Abstract
Alopecia areata is a cell-mediated autoimmune disease of humans and many domestic and laboratory animal species. C3H/HeJ inbred mice spontaneously develop alopecia areata at a low frequency (approximately 20% by 12 mo of age). Transferring full-thickness skin grafts from affected, older mice to young mice of the same strain reliably reproduces alopecia areata, thus enabling investigators to study disease pathogenesis or intervention with a variety of therapeutic approaches. We here describe in detail how to perform full-thickness skin grafts and the follow-up procedures necessary to consistently generate mice with alopecia areata. These engrafted mice can be used to study the pathogenesis of cell-mediated autoimmune disease and for drug-efficacy trials. This standard protocol can be used for many other purposes when studying abnormal skin phenotypes in laboratory mice.
Abbreviations: AA, alopecia areata; DEBR, Dundee experimental bald rat
Alopecia areata (AA) is a nonscarring, cell-mediated, autoimmune disease that causes hair loss in humans. At any time, between 0.05% and 0.1% of people express some form of the disease.3,19,20 Hair loss has been characterized as patchy (alopecia areata), total loss on the top of the head (alopecia totalis), or total loss of all body hair (alopecia universalis). Progress in understanding the pathogenesis and genetics of AA as well as the means to develop and test new therapies was severely hampered until the development of a spontaneous mouse (C3H/HeJ) disease model that very closely mimics the adult-onset form of AA.23,26 In addition to the laboratory mouse, several other species have been proposed as models for AA, but most are poorly characterized or not readily available. These include hair loss syndromes in dogs, cats, horses, cattle, and nonhuman primates and even a feather-loss syndrome in chickens.11 The Dundee experimental bald rat (DEBR) also has many features of AA.15-17
C3H/HeJ mice develop a spontaneous, complex polygenic, AA-like hair loss.25,30 Mouse AA undergoes stages of waxing and waning in terms of clinically evident areas of alopecia, and the extent of alopecia varies greatly between subjects, thus complicating the use of these spontaneous models as drug-screening tools. Full-thickness skin grafts initially were used as a tool to decipher whether the inflammation seen histologically was driving the skin lesions or whether the skin abnormalities caused changes that resulted in localized, chronic inflammation.10 To this end, we grafted affected skin to severely immunodeficient (Prkdcscid) mutant mice congenic on the C3H/HeJ background and to histocompatible C3H/HeJ mice of the same sex as the donor. We found that full-thickness skin grafts could be used to initiate AA in histocompatible recipients in a controlled and predictable manner. Hair regrew in the immunodeficient mice, but it regrew white rather than agouti,10 a feature also seen in human AA and in injured mouse skin because of damage to melanocyte stem cells.13 Both the spontaneous and graft-induced forms of this mouse model have been used extensively to test hypotheses regarding disease mechanisms and responses to various treatments and to refute the association of AA with suspected infectious or antigenic challenges.5,9,22,27 This graft-initiated mouse model is now readily available as individual mice or for contract drug-efficacy trials (The Jackson Laboratory, West Sacramento, CA; http://jaxmice.jax.org/services/alopecia_areata.html;http://jaxmice.jax.org/library/notes/504/504b.html).
We here describe how to perform full-thickness skin grafts in mice, to enable investigators to reliably reproduce this AA model system in their own laboratories.
Materials and Methods
Mice.
For this AA mouse model, C3H/HeJ mice (10-wk-old recipients or affected donors [age, 8 to 10 mo or older]) are available from The Jackson Laboratory (Bar Harbor, ME). Female mice are typically, but not exclusively, used because AA is somewhat more frequent and severe in female than male patients.26
Mice need to be maintained under carefully controlled husbandry conditions, with particular attention to diet. Rodent diets high in phytoestrogens have a profound negative effect on the success of graft methods.12 Our mouse colony is maintained in a humidity-, temperature-, and light- (12:12-h) controlled vivarium under SPF conditions (http://jaxmice.jax.org/html/health/quality_control.shtml#Animalhealth).26 At our facility, mice are routinely housed in double-pen polycarbonate cages (floor area, 330 cm2) at a maximal capacity of 4 mice per pen. Mice are allowed free access to autoclaved food (NIH 31, 6% fat; LabDiet 5K52, Purina Mills, St Louis, MO) and acidified water (pH 2.8 to 3.2). After surgery, mice are individually housed until the graft sites have healed.
