Abstract
Surgical models in animals are used extensively to study small molecules and devices for lumbar intervertebral disc repair, replacement, and fusion. Although the ventral lumbar animal models themselves are well described, critical assessment of morbidity and mortality avoidance when using the models have not been reported. Hypothesizing that technique modifications and the relative prevalence and severity of complications would be correlated, we collected and examined peri- and postoperative data stratified by surgical technique. We here report complications associated with the transperitoneal approach to the lumbar spine in 268 Lewis rats and offer data-driven suggestions regarding complication avoidance through technique modification. Compared with wider exposure, limiting the width of exposure to a maximum of 3 mm resulted in fewer neurologic complications in the lower limbs. In addition, avoiding extracorporeal reflection of the small intestine during the exposure was associated with lower incidence of postoperative gastrointestinal distress and fewer situations requiring euthanasia. These findings underscore the importance of detailed approaches in minimizing postoperative morbidity and attrition in surgical models.
Spine fusion is the definitive method for treatment of progressive spinal deformity, instability, and intractable pain unresponsive to nonsurgical management. Spine fusion surgery in humans is associated with numerous risks and complications, including those associated with the surgical anatomic approach used2,3,13,25 and those related to the success or failure of the fusion or clinical outcomes.12,14,31,32 Research efforts and surgical technique modifications to reduce complications associated with spine fusion have resulted in advances in minimally invasive methods to access the spine and achieve fusion,2,19,20 biologic agents to improve fusion success rates,4,17 and surgical models in animals to assess new devices and agents.5,6,15,22,28 As has been reported for human medical care,1,8,9,23 nonhuman preclinical studies frequently report on the primary endpoints associated with the use of the models but fail to adequately document morbidity and mortality encountered, particularly as may be attributed to surgical technique. In regard to ventral lumbar spine access in rats, data is emerging to help minimize morbidity and mortality associated with the ventral transabdominal access to the spine.26
Surgical models in animals that provide access to the intervertebral disc and ventral spine have been described for several species.11,21,24 Owing to their low cost, wide availability, tolerance for surgical procedures, and relative ease for caging and care, rats have become a popular model for intervertebral disc and spine research. Descriptions of safe and reproducible transperitoneal access to the rat lumbar spine was reported by 3 groups almost simultaneously for autogenous nucleus pulposus cell delivery,16 gene delivery,18 and potential intervertebral tissue engineering26 and fusion15 applications. Each group reported that the animals tolerated the procedure well, but none detailed any complications encountered other than death; subsequent studies using ventral lumbar access describe neither complications nor proven methods to avoid morbidity.7,27,29,30
We have used the ventral transperitoneal approach to the rat lumbar spine in pilot studies and 2 experimental studies while modifying the surgical technique to reduce surgical morbidity and improve survival. The current observational study reviews morbidity and mortality data from that work with the goal of defining parameters what would minimize morbidity and mortality associated with use of the model. We describe the relevant surgical anatomy, identification of segmental levels, and typical surgical technique and explain modifications to the surgical technique and their effects on morbidity and mortality.
Materials and Methods
All animal experiments were performed under IACUC-approved protocols. Male Lewis (LEW/HanHsd) rats (n = 268; weight, 250 to 350 g) were obtained from Harlan Laboratories (Indianapolis, IN). Animals were housed in pairs inside an individually ventilated caging rack for rats (Thoren Caging Systems, Hazelton PA). Rats were bedded directly on Beta-Chip contact bedding (NEPCO, Warrensburg, NY), which is a heat-treated (kiln-dried), hardwood bedding that is produced specifically for use as laboratory animal bedding. Rats were fed a commercial diet (Laboratory Rodent Diet no. 5001, PMI, St Louis, MO) ad libitum. Water was acidified (hydrochloric acid) to pH 2.7 and delivered via water bottle ad libitum. Only SPF, virus-antibody–negative rats that were purpose bred-for research were used and housed in the animal facility. The resident rat colony health status was monitored through an inhouse rodent sentinel monitoring program. New rats were afforded 4 d of acclimation before undergoing any survival surgery. Rat room temperature was maintained at 20.5 to 22.2 °C (69 to 72 °F), with 30% to 70% humidity and a 12:12-h light:dark cycle.
