater heart rate suppression to fetal stimulation (β = 1.53, SE = .30, t = 5.08, p < .05).
Comparisons across gestational age
Table 1 presents t-values that evaluate change in pre-condition baseline levels from 24 to 36 weeks. As expected, baseline fetal heart rate declined and maternal heart rate increased; the remaining measures did not change significantly. Modeling of delta values to the stimulation was used to ascertain whether the fetal and maternal responses were different over time. As expected, since there was a significant fetal heart rate response at 36 but not 24 weeks, the interaction between gestational age and fetal heart rate was significant (β = −0.049, SE = .013, t = −3.80, p < .01). Although a fetal motor response was observed at both gestational ages, interaction results indicated that the responses were somewhat different (β = 0.045, SE = .008, t = 5.39, p < .01). Specifically, the elevation in motor activity to the stimulus persisted for a longer duration at 24 weeks than at 36 weeks.
Figure 3a shows the maternal heart rate delta values to the fetal stimulus condition by gestational age. The suppression pattern observed for maternal heart rate did not change in magnitude over time (β = −0.0008, SE = .001, t = −0.79, p = .43). The maternal skin conductance response (Figure 3b) was greater at 36 weeks than at 24 weeks (β = 0.002, SE = .0001, t = 5.68, p < .01).
Figure 3.
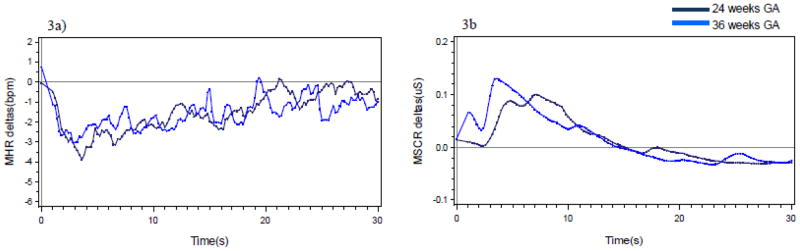
Mean change (delta) in maternal heart rate (a) and skin conductance response (b) to fetal stimulus at each gestational age.
Discussion
To our knowledge, this is the first demonstration of an experimentally elicited maternal response to human fetal behavior. As compared to the sham period, the evoked fetal response generated transient maternal heart rate suppression coupled with transient sympathetic activation at both 24 and 36 weeks. Moreover, the significant correspondence between the magnitude of the fetal motor response and the magnitude of the maternal skin conductance response at 36 weeks further supports fetal movement, evoked by the auditory stimulus, as the signal. While we are unable to determine a physiological mechanism for this association, and it is possible that currently undocumented neuroendocrinological signals from fetus to woman may play a role, perhaps the most obvious explanation would involve perturbations to the uterine wall. Although it has been known for some time that the uterine wall contains adrenergic innervations [58], the interplay between mechanical and endocrine processes in uterine activity remains a topic of active research [59]. The precise role, sensitivity, and direction of the pathways in which the mechanical stimulation of the uterine wall might elicit a sympathetic response in pregnant women are currently unknown. In addition, although pregnant women tend to become hyporesponsive to external stimulation with advancing gestation [60–62], here we found a greater maternal electrodermal response at the latter gestational age. This could be consistent with an interpretation that movements by larger fetuses provide more intense stimulation.
While the electrodermal response to elicited fetal movement is consistent with the observations made previously through time series analysis during spontaneous fetal movement [32, 34], the observed decrease in maternal heart rate was in contrast to the accelerative association observed in those reports with spontaneous activity. This may be attributable to differences in the characteristics of spontaneous movements versus those elicited by sudden onset of a disruptive stimulus. We suggest that this difference may be reconciled by the expression of a subtle maternal orienting response, as opposed to a defensive response, to fetal movement due to heightened maternal anticipation during the experiment. As a result, women may have been more sensitized to detecting abrupt onset of fetal movement in contrast to the experience of unexpected periods of spontaneous movement encountered during daily activities.