All procedures were done with the approval of The Jackson Laboratory Animal Care and Use Committee.
Recommended surgical procedure.
Donor mice.
Donor mice with generalized hair loss and remnants of hair are euthanized by CO2 asphyxiation, according to the recommendations of the American Veterinary Medical Association6 and The Jackson Laboratory Animal Care and Use Committee.1,24 Representative areas are biopsied to confirm the diagnosis (see Histopathology). Areas of skin with hair loss are surgically prepared and then harvested in approximately 1-cm2 units. Specifically, the dorsal skin is shaved with electric clippers; remaining cut hairs are removed by using adhesive tape; and skin is disinfected with 70% ethanol and surgical iodine. Ethanol and iodine are applied to the center of the surgical field and worked centrifugally outward. This procedure is done using aseptic surgical methods for harvesting grafts on the dorsum and repeated before harvesting on the ventrum. The donor mouse is placed on a platform under a dissection microscope. Grafts (diameter, 1 cm or less) are removed by elevating a fold of skin from the desired site with sterile forceps. A circular or oblong piece of skin then is excised (Micro Dissecting Curved, Sharp Scissors, Roboz Surgical Instrument, Gaithersburg, MD); grafts are harvested from both the ventral and dorsal surfaces of the donor mice.
The excised grafts are placed epidermal side down on a sterile flat surface, and all subcutaneous fascia is scraped away by using forceps. The grafts then are transferred to a culture dish (diameter, 100 mm) containing sterile 0.9% saline solution (Am Tech, St Joseph, MO) at room temperature and placed dermal side down. The grafts are stored in saline for as long as 4 h at room temperature until grafted onto recipient mice.
Recipient mice.
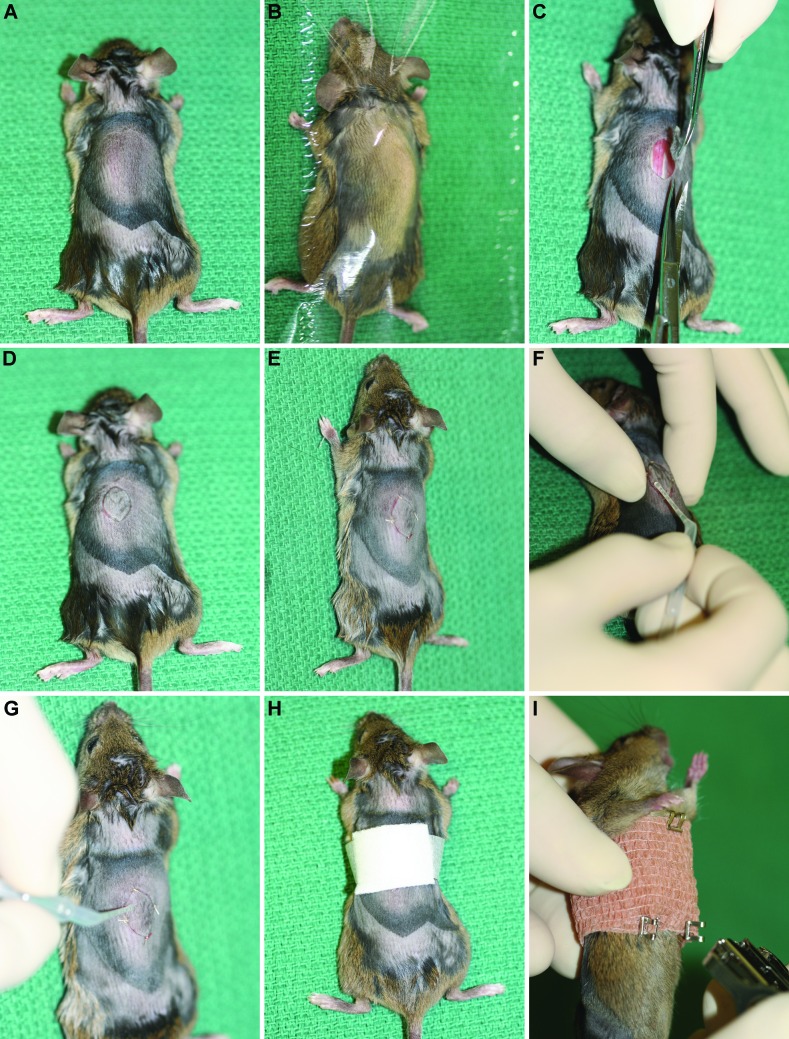
Recipient mice are anesthetized by using intraperitoneal tribromoethanol (0.2 mL per 10 g body weight; Aldrich, Milwaukee, WI).1 Isoflurane gas anesthesia can be used as an alternative to tribromoethanol. Recipient mice receive carprofen subcutaneously (0.1 mL per 10 g body weight; Rimadyl, Pfizer Animal Health, New York, NY) immediately after the administration of tribromoethanol, to alleviate pain or distress. Lubricating ointment (Puralube, Pharmaderm Animal Health, Melville, NY) is applied over the eyes. The dorsal skin surface at the level of the thoracolumbar junction is prepared for surgery, as done previously for the donor skin site. Anesthetized recipient mice are positioned in ventral recumbency to provide easy access to the graft