Survival surgery and postoperative analgesia were completed according to institutional protocols. Anesthesia comprised ketamine (100 mg/mL; 65 mg/kg IP) and acepromazine (10 mg/mL; 2.5 mg/kg IP); a single dose routinely provided 20 to 30 min of surgical anesthesia. When necessary, anesthesia was prolonged through the administration of isoflurane via nose cone. Rats were placed on a temperature-controlled (heated) operating-room table during the surgical procedure, to maintain body temperature. Immediately postoperatively, animals were hydrated, warmed and given pain medication. Directly from the operating-room table, rats received sterile lactated Ringers (10 mL/kg SC) hourly until ambulatory and able to reach their water bottles. Rats were placed in a warmed ICU cage or in a recovery cage maintained on warming blanket. Upon the rats’ recovery from anesthesia, initial pain was managed with buprenorphine (0.03 mg/kg SC; Buprenex, Reckitt Benckiser, Richmond, VA). Ongoing pain was controlled with buprenorphine (0.03 mg/kg SC) every 8 to 12 h for 48 h postoperatively. Additional analgesia beyond 48 h was given at the discretion of the veterinary staff after consultation with investigators.
For postoperative housing, rats were placed in clean cages on cage liners (Techboard, Shepherd Specialty Papers, Kalamazoo, MI); no bedding was used throughout buprenorphine administration. For the first 48 postoperative hours, irradiated trail mix and soft dietary supplement (DietGet Recovery, Clear H2O, Portland, ME) were placed with the rats in cages, to facilitate their access to food; rats were routinely ambulatory and appetent, and by the fifth postoperative day, appetite and attitude approximated preoperative levels. Prophylactic or postoperative antibiotics were not administered for these procedures, which were performed under aseptic conditions.
Rats were anesthetized as described, the abdomen was clipped free of hair and sterilely prepared with povidone–iodine and alcohol, and they underwent transperitoneal exposure of lumbar levels L4 through L6, according to a technique that incorporated minor modifications of previously published methods.16,18,26 A ventral abdominal incision was created from the xyphoid process to pubis (approximately 4 cm) along the midline, and the abdominal wall was entered in the midline through the median raphe, by using forceps to elevate the abdominal musculature and a scalpel to sharply incise the raphe. In this way, the abdominal contents safely fell away from the incision once the peritoneal cavity was entered, and the remainder of the midline muscular laparotomy was completed by using dissecting scissors under direct vision. Abdominal contents tended to limit visualization, and technique modifications were attempted to reposition the viscera and improve exposure. To this end, a custom wire retractor was made to allow the vicera to be swept to the animal's right side within the peritoneal cavity, and either the cecum and mobile large intestine (n = 212, nonconsecutive), or both the large and small intestines (n = 46, consecutive) were reflected extracorporeally onto sterile saline-soaked 4×4-in.2 gauze and covered with a sterile saline-soaked 4×4 in.2 gauze. This step provided access to the posterior peritoneal cavity and visualization of the psoas muscles, inferior vena cava, abdominal aorta, ureters, and urinary bladder. Perioperative morbidity was stratified with respect to extracorporeal reflection of either the cecum only or the cecum and small intestines combined.
Deep dissection in the posterior abdominal wall involved a relatively bloodless plane between the psoas muscles in the midline. The posterior peritoneal membrane was bluntly separated by using sterile cotton-tipped wooden applicators, and the great vessels were swept to the animal's right. A posteriorly directed dissection in the midline was performed bluntly by using cotton tipped wooden applicator sticks or sharply by using a Penfield #1 or 3-0 straight curette. Dissection from midline initially was performed widely to provide maximal visual landmarks for treatment delivery but later was strictly limited to 1 to 1.5 mm from midline as suggested.26 Morbidity was stratified with respect to midline-constrained or wide central exposure. Vertebral discs or bodies within the exposure area were easily visualized by their respective white or reddish color and as respective ‘peaks’ or ‘valleys’, as assessed by visual inspection or palpation. Specific to the experiments that used these rats, the vertebral endplates were punctured by using a 22-gauge needle, which was passed through the ventral L5 body proximally into the L4 body (through the L4L5 disc) and distally into the L6 body (through the L5L6 disc); 2 to 3 passages per disc were performed.10 An intraoperative high-definition X-ray image (model MX-20, Faxitron X-Ray, Wheeling, IL) was obtained with the 22-gauge needle traversing the L5L6 disc, to confirm the segmentation of the instrumented levels. Treatments were delivered to the intervertebral disc by using a 25-gauge needle by ventral annulus fibrosus puncture, 2-mm needle advancement, and 20- to 30-μL injection into the disc interior. In a subset of injections, a portion of the injectate was noted to extrude from the site of implantation and into the exposure; no correlation between the particular treatment delivered and complications was noted (data not shown). The muscular abdominal wall was closed in a single layer by using a running locked pattern in 4-0 absorbable suture, and the skin was approximated by using an interrupted horizontal mattress pattern in 4-0 nylon. Surgeries initially took as long as 60 min, but familiarity with the technique rapidly reduced operation times to 20 to 30 min; all surgeries were performed by a single surgeon.