Although the focus of this report was not on the development of the fetal responsiveness to stimulation per se, the airborne stimulus used in this procedure effectively elicited a fetal response. Fetal motor activity was elicited at both 24 and 36 weeks while a fetal heart rate response was isolated to the latter gestational age. This is consistent with other reports that note changes in responsiveness to airborne or vibratory stimuli with advancing maturation [38, 41, 48, 63]. While the significantly greater fetal motor response observed at 24 weeks may be somewhat counter-intuitive to a maturational phenomenon, it was attributable to a prolonged period of motor activity following stimulation. As such, this can be regarded as indicative of a less well-regulated response earlier in gestation, as opposed to a more self-limiting motor reaction near term. We did not observe a sex difference in fetal reactivity at either gestational age as has been reported in one study [40] but not in another [49]. The presence or absence of sex differences in fetal responses to stimulation awaits confirmation by additional research with larger samples.
The consequences of both elicited and spontaneous fetal motor activity for the pregnant woman are unknown. We have previously suggested that periodic perturbations to maternal autonomic processes constitute fetal signaling [32]. Fetal movement is a ubiquitous signal since the fetus moves approximately once per minute during the second half of gestation [64–66]. We offer the possibility that repeated perturbations to the maternal autonomic nervous system may unconsciously redirect maternal attention from the broader external environment towards the intrauterine one. Persistent but intermittent sympathetic signals provided by the fetus may serve to entrain maternal arousal patterns to fetal behavior with implications for the impending demands of caring for a neonate. Despite the repetitive nature of fetal motor activity, women exhibit greater skin conductance responsivity at the 36 week visit compared to the earlier one; suggesting that the signal remains salient, particularly for nulliparous women who are experiencing it for the first time. We have previously shown that women also do not habituate to spontaneous fetal movement [34]. Pregnancy has been associated with decrements in memory performance that have typically be attributed to hormonal changes[67]. We further suggest that the repeated, but unattributed, sympathetic activation of the mother by the fetus may negatively affect cognitive performance by interfering with parasympathetic processes that are required for maintenance of attention. From this perspective, the working memory interference observed during pregnancy can be interpreted not in terms of a deficit, but rather as a relatively minor cost of enhanced sensitization to the fetus in preparation for motherhood. A fairly small but emerging literature is aimed at identifying the ways in which the maternal nervous system and corresponding functional changes in cognitive and psychological performance is benefitted by pregnancy in a manner that maximizes rearing of offspring [68–70].
Does the fetus program the mother? While much attention has been recently devoted to maternal “programming” effects on the fetus [71], there is increasing evidence of the role that the fetus plays in its own development, which includes providing feedback to maternal systems [72]. In addition to spontaneous behaviors, the fetus can mount neurohormonal responses that are independent of maternal responses [73]. Both processes can maximize intrauterine environmental adaptation, generate epigenetic shaping of the sensory, skeletal, and nervous systems, stimulate parturition, and provide preparation for successful transition to the postnatal environment [29–31, 74, 75]. The construct of the predictive adaptive response [76] attributes developmental success to the degree to which the postnatal environment is consistent with the prenatal environment of a developing organism. This perspective has received recent empirical support with respect to matching of prenatal and postnatal maternal psychological state [77]. As such, one could envision that a fetus that reliably evokes a maternal physiological response, or a mother that reliably mounts a physiological response, may become a better adapted pair after birth. This possibility cannot be addressed by the current analysis, which focuses on group comparisons between stimulus and control conditions. However, examination of individual differences in maternal and fetal responsiveness along with consistency over time in relation to postnatal interaction patterns is underway within our research program.