site (Figure 1 A). The dorsal skin is covered with a sterile clear plastic drape (Figure 1 B; Medical Concepts Development, Woodbury, MN; Vi-Drape; http://medconceptsdev.com/category/103-incisesurgical-film.aspx) that has a hole cut in it to expose the surgical site. A fold of skin over the graft site is elevated by using forceps, and a circular or oblong piece of skin (diameter, 1 cm or less) is excised by using curved scissors (Figure 1 C). The donor graft then is placed in the recipient site (Figure 1 D). The graft is oriented 180° from normal, to allow the hair to grow in the opposite direction of the recipient's hair and for easy identification of the site after healing.10
Figure 1.
Surgical approach. (A) The dorsal skin of the graft recipient has been shaved by using electric clippers, and the site is disinfected for surgery. Note the differences in skin color, which depict areas in anagen (dark) compared with telogen (light).29 (B) The site is draped at this point (as a routine part of the procedure), but the drapes were not used for the remaining images, for the purposes of illustration. (C) The skin is picked up (tented) with forceps, and an elliptical incision is made with curved iris scissors. (D)The oval donor skin sample is turned 180° and placed in the wound on the back of the recipient mouse when used as a control. (E) The graft is sutured in 4 corners, and then (F, G) the edges are glued. (H) A nonstick dressing pad, is applied over the surgical site and taped in place. (I) Coban wrap is cut to fit the mouse, wrapped completely around the thorax, and fixed with wound clips.
To fix the graft in place, we use 4 simple interrupted sutures (5-0 Dexon S, Davis and Geck, Manati, Puerto Rico) on opposite sides of the graft (Figure 1 E). Surgical glue (Surgi-lock 2oc, Meridian Animal Health, Omaha, NE) is applied to the edges of the graft (Figure 1 F and G). After the graft is secured in position, a 0.1% solution of bupivacaine is dripped topically onto the graft site, which is then covered with a sterile dressing pad (Tefla, Kendall, Boston, MA; Figure 1 H), secured with surgical tape (3M Medical Surgical Division, St Paul, MN), and covered with self-adhesive bandage (Coban, 3M) to create a pressure bandage (Figure 1 I). Care is taken when wrapping the mouse with the self-adhesive bandage, to preserve normal respiration and locomotion. Wound clips are placed in the skin at the back of the neck, the thorax, and on each side of the pelvis to hold the bandages in place and therefore make it more difficult for the mouse to remove the bandage (Figure 1 I).
Support during recovery.