Morbidity and mortality endpoints measured included the following: required euthanasia for any reason, death prior to incision attributable to anesthesia, death resulting from excessive intraoperative bleeding, perioperative death from unknown cause, postoperative gastrointestinal distress (wasting, distension, abdominal wall bruising), wound problems (dehiscence, stitch abscess), and surgery at incorrect spinal level. Neurologic complications were recorded as rats mobilized after anesthesia (that is, ‘initial neurologic complications’) for profound weakness of one or both legs (that is loss of hip flexion, calcaneal or footdrop gaits) or milder gait changes (that is, subtle limp, voluntary nonweightbearing). Rats were monitored closely for either resolution within 7 d from the date of surgery (that is, ‘transient complication’) or persistence beyond 1 wk (that is, ‘persistent complication’). Neurologic complications first noticed after 1 wk were classified as persistent also. Note that the number of initial neurologic complications represents the total number of complications observed (that is, transient and persistent), with the exception of one late-presenting persistent deficit. No rat exhibited neglect of or mutilating behavior toward an extremity during any of the experiments. Data were analyzed statistically to enable comparisons. The data collected were dichotomous, with endpoints either present or absent. A 2-tailed Fisher exact test was used to compare the data, with significance set at a P value of less than 0.05.
Results
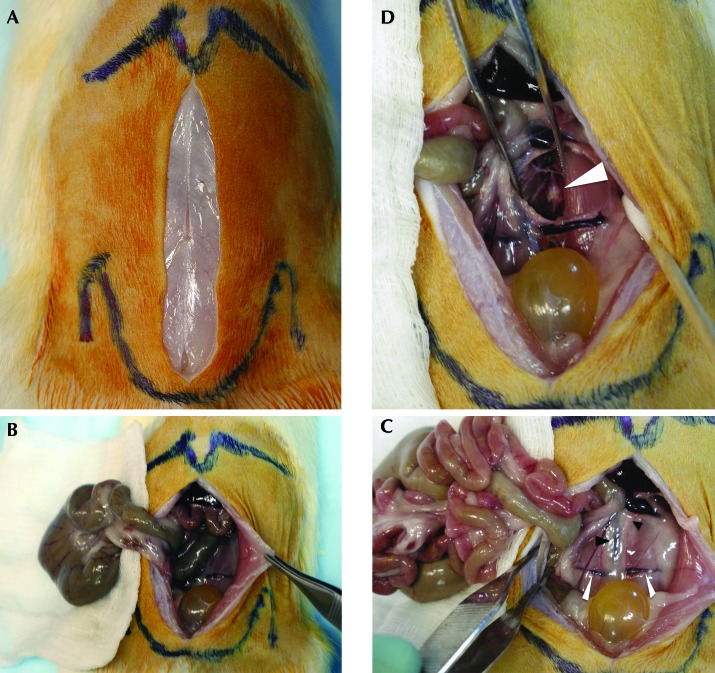
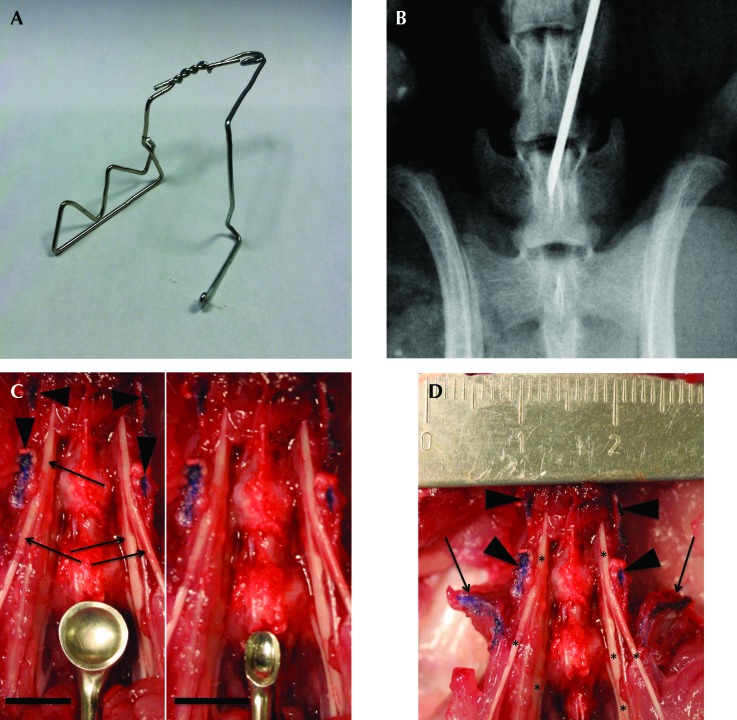
The surgical anatomy and typical ventral lumbar spine exposure is reproducible for the L3 to S1 segment. Bony surface landmarks for the incision and exposure were the xiphoid process proximally, and the iliac wings and pubis distally (Figure 1 A). When required for optimization of the exposure, the cecum (Figure 1 B) or cecum and small intestine (Figure 1 C) were reflected outside the peritoneal cavity and was placed on and under sterile, saline-soaked gauze pads. Blunt dissection in the retroperitoneum was performed between the left ureter and great vessels, with the goal of mobilizing the aorta and vena cava to the rat's right side (Figure 1 D). A relatively consistent vein transversely crossing the psoas (the transverse iliopsoas vein, Figure 1 C) was safely suture-ligated to control bleeding as needed; other bleeding in the deep exposure