The longer-term consequences of repeated cardiovascular and sympathetic responses generated by fetal movements for women after pregnancy and independent of caregiving are unknown, consistent with the limited understanding of how pregnancy affects women’s health and well-being over the lifespan. However, studies that compare non-pregnant women to women during pregnancy and/or longitudinal studies during pregnancy have found alterations in autonomic balance and sympathetic activation during pregnancy [78, 79] which increase as pregnancy advances [55, 78]. Such findings are consistent with a cumulative effect of fetal stimulation over time but the many other significant physiological changes that accompany pregnancy are likely to also be contributory.
The isolation of a maternal response in the current study to specific fetal behavior confirms the bidirectional nature of the developing maternal-fetal relationship. Modern models of maternal-infant or maternal-child interaction are complex and generally incorporate dynamic influences that flow in both directions and continuously feedback to further influence each member of the dyad. Such conceptually complex, transactional processes often strain methodological and statistical capabilities to directly assess them and investigators necessarily rely on evaluating unidirectional or sequential effects. Here we have shown a unidirectional, upstream, effect from fetus to pregnant woman within a research context that has traditionally examined downstream effects from pregnant woman to fetus. The prenatal period remains one of the last developmental frontiers and leaves much to be discovered about the complex dynamic between fetus and pregnant woman and the implications for subsequent psychophysiologic regulation of each.
Acknowledgments
This research was conducted with funding provided by The Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, R01 HD 27592-19, awarded to the first author.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bell RQ. A reinterpretation of the direction of effects in studies of socialization. Psychol Rev. 1968;75:81–95. doi: 10.1037/h0025583. [DOI] [PubMed] [Google Scholar]
- 2.Feldman R. Parent-infant synchrony: biological foundations and developmental outcomes. Curr Dir Psychol Sci. 2007;16:340–5. [Google Scholar]
- 3.Field T. In: Psychobiological attunement in close relationships. Featherman D, Lerner R, Perlmutter M, editors. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. pp. 1–25. [Google Scholar]
- 4.Harrist A, Waugh R. Dyadic synchrony: its structure and function in children’s development. Dev Rev. 2002;22:555–92. [Google Scholar]
- 5.Kohanska G, Akson N. Development of mutual responsiveness between parents and their young children. Child Dev. 2004;75:1657–76. doi: 10.1111/j.1467-8624.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- 6.Davis E, Glynn L, Waffarn F, Sandman C. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2010;52:119–29. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gluckman P, Hanson M, Cooper C, Thornburg K. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellison P. Fetal programming and fetal psychology. Infant Child Dev. 2010;19:6–20. [Google Scholar]
- 9.Phillips D. Programming of adrenocortical function and the fetal origins of adult disease. J Endocrinol Invest. 2001;24:742–6. doi: 10.1007/BF03343920. [DOI] [PubMed] [Google Scholar]