After surgery, mice are placed in ventral recumbency on a clean paper towel within the cage. Sterile saline (1 to 2 mL per 10 g body weight) is given subcutaneously to correct fluid deficits. A 40-W light bulb is placed over one end of the cage to warm the mice until they have recovered completely from anesthesia. To prevent overheating, mice should never be placed directly under the light. In addition, placing a thermometer in the cage to monitor the temperature will help to ensure the mice do not overheat. The mice are transferred back to the isolator caging after they have recovered from anesthesia.
Postsurgical care.
Mice should receive sulfamethoxazole (200 mg sulfamethoxazole plus 40 trimethoprim in 225 mL water; Schein Pharmaceutical, Florham, NJ) in their drinking water for 1 wk after surgery, to minimize the risk of infection. The mice are observed daily for the first week after surgery, to assure that the animals are eating, drinking, and behaving normally and that they have not removed the bandage or destroyed the graft site. If the bandage has been removed, the dressing pad and self-adhering bandage are reapplied. Each mouse is observed for signs of pain or distress and is treated accordingly as needed. Mice that are in distress or pain should receive carprofen every 24 h for 2 to 3 d. Signs of distress or pain may include, but not be limited to, aggressive behavior when handled, decreased food and water intake, decreased activity, hiding and burrowing. Placing food pellets or moistened crushed food pellets on the bottom of the cage will encourage the mice to eat.
Bandages, sutures, and wound clips are removed 7 to 9 d after surgery, and recipient mice are separated into individual cages. This separation is done so that the mice do not remove the unhealed grafts from each other. The graft sites and adjacent skin are monitored for hair growth or loss during the subsequent 20-wk observation period.
Control mice.
Unaffected controls are created by using age- and sex-matched C3H/HeJ mice with no clinical evidence of AA. Skin is prepared as described earlier, and the surgery is done in the same way. The control mouse serves as both donor and recipient: the skin is removed as described, rotated 180°, and grafted back onto the same mouse.
Histopathology.
When the skin from the donor mice is harvested for engraftment, additional representative samples should be collected from areas adjacent to the excised sites and placed on aluminum foil. The skin is flattened on the foil to eliminate wrinkles, oriented in an craniocaudal direction by labeling the foil, fixed in toto by immersion in Fekete acid–alcohol–formalin solution (100 mL 70% ethanol, 5 mL glacial acetic acid, and 10 mL 37% to 40% formalin or other fixative) overnight, and then transferred to 70% ethanol for storage until trimming.21 The skin is trimmed longitudinally in a craniocaudal orientation, to follow the natural angle of hair follicles emerging from the skin. Tissues are processed through graded ethanols and solvents, embedded in paraffin, and sectioned at 6 µm; slides are stained with hematoxylin and eosin.
Results
As verified previously through gene expression studies, histology, and electron microscopy,2,14 wounds usually heal uneventfully within 2 wk. Both AA recipients and sham grafted mice regrow hair within the shaved surgical sites. The AA mice begin to show evidence of hair loss initially adjacent to the skin graft site as well as in the axillary and inguinal regions of the ventral skin from as early as 5 to 6 wk after surgery but usually by 8 to 10 wk. Ventral alopecia expands centrifugally, and patchy alopecia develops on the dorsal skin. Mice are completely bald, with just stubble, by 20 wk after surgery.
To date we have completed nearly 1000 skin-graft surgeries, with a failure rate of less than 10%. Failures primarily are due to loss of the skin grafts when mice remove bandages and destroy the grafted skin. Control mice need to be followed carefully to ensure that they do not develop AA spontaneously, given that the C3H/HeJ inbred strain has a spontaneous background rate of approximately 20% in mice 12 mo of age and older, but AA can start at 6 mo of age or earlier.26 The control mice that develop AA cannot be used as controls; however relatively few (approximately 2%) develop AA, which typically occurs in much older mice.