was well controlled with direct pressure from a cotton-tipped applicator. The midline vertebral bodies and discs of the spine were easily palpated, with discs identifiable as ventral protrusions along the ventral vertebral column. The proximal iliac wings were easily palpated during the exposure, and they were used to identify the L5L6 disc that lies directly between them, with the use of an intraoperative radiograph to confirm spine segmentation (Figure 2 B). Initially a Penfield #1 and later a 3-0 straight curette (Figure 2 C) was used to mobilize the psoas musculature from the ventral spine midline for 1 to 1.5 mm in each direction, thus providing visualization of the ventral vertebral bodies and discs (Figure 1 D) while limiting potential trauma to the nearby lumbar nerves and plexus (Figure 2 D). The use of this technique routinely yielded safe access from the L3L4 through L6S1 level. One rat died intraoperatively from uncontrolled bleeding (0.4%), another had surgery at the incorrect spinal level, and 2 rats had wound complications (0.8%), with one dehiscence and one superficial infection that responded to local care. In addition, 8 perioperative deaths occurred prior to incision and were attributed to anesthesia (3%), and 20 (7.5%) additional perioperative deaths occurred secondary to respiratory distress recognized during wound closure (n = 10; 50%) or during recovery from sedation (n = 10; 50%), with no cause found at necropsy and no correlation between death and the specific treatment delivered or surgical technique used.
Figure 1.
Photographs from a necropsy dissection simulating surgical technique. (A) The skin incision has been created, and palpated bony landmarks are outlined: xiphoid process projecting distally at the top of the image and iliac wings prominent laterally in vicinity of the pelvis meeting in the midline distally at the pubis. (B) The cecum (large intestine) has been extraperitoneally reflected onto saline-moistened 4×4 gauze pads. (C) The cecum and small intestine have both been reflected extraperitoneally; note the increased visualization of the retroperitoneum and traversing iliolumbar veins (white arrowheads). Black arrowheads indicate the left ureter (small) and aorta–inferior vena cava (large). (D) The ureter and great vessels have been dissected medially and the left psoas laterally; the forceps maintain the deep exposure in the dissection plane, and the arrowhead indicates the ventral disc annulus.
Figure 2.