- 10.Entringer S, Buss C, Wadhwa P. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of emprical findings. Curr Opin Endocrinol Diabetes Obes. 2010;17:507–16. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Bergh B, Mulder E, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioral development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29:237–58. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Visser G, Mulder E, Ververs FT. Fetal behavioral teratology. J Matern Fetal Neonatal Med. 2010;23:14–6. doi: 10.3109/14767058.2010.517717. [DOI] [PubMed] [Google Scholar]
- 13.DiPietro JA, Hilton SC, Hawkins M, Costigan KA, Pressman EK. Maternal stress and affect influence fetal neurobehavioral development. Dev Psychol. 2002;38:659–68. [PubMed] [Google Scholar]
- 14.Groome LJ, Swiber MJ, Bentz LS, Holland SB, Atterbury JL. Maternal anxiety during pregnancy: Effect on fetal behavior at 38 and 40 weeks of gestation. J Dev Behav Pediatr. 1995;16:391–6. [PubMed] [Google Scholar]
- 15.Van den Bergh B. The influence of maternal emotions during pregnancy on fetal and neonatal behavior. Pre- Perinat Psychol. 1990;5:119–30. [Google Scholar]
- 16.Field T, Diego M, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, Bendell D. Pregnancy anxiety and comorbid depression and anger: effects on the fetus and neonate. Depress Anxiety. 2003;17:140–51. doi: 10.1002/da.10071. [DOI] [PubMed] [Google Scholar]
- 17.Class Q, Buss C, Davis E, Gierczak M, Pattillo C, Chicz-DeMet A, Sandman C. Low levels of cortioctropin-releasing homone during early pregnancy are associated with precocious maturation of the human fetus. Dev Neurosci. 2008;30:419–26. doi: 10.1159/000191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandman CA, Wadhwa PD, Chica-DeMet A, Dunkel-Schetter C, Porto M. Maternal stress, HPA activity, and fetal/infant outcome. Ann N Y Acad Sci. 1997;814:266–75. doi: 10.1111/j.1749-6632.1997.tb46162.x. [DOI] [PubMed] [Google Scholar]
- 19.DiPietro JA, Kivlighan KT, Costigan KA, Laudenslager ML. Fetal motor activity and maternal cortisol. Dev Psychobiol. 2009;51:505–12. doi: 10.1002/dev.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Copher DE, Huber C. Heart rate response of the human fetus to induced maternal hypoxia. Am J Obstet Gynecol. 1967;98:320–35. doi: 10.1016/0002-9378(67)90151-2. [DOI] [PubMed] [Google Scholar]
- 21.Benson P, Little BC, Talbert DG, Dewhurst J, Priest RG. Foetal heart rate and maternal emotional state. Br J Med Psychol. 1987;60:151–4. doi: 10.1111/j.2044-8341.1987.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 22.DiPietro J, Costigan K, Gurewitsch E. Fetal response to induced maternal stress. Early Hum Dev. 2003;74:125–38. doi: 10.1016/j.earlhumdev.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Fink NS, Urech C, Berger CT, Hoesli I, Holzgreve W, Bitzer J, Alder J. Maternal laboratory stress influences fetal neurobehavior: Cortisol does not provide all answers. J Matern Fetal Neonatal Med. 2010;23:488–500. doi: 10.3109/14767050903300985. [DOI] [PubMed] [Google Scholar]
- 24.Araki M, Nishitani S, Ushimaru K, Masuzaki H, Oishi K, Shinohara K. Fetal response to induced maternal emotions. J Physiol Sci. 2010;60:213–2020. doi: 10.1007/s12576-010-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, Tager F, Fifer WP. Fetal heart rate reactivity differs by women’s psychiatric status: An early marker for developmental risk? J Am Acad Child Adolesc Psychiatry. 2004;43:283–90. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 26.DiPietro JA, Costigan KA, Nelson P, Gurewitsch ED, Laudenslager ML. Fetal responses to induced maternal relaxation during pregnancy. Biol Psychol. 2008;77:11–9. doi: 10.1016/j.biopsycho.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink NS, Urech C, Isabel F, Meyer A, Hoesli I, Bitzer J, Alder J. Fetal response to abbreviated relaxation techniques. A randomized controlled study. Early Hum Dev. 2011;87:121–7. doi: 10.1016/j.earlhumdev.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Als H. Toward a synactive theory of development: Promise for the assessment and support of infant individuality. Infant Ment Health J. 1982;3:229–43. [Google Scholar]