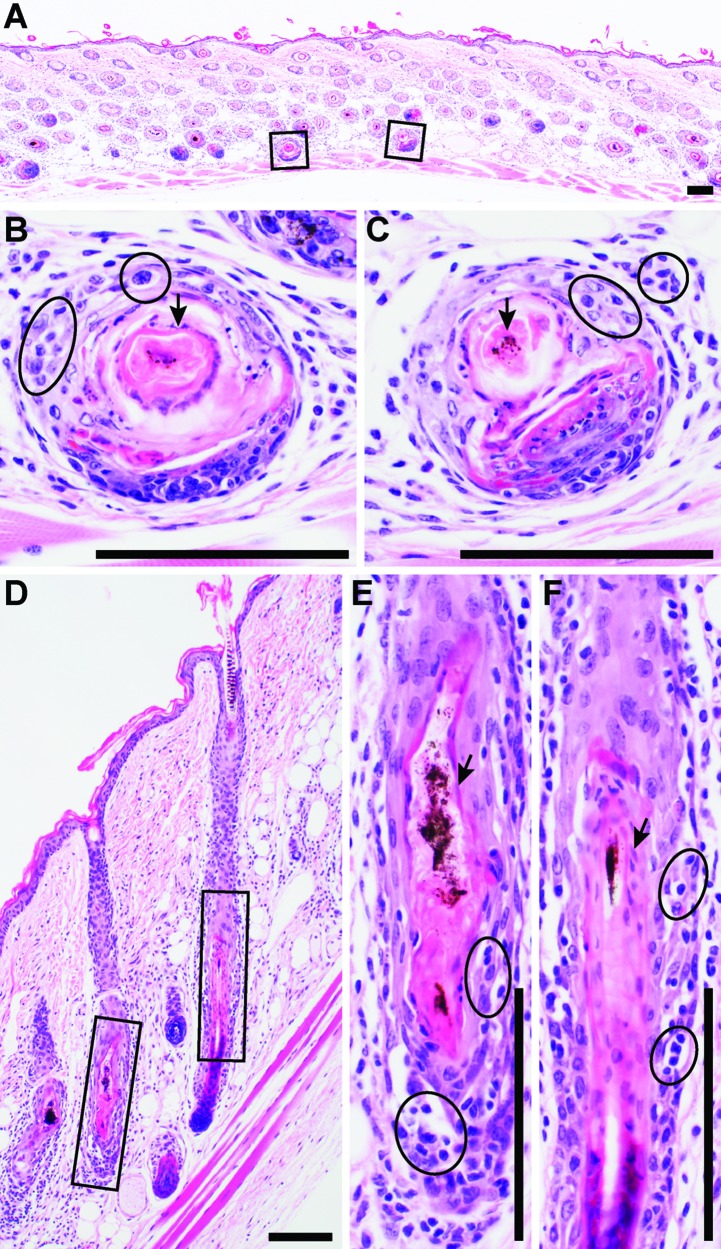
Histologic sections can be interpreted in either horizontal–oblique (Figure 2 A through C) or vertical (Figure 2 D through F) orientation. Compared with horizontal–oblique samples, vertical sections often are interpreted more easily, because most histology textbooks present hair follicles in vertical orientation. Histologic sections from mice with AA reveal that most or all hair follicles in the late anagen or early catagen stage of the hair cycle have variable numbers of lymphocytes and few inflammatory cells of other types that infiltrate into and around the hair follicles between the hair bulb and sebaceous glands, depending on the stage of disease (Figure 2 A through F).2 Follicular dystrophy in the precortical region of the hair bulb can be seen to various degrees (Figure 2 B and C).26,28 Dystrophic hair shafts become severely deformed and weakened, sometimes in association with mild to moderate hyperplasia of the surrounding root sheaths (Figure 2 E and F). Hair follicles in the grafted skin in the control mice are in all stages of the hair cycle but are most often in telogen. This characteristic can be problematic in gene expression studies, for which it is important to match the hair cycle between mutant and control mice as closely as possible.
Figure 2.
Histologic features of alopecia areata in C3H/HeJ mice. Skin sections can be interpreted in either (A–C) horizontal–oblique or (D–F) vertical orientation. Although the vertical orientation is easiest to interpret, samples in horizontal–oblique orientation are obtained most often. A mixed inflammatory cell infiltrate consisting primarily of CD4+, CD8+, and NK cells and fewer granulocytes are present surrounding and infiltrating late anagen to early catagen hair follicles (circles in panels B, C, E, and F). Follicular dystrophy (boxed areas in panels A and D) can be relatively mild (arrows in panels B and C) or cause severe disruption of hair shafts (arrows in panels E and F). These processes surround the follicle from the bulb to the level of the sebaceous gland. Bulb involvement is limited to very short intervals at the very end of the anagen stage of the hair cycle. Hematoxylin and eosin stain; bar, 100 µm.