Photograph of retractor, intraoperative radiograph, and photographs showing relevant surgical anatomy. (A) Photograph shows the retractor used during the laparotomies, which was fashioned from 0.072-gauge steel wire. The spring is placed cranially, viscera not reflected extraperitoneally are placed behind the ‘sawtooth’ broad retractor arm and are retracted to the animal's right; the smaller arm is used to push against the left side of the skin and body wall incision to maintain position. Visualization is optimized, and the midline is not blocked for intraoperative radiographic assessment of segmentation relative to the pelvis. (B) A Faxitron high-definition radiograph showing a 22-gauge needle with tip in the L6 vertebral body, definitively confirming spine segmentation. (C) Photograph shows a Penfield no. 1 dissector (left) and 3-0 straight curette (right) in proximity to the lumbar nerves and plexus (black arrows in left image) after removal of the psoas muscle. Note that the ventral surfaces and tips of the transverse processes have been colored with a blue surgical marker (black arrowheads in left image); the black scale line in each photo indicates 5 mm. The 3-0 curette is much smaller than is the Penfield no. 1 dissector, possibly making the curette safer for deep dissection. (D) Photograph of ventral lumbar spine with nerves dissected and ruler in place to illustrate relative scale. Note the relation of the neuroelements (asterisks) immediately medial to the transverse processes (arrowheads), the small distance from midline available for safe dissection before encountering the nerves, and the entry of nerves past the iliac wings (arrows) into the pelvis. As shown, the transverse processes limit the total possible lateral dissection of the ventral spine to approximately 1cm, but because they act as a hard surface where nerve trauma can be induced iatrogenically with the dissecting tools, dissection must be kept close to midline.
Neurologic complications after surgical exposure decreased when surgical dissection was limited to a maximal width of 3 mm. In pilot experiments, 70% (7 of 10) of rats with wide exposure of the ventral spine to the base of the transverse processes developed initial postoperative neurologic deficits, with only 2 of the 7 rats recovering to normal function by 1 wk after surgery and therefore having transient deficits (Table 1). However, when midline dissection was maintained to a maximal width of 1 to 1.5 mm in each direction (2 to 3 mm total), the rates of initial postoperative neurologic deficits (28 of 258, or 10.9%) and persistent deficits (11 of 258, or 4.3%; P < 0.001) varied, but the rate of transient deficits (17 of 258; 6.6%) did not differ (P = 0.152). There was no difference between groups in regard to probability for return to normal neurologic function at 1 wk (28.6% for wide compared with 60.7% for narrow dissection, P = 0.208). When present, postoperative neurologic improvement and gait normalization occurred spontaneously and required no intervention other than routine care. Perioperative neurologic morbidity did not elicit limb mutilation behavior in any of the rats, and no euthanasia was required due to neurologic status. Profound persistent unilateral or bilateral leg weakness resulting in a flail limb occurred in 5 rats in the wide-dissection group and in 2 rats in the limited-exposure group; these cases were included in the statistics regarding gait alteration, described previously. Late (that is, 7 wk postoperatively) paralysis of a hindlimb occurred in one rat; the neurologic deficit did not require euthanasia. In addition, neither bone destruction nor instability was identified through inspection and palpation at necropsy, nor did imaging reveal prolific bone formation suggestive of iatrogenic stenosis. If the first 10 rats are excluded due to width of dissection technique refinement, a trend (P = 0.069) toward a ‘learning curve’ effect for neurologic complication avoidance became apparent between the first large study (16 of 105 rats; 15.2%) compared with the second study (12 of 153; 7.8%). This finding suggests that familiarity with the surgical technique may decrease neurologic complications.
Table 1.