- 29.Prechtl HFR. In: Continuity and change in early neural development. Prechtl H, editor. Philadelphia, PA: J.B. Lippincott Co; 1984. pp. 1–15. [Google Scholar]
- 30.Smotherman WP, Robinson SR. The development of behavior before birth. Dev Psychol. 1996;32:425–34. [Google Scholar]
- 31.Visser GHA. Fetal behaviour: a commentary. Neurobiol Aging. 2004;24:S47–S9. [Google Scholar]
- 32.DiPietro JA, Irizarry RA, Costigan KA, Gurewitsch ED. The psychophysiology of the maternal-fetal relationship. Psychophysiology. 2004;41:510–20. doi: 10.1111/j.1469-8986.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 33.Johnson TRB, Jordan ET, Paine LL. Doppler recordings of fetal movement: II. Comparison with maternal perception. Obstet Gynecol. 1990;76:42–3. [PubMed] [Google Scholar]
- 34.DiPietro J, Caulfield LE, Irizarry RA, Chen P, Merialdi M, Zavaleta N. Prenatal development of intrafetal and maternal-fetal synchrony. Behav Neurosci. 2006;120:687–701. doi: 10.1037/0735-7044.120.3.687. [DOI] [PubMed] [Google Scholar]
- 35.Busnel M, Granier-Deferre C, Lecanuet J. Fetal audition. Annnals of New York Academy of Science. 1992;662:118–34. doi: 10.1111/j.1749-6632.1992.tb22857.x. [DOI] [PubMed] [Google Scholar]
- 36.Moon C, Fifer W. Evidence of transnatal auditory learning. J Perinatol. 2000;20:S36–S43. doi: 10.1038/sj.jp.7200448. [DOI] [PubMed] [Google Scholar]
- 37.Sontag LW, Wallace RF. The movement response of the human fetus to sound stimuli. Child Dev. 1935;6:253–8. [Google Scholar]
- 38.Grant-Buettler M, Glynn L, Salisbury A, Davis E, Holliday C, Sandman C. Development of fetal movement between 26 and 36 weeks’ gestation in response to vibro-acoustic stimulation. Front Psychol. 2011;2:Article 350. doi: 10.3389/fpsyg.2011.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dirix C, Nijhuis J, Jongsma H, Hornstra G. Aspects of fetal learning and memory. Child Dev. 2009;80:1251–8. doi: 10.1111/j.1467-8624.2009.01329.x. [DOI] [PubMed] [Google Scholar]
- 40.Buss C, Davis E, Class Q, Gierczak M, Pattillo C, Glynn L, Sandman C. Maturation of the human fetal startle response: evidence for sex-specific maturation of the human fetus. Early Hum Dev. 2009;85:633–8. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiPietro J, Hodgson DM, Costigan KA, Hilton SC, Johnson TRB. Fetal neurobehavioral development. Child Dev. 1996;67:2553–67. [PubMed] [Google Scholar]
- 42.Zimmer EZ, Divon MY. Fetal vibroacoustic stimulation. Obstet Gynecol. 1993;81:451–7. [PubMed] [Google Scholar]
- 43.Visser GHA, Mulder EJH. The effect of vibroacoustic stimulation on fetal behavioral state organization. Am J Ind Med. 1993;23:531–9. doi: 10.1002/ajim.4700230403. [DOI] [PubMed] [Google Scholar]
- 44.Kisilevsky BS, Muir DW, Low JA. Maturation of human fetal responses to vibroacoustic stimulation. Child Dev. 1992;63:1497–508. [PubMed] [Google Scholar]
- 45.Gagnon R, Foreman J, Hunse C, Patrick J. Effects of low frequency vibration on human term fetuses. Am J Obstet Gynecol. 1989;162:1479–85. doi: 10.1016/0002-9378(89)90908-3. [DOI] [PubMed] [Google Scholar]