Discussion
Alopecia areata is an autoimmune disease with focal, perifollicular, and intrafollicular inflammation of late anagen to early catagen stage hair follicles. The AA phenotype can be transferred from spontaneously affected mice to normal-haired recipients of the same histocompatible strain by using skin grafts or subcutaneous injection of lymph node or spleen cells.2,14 The transfer of AA is presumed to be mediated by passenger lymphocytes or antigen-presenting cells, inducing autoreactivity by the host immune system given that AA transfer is ineffective in immunodeficient mice. Both CD4+ and CD8+ T cells play a role in the pathogenesis of AA.2,14 Placing full-thickness, AA-affected skin grafts onto histocompatible mice reproducibly induces an AA-like disease in C3H/HeJ mice. The same protocol can be used in A/J mice, another inbred strain that develops a similar AA-like disease, although the time to onset of disease in graft recipients is much prolonged.8 Most C3H/HeJ engrafted mice develop generalized alopecia (similar to human alopecia universalis), as evidenced by a very thin hair coat, by 20 wk after surgery. The surgical grafting methods described here reliably and predictably produce recipient mice with AA. Grafted mice with AA can be used as donors to generate more mice with AA, making it relatively easy to generate large numbers of mice for future studies. Mice receiving skin grafts from mice with AA developed extensive peri- and interfollicular inflammation, primarily lymphocytic, in and around anagen follicles and located in extensive areas around the graft and at distant sites. Hair follicles from AA graft recipients in late anagen and early catagen have a prominent inflammatory component but also exhibit marked follicular dystrophy, with disintegration of the hair fiber in the matrix above the hair bulb.28
During the 15 y that we have used this surgically initiated form of alopecia areata, the surgeries failed or the mice did not develop AA within 10 to 20 wk after surgery in fewer than 10% of cases. The reasons for these failures include bandage failures, poor surgical technique (for trainees), and minor histoincompatibility issues when using insipient congenic stocks as recipients. In these congenic mice, evaluation of the graft sites revealed loss of the grafts, and the mice were discarded from use in any experiments.
By selecting mice that maintain grafts, disease onset can be prevented by blocking the lymphocyte costimulatory cascade,2 or the mice can be used in a variety of preclinical drug efficacy trials.22 In addition, success rates at other institutions have varied, even when surgeries were done by the same person that did them at The Jackson Laboratory, due to dietary soy oil intake variations between diets at different institutions in which C3H/HeJ mice apparently increases their resistance to AA induction through skin grafting in an approximately dose-dependent manner.12 The properties of soy-derived isoflavones such as genistein may have the potential to influence the susceptibility level of C3H/HeJ mice to the onset of AA.12 In other studies, dietary differences of phytoestrogens were proven to be one environment factor with the potential to significantly influence susceptibility or resistance to various diseases.18,31 Dietary phytoestrogens have not caused problems at our facility but may become important at other institutions that use different commercial diets.
Regardless of surgical method, a few control mice typically develop AA after surgery. This result most likely is not due to the surgical procedure itself but rather to the normal background frequency of AA in C3H/HeJ mice.26 Alternatively, the role of psychologic factors in the pathogenesis of AA has long been the subject of debate. One study7 documented mental trauma or stress as the cause of AA attacks in only 4.8% of 115 human patients with AA. Other research4,32 has shown similar results in human patients.
The C3H/HeJ inbred strain of mice is a useful model for studying the adult-onset form of human alopecia areata. The surgical model we have described here provides an easily reproducible and highly predictable system for assessing theories of disease mechanisms and for preclinical drug testing.22
Acknowledgments
This work was supported by grants from the National Alopecia Areata Foundation and the NIH (AR056635). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Jackson Laboratory Shared Scientific Services were supported in part by a Basic Cancer Center Core Grant from the National Cancer Institute (CA34196).