Neurologic morbidity by technique
| No. of rats with |
|||||
| Total no. of rats | Initial deficit | Persistent deficit | Transient deficit | Euthanasia due to deficit | |
| Wide dissection | 10 | 7 | 5 | 2 | 0 |
| Narrow dissection: study 1 | 105 | 16 | 5 | 11 | 0 |
| Narrow dissection: study 2 | 153 | 12 | 6 | 6 | 0 |
Gastrointestinal complications after ventral lumbar spinal surgery were minimized when the small intestines were maintained within the abdomen. Of the 258 rats in the 2 large studies, 212 animals underwent exposure with extracorporeal reflection of the cecum (large intestine) to improve visualization; an additional 46 rats underwent extracorporeal reflection of both the cecum and the mobile portions of the small intestine (Table 2). Gastrointestinal complications that occurred during the postsurgical monitoring period included failure to thrive requiring euthanasia, abdominal distension, abdominal bruising (bluish discoloration of the abdominal wall), and spontaneous death (with necropsy demonstrating ascites, empty bowel, or bowel with blanched appearance consistent with nonviability). Gastrointestinal complications were more frequent in the cecum–small intestine group, with 10 of 46 animals affected compared with none affected among the 212 rats in the cecum-only group (P < 0.001). Neurologic complication rates (P = 0.79) and overall mortality rates (P = 0.08) did not differ between groups, but cases of postoperatively required euthanasia were more numerous (P = 0.04) in the cecum–small intestine group.
Table 2.
Morbidity and mortality of rats by technique
| Technique | Total no. of deaths | No. of anesthesia-related deaths | No. that required euthanasia | No. of respiratory deaths or cause unknown | No. with gastrointestinal distress | No. with initial neurologic deficit |
| Cecum and small | 9 (19.6%) | 2 (4.3%) | 3 (6.5%) | 4 (8.7%) | 10 (21.7%) | 4 (8.6%) |
| intestine (n = 46) | ||||||
| Cecum only (n = 212) | 21 (9.9%) | 2 (0.9%) | 2 (0.9%) | 16 (7.5%) | 0 (0%) | 24 (11.3%) |
| P | 0.08 | 0.15 | 0.04 | 0.99 | <0.001 | 0.79 |
| Overall rate for both | 11.6% | 1.6% | 1.9% | 7.8% | 3.9% | 10.9% |
| techniques combined |
Discussion
This study describes our experience of using a transperitoneal approach to the ventral lumbar spine in young adult Lewis rats. Through relatively minor modifications in surgical technique, postoperative gastrointestinal distress, postoperative neurologic impairments, and required euthanasias were significantly decreased compared with rates associated with the traditional approach. We hope that these findings help to minimize morbidity and mortality when other investigators use this model and that future studies lead to additional suggestions in technique refinement to improve the method.
Previous use of the ventral transperitoneal lumbar exposure was reported to be associated with complications,16,26 but the complications were not stratified according to technique or evaluated for improvement in outcomes. Postoperative neurologic impairment was recognized and found to decrease with increased surgical experience, but learning was not otherwise examined with regard to overall occurrence of complications or differences between less and more experienced surgeons.16 An independent report stated that when dissection was maintained to within 1.5 mm of midline, no neurologic complications were observed in the 30 rats that underwent the procedure, but no explanation for the 1.5-mm threshold was given nor were the consequences when dissection was continued lateral to that point.26 Our data indicate that for wide dissection to the transverse processes, initial neurologic complications occur in as many as 70% of subjects, but when dissection was kept in midline and to a maximal width of 3 mm, the initial neurologic injury rate was significantly lower, at 10.9%, and persistent neurologic injury decreased from 50% to 3.5%. This 3-mm width can be estimated intraoperatively as being approximately equal to the height of the disc, from bony endplate to bony endplate, visualized within the exposure, and this lateral limit of the dissection maintains the exposure at a reproducibly safe distance from the nearby lumbar plexus (Figure 2 D). In addition, prior detailed description of the handling of the intestines during the exposure suggested that the mobile bowel could be “reclined” extracorporeally during the procedure, with “no sign that the digestive tract suffered.”26 Our data indicate that extracorporeal reflection of the large intestine is well tolerated, but that reflection of both the large and small intestines results in significantly higher rates of gastrointestinal distress and required euthanasia, leading to our current practice of maintaining the small intestine within the abdomen during the procedure and extracorporeal reflection of only the cecum.
This investigation shows that minor technique modifications significantly decrease adverse complications and the need for euthanasia in rats. More generally our findings suggest that systematic monitoring of morbidity and mortality during technique refinement can generate evidence-based data-driven technique optimization, resulting in fewer complications and less animal distress. Because respiratory distress remained a complication even after our modification of the surgical technique, we will attempt to further reduce neurologic morbidity and perioperative mortality due to respiratory distress in subsequent studies.