- 46.Groome LJ, Mooney DM, Holland SB, Smith YD, Atterbury JL, Dykman RA. Temporal pattern and spectral complexity as stimulus parameters for eliciting a cardiac orienting reflex in human fetuses. Percept Psychophys. 2000;62:313–20. doi: 10.3758/bf03205551. [DOI] [PubMed] [Google Scholar]
- 47.Lecanuet J, Granier-Deferre C, Busnel M. Fetal cardiac and motor response to octave-band noises as a function of central frequency, intensity and heart rate variability. Early Hum Dev. 1988;18:81–93. doi: 10.1016/0378-3782(88)90045-x. [DOI] [PubMed] [Google Scholar]
- 48.Kisilevsky B, Pang L, Hains S. Maturation of human fetal responses to airborne sound in low- and high-risk fetuses. Early Hum Dev. 2000;58:179–95. doi: 10.1016/s0378-3782(00)00075-x. [DOI] [PubMed] [Google Scholar]
- 49.Hepper P. Sex differences in fetal habituation. Developmental Science. 2012;15:373–83. doi: 10.1111/j.1467-7687.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- 50.Hepper P, Scott D, Shahidullah S. Newborn and fetal response to maternal voice. J Reprod Infant Psychol. 1993;11:147–53. [Google Scholar]
- 51.Zimmer EZ, Fifer WP, Kim Y, Rey HR, Chao CR, Myers MM. Response of the premature fetus to stimulation by speech sounds. Early Hum Dev. 1993;33:207–15. doi: 10.1016/0378-3782(93)90147-m. [DOI] [PubMed] [Google Scholar]
- 52.Lecanuet JP, Granier-Deferre C, Jacquet A, Busnel MC. Decelerative cardiac responsiveness to acoustical stimulation in the near term fetus. Q J Exp Psychol. 1992;44:279–303. doi: 10.1080/02724999208250616. [DOI] [PubMed] [Google Scholar]
- 53.Kisilevsky B, Hains S, Jacquet A, Granier-Deferre C, Lecanuet J. Maturation of fetal resonses to music. Dev Sci. 2004;7:550–9. doi: 10.1111/j.1467-7687.2004.00379.x. [DOI] [PubMed] [Google Scholar]
- 54.Venables PH. Autonomic activity. Ann N Y Acad Sci. 1991;620:191–207. doi: 10.1111/j.1749-6632.1991.tb51584.x. [DOI] [PubMed] [Google Scholar]
- 55.DiPietro J, Costigan KA, Gurewitsch ED. Maternal physiological change during the second half of gestation. Biol Psychol. 2005;69:23–38. doi: 10.1016/j.biopsycho.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Nijhuis JG, Prechtl HFR, Martin CB, Bots RSG. Are there behavioural states in the human fetus? Early Hum Dev. 1982;6:47–65. doi: 10.1016/0378-3782(82)90106-2. [DOI] [PubMed] [Google Scholar]
- 57.Maeda K, Tatsumura M, Utsu M. Analysis of fetal movements by Doppler actocardiogram and fetal B-mode imaging. Clin Perinatol. 1999;26:829–51. [PubMed] [Google Scholar]
- 58.Owman C, Rosengren E, Sjoberg N. Adrenergic innervation of the human fetal reproductive organs: a histochemical and chemical investigation. Obstet Gynecol. 1967;30:763–73. [PubMed] [Google Scholar]
- 59.Shynlova O, Tsui P, Jaffer S, Lye S. Integration of endocrine and mechanical signals in the regulation of myometiral functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144S:S2–S10. doi: 10.1016/j.ejogrb.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 60.Entringer S, Buss C, Shirtcliff EA, Cammack AL, Yim IS, Chicz-DeMet A, Sandman CA, Wadhwa PD. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010;13:258–68. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glynn LM, Schetter CD, Wadhwa PD, Sandman CA. Pregnancy affects appraisal of negative life events. J Psychosom Res. 2004;56:47–52. doi: 10.1016/S0022-3999(03)00133-8. [DOI] [PubMed] [Google Scholar]
- 62.Nierop A, Klinkenberg ABA, Nater U, Zimmermann R, Ehlert U. Prolonged salivary cortisol recovery in second-trimester pregnant women and attenuated salivary alpha-amylase response to psychosocial stress in human pregnancy. J Clin Endocrinol Metab. 2006;91:1329–35. doi: 10.1210/jc.2005-1816. [DOI] [PubMed] [Google Scholar]