References
- 1.Boggess D, Silva KA, Landel C, Mobraaten L, Sundberg JP. 2006. Approaches to handling, breeding, strain preservation, genotyping, and drug administration for mouse models of cancer, p 3–14. In: Holland EC. Mouse models of human cancer. Hoboken (NJ): John Wiley and Sons [Google Scholar]
- 2.Carroll JM, McElwee KJ, King LE, Byrne MC, Sundberg JP. 2002. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol 119:392–402 [DOI] [PubMed] [Google Scholar]
- 3.Gollinck H, Orfanos CE. 1990. Alopecia areata: pathogenesis and clinical picture, p 529–569. In: Orfanos CE, Happle R. Hair and hair diseases. Berlin (Germany): Springer–Verlag [Google Scholar]
- 4.Gupta MA, Gupta AK, Watteel GN. 1997. Stress and alopecia areata: a psychodermatologic study. Acta Derm Venereol 77:296–298 [DOI] [PubMed] [Google Scholar]
- 5.King LE, McElwee KJ, Sundberg JP. Alopecia areata, p 280–312. In: Nickoloff BJ Nestle FO Dermatologic immunity: current directions in autoimmunity. Basel (Switzerland): Karger; [DOI] [PubMed] [Google Scholar]
- 6.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre CB, Gwaltney-Bran S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R. 2013. AVMA guidelines for the euthanasia of animals: 2013 edition. [Cited 03 September 2013]. Available at: http://works.bepress.com/cheryl_greenacre/14 [Google Scholar]
- 7.Macalpine I. 1958. Is alopecia areata psychosomatic? A psychiatric study. Br J Dermatol 70:117–131 [DOI] [PubMed] [Google Scholar]
- 8.McElwee K, Boggess D, Miller J, King L, Sundberg J. 1999. Spontaneous alopecia areata-like hair loss in 1 congenic and 7 inbred laboratory mouse strains. J Investig Dermatol Symp Proc 4:202–206 [DOI] [PubMed] [Google Scholar]
- 9.McElwee KJ, Boggess D, Burgett B, Bates R, Bedigian HG, Sundberg JP, King LE. 1998. Murine cytomegalovirus is not associated with alopecia areata in C3H/HeJ mice. J Invest Dermatol 110:986–987 [DOI] [PubMed] [Google Scholar]
- 10.McElwee KJ, Boggess D, King LE, Sundberg JP. 1998. Experimental induction of alopecia areata-like hair loss in C3H/HeJ mice using full-thickness skin grafts. J Invest Dermatol 111:797–803 [DOI] [PubMed] [Google Scholar]
- 11.McElwee KJ, Boggess D, Olivry T, Oliver RF, Whiting D, Tobin DJ, Bystryn JC, King LE, Jr, Sundberg JP. 1998. Comparison of alopecia areata in human and nonhuman mammalian species. Pathobiology 66:90–107 [DOI] [PubMed] [Google Scholar]
- 12.McElwee KJ, Niiyama S, Freyschmidt-Paul P, Wenzel E, Kissling S, Sundberg JP, Hoffmann R. 2003. Dietary soy oil content and soy-derived phytoestrogen genistein increase resistance to alopecia areata onset in C3H/HeJ mice. Exp Dermatol 12:30–36 [DOI] [PubMed] [Google Scholar]
- 13.McElwee KJ, Silva K, Beamer WG, King LE, Sundberg JP. 2001. Melanocyte and gonad activity as potential modifying factors in C3H/HeJ mouse alopecia areata. Exp Dermatol 10:420–429 [DOI] [PubMed] [Google Scholar]
- 14.McElwee KJ, Silva K, Boggess D, Bechtold L, King LE, Sundberg JP. 2003. Alopecia areata in C3H/HeJ mice involves leucocyte-mediated root sheath disruption in advance of overt hair loss. Vet Pathol 40:643–650 [DOI] [PubMed] [Google Scholar]
- 15.Michie HJ, Jahoda CAB, Oliver RF, Johnson BE. 1991. The DEBR rat: an animal model of human alopecia areata. Br J Dermatol 125:94–100 [DOI] [PubMed] [Google Scholar]