Acknowledgments
We thank the staff of the Hospital for Special Surgery CLAS facility, including Theresa Cunningham, Xiomora Santiago, and Mariel Nigro. This study was supported in part by the Arthritis Foundation (New York Chapter; MEC); an NIH grant (5-P30-AR46121-10) for the Musculoskeletal Repair and Regeneration Core Center (MEC); and the North American Spine Society (MEC). The authors have no financial conflicts that influenced the work described here.
References
- 1.Audige L, Goldhahn S, Daigl M, Goldhahn J, Blauth M, Hanson B. 2011. How to document and report orthopedic complications in clinical studies? A proposal for standardization. Arch Orthop Trauma Surg [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Bergey DL, Villavicencio AT, Goldstein T, Regan JJ. 2004. Endoscopic lateral transpsoas approach to the lumbar spine. Spine (Phila Pa 1976) 29: 1681–1688 [DOI] [PubMed] [Google Scholar]
- 3.Bertagnoli R, Zigler J, Karg A, Voigt S. 2005. Complications and strategies for revision surgery in total disc replacement. Orthop Clin North Am 36:389–395 [DOI] [PubMed] [Google Scholar]
- 4.Boden SD, Kang J, Sandhu H, Heller JG. 2002. Use of recombinant human bone morphogenetic protein 2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 27: 2662–2673 [DOI] [PubMed] [Google Scholar]
- 5.Boden SD, Schimandle JH, Hutton WC. 1995. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine (Phila Pa 1976) 20: 412–420 [DOI] [PubMed] [Google Scholar]
- 6.Boden SD, Titus L, Hair G, Liu Y, Viggeswarapu M, Nanes MS, Baranowski C. 1998. Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP1). Spine (Phila Pa 1976) 23: 2486–2492 [DOI] [PubMed] [Google Scholar]
- 7.Boxberger JI, Auerbach JD, Sen S, Elliott DM. 2008. An in vivo model of reduced nucleus pulposus glycosaminoglycan content in the rat lumbar intervertebral disc. Spine (Phila Pa 1976) 33: 146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carragee EJ, Hurwitz EL, Weiner BK. 2011. A critical review of recombinant human bone morphogenetic protein 2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 11:471–491 [DOI] [PubMed] [Google Scholar]
- 9.Crudu V, Blankenship J, Berger P, Scott T, Skelding K. 2011. Complications related to access site after percutaneous coronary interventions: are the adverse events underreported? Catheter Cardiovasc Interv 77:643–647 [DOI] [PubMed] [Google Scholar]
- 10.Damle SR, Rawlins BA, Boachie-Adjei O, Crystal RG, Hidaka C, Cunningham ME. 2013. Lumbar spine intervertebral disc gene delivery: a pilot study in Lewis rats. HSS J 9:36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott DM, Yerramalli CS, Beckstein JC, Boxberger JI, Johannessen W, Vresilovic EJ. 2008. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine 33:588–596 [DOI] [PubMed] [Google Scholar]
- 12.Floman Y, Micheli LJ, Penny JN, Riseborough EJ, Hall JE. 1982. Combined anterior and posterior fusion in 73 spinally deformed patients: indications, results, and complications. Clin Orthop Relat Res (164):110–122 [PubMed] [Google Scholar]
- 13.Flynn JC, Price CT. 1984. Sexual complications of anterior fusion of the lumbar spine. Spine (Phila Pa 1976) 9: 489–492 [DOI] [PubMed] [Google Scholar]
- 14.Fritzell P, Hagg O, Wessberg P, Nordwall A. 2002. Chronic low-back pain and fusion: a comparison of 3 surgical techniques: a prospective multicenter randomized study from the Swedish Lumbar Spine study group. Spine (Phila Pa 1976) 27: 1131–1141 [DOI] [PubMed] [Google Scholar]
- 15.Gruber HE, Gordon B, Williams C, Ingram JA, Norton HJ, Hanley EN., Jr 2009. A new small animal model for the study of spine fusion in the sand rat: pilot studies. Lab Anim 43:272–277 [DOI] [PubMed] [Google Scholar]