- 63.Gagnon R, Hunse C, Carmichael L, Fellows F, Patrick J. Human fetal responses to vibratory acoustic stimulation from twenty-six weeks until term. Am J Obstet Gynecol. 1987;157:1375–81. doi: 10.1016/s0002-9378(87)80227-2. [DOI] [PubMed] [Google Scholar]
- 64.Roberts AB, Griffin D, Mooney R, Cooper DJ, Campbell S. Fetal activity in 100 normal third trimester pregnancies. Br J Obstet Gynaecol. 1980;87:480–4. doi: 10.1111/j.1471-0528.1980.tb04582.x. [DOI] [PubMed] [Google Scholar]
- 65.DiPietro JA, Caulfield LE, Costigan KA, Merialdi M, Nguyen RHN, Zavaleta N, Gurewitsch E. Fetal neurobehavioral development: a tale of two cities. Dev Psychol. 2004;40:445–56. doi: 10.1037/0012-1649.40.3.445. [DOI] [PubMed] [Google Scholar]
- 66.Nasello-Paterson C, Natale R, Connors G. Ultrasonic evaluation of fetal body movements over twenty-four hours in the human fetus at twenty-four to twenty-eight weeks’ gestation. Am J Obstet Gynecol. 1988;158:312–6. doi: 10.1016/0002-9378(88)90145-7. [DOI] [PubMed] [Google Scholar]
- 67.Glynn LM. Giving birth to a new brain: Hormone exposures of pregnancy influence human memory. Psychoneuroendocrinology. 2010b;35:1148–55. doi: 10.1016/j.psyneuen.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 68.Kinsley CH. The neuroplastic maternal brain. Horm Behav. 2008;54:1–4. doi: 10.1016/j.yhbeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 69.Maeng L, Shors T. Once a mother, always a mother: maternal experience protects femals from the negative effects of stress on learning. Behav Neurosci. 2012;126:137–41. doi: 10.1037/a0026707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci. 2010;124:695–700. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barker D. In utero programming of chronic disease. Clinical Sciences. 1998;2:115–28. [PubMed] [Google Scholar]
- 72.Glynn L, Sandman C. Prenatal origins of neurological development: a critical period for fetus and mother. Cur Dir Psychol Sci. 2011;20:384–9. [Google Scholar]
- 73.Gitau R, Fisk N, Teixeira J, Cameron A, Glover V. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86:104–9. doi: 10.1210/jcem.86.1.7090. [DOI] [PubMed] [Google Scholar]
- 74.Petraglia F, Imperatore A, Challis JRG. Neuroendocrine mechanisms in pregnancy and parturition. Endocr Rev. 2010;31:783–816. doi: 10.1210/er.2009-0019. [DOI] [PubMed] [Google Scholar]
- 75.Challis J, Lye S, Gibb W, Whittle W, Patel F, Alfaidy N. Understanding preterm labor. Ann N Y Acad Sci. 2001;943:225–34. doi: 10.1111/j.1749-6632.2001.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 76.Gluckman PD, Hanson MA, Spencer HG, Bateson P. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proc Biol Sci. 2005;272:671–7. doi: 10.1098/rspb.2004.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sandman C, Davis E, Glynn L. Prescient human fetuses thrive. Psychol Sci. 2012;23:93–100. doi: 10.1177/0956797611422073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuo CD, Chen GY, Yang MJ, Lo HM, Tsai YS. Biphasic changes in autonomic nervous activity during pregnancy. Br J Anaesth. 2000;84:323–9. doi: 10.1093/oxfordjournals.bja.a013433. [DOI] [PubMed] [Google Scholar]
- 79.Klinkenberg AV, Nater UM, Nierop A, Bratsikas A, Zimmerman R, Ehlert U. Heart rate variability changes in pregnant and non-pregnant women during standardized psychosocial stress. Acta Obstet Gynecol Scand. 2009;88:77–82. doi: 10.1080/00016340802566762. [DOI] [PubMed] [Google Scholar]