- 16.Michie HJ, Jahoda CAB, Oliver RF, Poulton TA. 1990. Immunobiological studies on the alopecic (DEBR) rat. Br J Dermatol 123:557–567 [DOI] [PubMed] [Google Scholar]
- 17.Oliver RF, Jahoda CAB, Horne KA, Michie HJ, Poulton T, Johnson BE. 1991. The DEBR rat model for alopecia areata. J Invest Dermatol 96:97S. [DOI] [PubMed] [Google Scholar]
- 18.Regal JF, Fraser DG, Weeks CE, Greenberg NA. 2000. Dietary phytoestrogens have antiinflammatory activity in a guinea pig model of asthma. Proc Soc Exp Biol Med 223:372–378 [DOI] [PubMed] [Google Scholar]
- 19.Safavi K. 1992. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol 128:702. [DOI] [PubMed] [Google Scholar]
- 20.Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ. 1995. Incidence of alopecia areata in Olmstead County, Minnesota 1975–1989. Mayo Clin Proc 70:628–633 [DOI] [PubMed] [Google Scholar]
- 21.Silva KA, Sundberg JP. 2012. Necropsy methods, p 779–806. In: Hedrich HJ The laboratory mouse. London (UK): Academic Press [Google Scholar]
- 22.Sun J, Silva KA, McElwee KJ, King LE, Sundberg JP. 2008. The C3H/HeJ mouse and DEBR rat models for alopecia areata: preclinical drug screening tools. Exp Dermatol 17:793–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundberg JP, Berndt A, Silva KA, Kennedy VE, Sundberg BA, Everts HE, Rice RH, King LE. Alopecia areata: updates from the mouse perspective. J Invest Dermatol Sym Proc. In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundberg JP, Boggess D. 2000. Systematic characterization of mouse mutations. Boca Raton (FL): CRC Press [Google Scholar]
- 25.Sundberg JP, Boggess D, Silva KA, McElwee KJ, King LE, Li R, Churchill G, Cox GA. 2003. Major locus on mouse chromosome 17 and minor locus on chromosome 9 are linked with alopecia areata in C3H/HeJ mice. J Invest Dermatol 120:771–775 [DOI] [PubMed] [Google Scholar]
- 26.Sundberg JP, Cordy WR, King LE. 1994. Alopecia areata in aging C3H/HeJ mice. J Invest Dermatol 102:847–856 [DOI] [PubMed] [Google Scholar]
- 27.Sundberg JP, McElwee KJ, Carroll J, King LE., Jr 2011. Hypothesis testing: CTLA4 costimulatory pathways critical in pathogenesis of human and mouse alopecia areata. J Invest Dermatol 131:2323–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundberg JP, McElwee KJ, King LE. 2003. Spontaneous and experimental skin-graft-transfer mouse models of alopecia areata, p 429–449. In: Chan LS Animal models of human inflammatory skin diseases. Boca Raton (FL): CRC Press [Google Scholar]
- 29.Sundberg JP, Silva KA. 2012. What color is the skin of a mouse? Vet Pathol 49:142–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundberg JP, Silva KA, Li R, King LE, Cox GA. 2004. Adult-onsetal opecia areata is a complex polygenic trait in the C3H/HeJ mouse model. J Invest Dermatol 123:294–297 [DOI] [PubMed] [Google Scholar]
- 31.Tomobe K, Philbrick DJ, Ogborn MR, Takahashi H, Holub BJ. 1998. Effect of dietary soy protein and genistein on disease progression in mice with polycystic kidney disease. Am J Kidney Dis 31:55–61 [DOI] [PubMed] [Google Scholar]
- 32.Van der Steen P, Boezeman J, Duller P, Happle R. 1992. Can alopecia areata be triggered by emotional stress? An uncontrolled evaluation of 178 patients with extensive hair loss. Acta Derm Venereol 72: 279–280 [PubMed] [Google Scholar]