- 16.Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, Banks D, Phieffer L, Coldham G, Hanley EN., Jr 2002. Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine 27:1626–1633 [DOI] [PubMed] [Google Scholar]
- 17.Johnsson R, Stromqvist B, Aspenberg P. 2002. Randomized radiostereometric study comparing osteogenic protein 1 (BMP7) and autograft bone in human noninstrumented posterolateral lumbar fusion: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 27: 2654–2661 [DOI] [PubMed] [Google Scholar]
- 18.Leo BM, Li X, Balian G, Anderson DG. 2004. In vivo bioluminescent imaging of virus-mediated gene transfer and transduced cell transplantation in the intervertebral disc. Spine 29:838–844 [DOI] [PubMed] [Google Scholar]
- 19.Lin PM, Cautilli RA, Joyce MF. 1983. Posterior lumbar interbody fusion. Clin Orthop Relat Res (180):154–168 [PubMed] [Google Scholar]
- 20.Lowe TG, Tahernia AD. 2002. Unilateral transforaminal posterior lumbar interbody fusion. Clin Orthop Relat Res (394):64–72 [DOI] [PubMed] [Google Scholar]
- 21.Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS. 2005. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 30:5–14 [DOI] [PubMed] [Google Scholar]
- 22.McAfee PC, Regan JJ, Farey ID, Gurr KR, Warden KE. 1988. The biomechanical and histomorphometric properties of anterior lumbar fusions: a canine model. J Spinal Disord 1:101–110 [PubMed] [Google Scholar]
- 23.Miller KL, Shafman TD, Anscher MS, Zhou SM, Clough RW, Garst JL, Crawford J, Rosenman J, Socinski MA, Blackstock W, Sibley GS, Marks LB. 2005. Bronchial stenosis: an underreported complication of high-dose external beam radiotherapy for lung cancer? Int J Radiat Oncol Biol Phys 61:64–69 [DOI] [PubMed] [Google Scholar]
- 24.Osti OL, Vernon-Roberts B, Fraser RD. 1990. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine (Phila Pa 1976) 15: 762–767 [DOI] [PubMed] [Google Scholar]
- 25.Pradhan BB, Nassar JA, Delamarter RB, Wang JC. 2002. Single-level lumbar spine fusion: a comparison of anterior and posterior approaches. J Spinal Disord Tech 15:355–361 [DOI] [PubMed] [Google Scholar]
- 26.Rousseau MA, Bass EC, Lotz JC. 2004. Ventral approach to the lumbar spine of the Sprague–Dawley rat. Lab Anim (NY) 33:43–45 [DOI] [PubMed] [Google Scholar]
- 27.Rousseau MA, Ulrich JA, Bass EC, Rodriguez AG, Liu JJ, Lotz JC. 2007. Stab incision for inducing intervertebral disc degeneration in the rat. Spine (Phila Pa 1976) 32: 17–24 [DOI] [PubMed] [Google Scholar]
- 28.Steffen T, Marchesi D, Aebi M. 2000. Posterolateral and anterior interbody spinal fusion models in the sheep. Clin Orthop Relat Res (371):28–37 [DOI] [PubMed] [Google Scholar]
- 29.Sugiura A, Ohtori S, Yamashita M, Inoue G, Yamauchi K, Koshi T, Suzuki M, Norimoto M, Orita S, Eguchi Y, Takahashi Y, Watanabe TS, Ochiai N, Takaso M, Takahashi K. 2008. Existence of nerve growth factor receptors, tyrosine kinase a, and p75 neurotrophin receptors in intervertebral discs and on dorsal root ganglion neurons innervating intervertebral discs in rats. Spine (Phila Pa 1976) 33:2047–2051 [DOI] [PubMed] [Google Scholar]
- 30.Takahashi Y, Aoki Y, Douya H, Ohtori S, Takahashi K. 2006. Projection field of primary afferent fibers innervating the ventral portion of the lumbar intervertebral disc in the spinal cord dorsal horn. Anat Sci Int 81:92–99 [DOI] [PubMed] [Google Scholar]
- 31.Tiusanen H, Hurri H, Seitsalo S, Osterman K, Harju R. 1996. Functional and clinical results after anterior interbody lumbar fusion. Eur Spine J 5:288–292 [DOI] [PubMed] [Google Scholar]
- 32.Tiusanen H, Seitsalo S, Osterman K, Soini J. 1996. Anterior interbody lumbar fusion in severe low back pain. Clin Orthop Relat Res (324):153–163 [DOI] [PubMed] [Google Scholar]