Abstract
Even though cardiovascular disease is the leading cause of death for men and women, the vast majority of animal studies use male animals. Because female reproductive hormones have been associated with cardioprotective states, many investigators avoid using female animals because these hormones are cyclical and may introduce experimental variability. In addition, no studies have investigated the specific effects of the estrous cycle on cardiac ischemic injury. This study was conducted to determine whether the estrous cycle stage influences the susceptibility to ischemic injury in rat hearts. Estrous cycle stage was determined by using vaginal smear cytology, after which hearts underwent either in vivo (surgical) or ex vivo (isolated) ischemia–reperfusion injury. For in vivo studies, the left anterior coronary artery was ligated for 25 min of ischemia and subsequently released for 120 min of reperfusion. Infarct sizes were 42% ± 6%; 49% ± 4%; 40% ± 9%; 47% ± 9% of the zone-at-risk for rats in proestrus, estrus, metestrus, and diestrus, respectively. For ex vivo studies, isolated, perfused hearts underwent global ischemia and reperfusion for 25 and 120 min, respectively. Similar to our in vivo studies, the ex vivo rat model showed no significant differences in susceptibility to infarction or extent of cardiac arrhythmia according to estrous stage. To our knowledge, these studies provide the first direct evidence that the stage of estrous cycle does not significantly alter cardiac ischemia–reperfusion injury in rats.
Abbreviations: VF, ventricular fibrillation; VT, ventricular tachycardia
Cardiovascular disease remains the leading cause of morbidity and mortality throughout the industrialized world, with ischemic heart disease being a major manifestation of cardiovascular disease. Many investigators use animal models to advance our understanding of the etiology and mechanisms involved. Although ischemic heart disease is the leading cause of death for both men and women, the overwhelming majority of studies use male animals. Perhaps the most common reason for this practice is that physiologic fluctuations in female reproductive hormones such as estrogen may be a confounding variable, given the influence of female reproductive hormones on various organ systems.25 Despite the assertion that cyclical variations in female reproductive hormones may confound experimental studies, few data are available that support estrous-cycle–dependent variations in susceptibility to ischemic heart injury.
Epidemiologic studies suggest that, compared with men, women have lower cardiac mortality prior to undergoing menopause.40 Consistent with human studies, experimental models in several species commonly show that the degree of cardiac injury in young female animals is lower than that in male counterparts.7,9,21,22,42 Exogenous administration of estrogen has a clear effect in reducing injury,14,15 but whether endogenous cyclical variations in female reproductive hormones affect cardiac injury is not known.
Rats and mice are commonly used species to examine cardiac ischemia–reperfusion injury. Unlike humans, rodents do not undergo menstruation, during which the uterine endometrium sloughs off and is expelled through the vagina, but rather the uterine lining of rodents is reabsorbed during an estrous cycle.24 The rat estrous cycle is typically 4 to 5 d in length and is defined by 4 separate stages: proestrus, estrus, metestrus, and diestrus. Proestrus is characterized by increasing levels of estrogen. At the end of proestrus, ovulation (signaled by luteinizing hormone) occurs and marks the beginning of the estrus cycle. During metestrus and diestrus, the uterine lining regenerates, and the cycle starts again.24,33 These stages induce changes in the composition of the epithelium of the vagina and the presence of inflammatory cells, which can easily be detected by using vaginal cytology.18,35
We conducted the current study to determine whether estrous cycle stage influences the susceptibility to ischemia–reperfusion injury in the rat heart. Because the stage of the estrous cycle may influence cardiac injury either directly (via a direct effect of circulating hormones), or indirectly (by inducing changes that are intrinsic to the heart), we used both in vivo and ex vivo models of injury.
Materials and Methods
Animals.
Female Sprague–Dawley rats (Rattus norvegicus) were obtained from a commercial vendor and kept on a 12:12-h light:dark cycle, with access to food (Prolab RMH 3000, Lab Diets, Purina Laboratory, St Louis, MO) and water ad libitum. Serologic evaluation of 2 dirty-bedding sentinel rats caged on the same rack gave negative results for serum antibodies to common rat pathogens (rat coronavirus, rat parvoviruses, rat theilovirus, Sendai virus, pneumonia virus of mice, Mycoplasma pulmonis, Pneumocystis carinii, and reovirus). Rats were ordered so that they were older than 50 d at the onset of experiments. Typically female rats begin cycling immediately after the vaginal orifice opens (at 32 to 36 d).12,24 To ensure regular cycling, female rats were housed in a room with at least one male rat for the duration of the study.12 Research was performed in an AAALAC-accredited facility, and animals use adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals.16 All studies received prior approval from the IACUC at East Carolina University.
Vaginal cytology examination.
To evaluate estrous stage in the rats, vaginal smears were collected for cytologic evaluation.18,35 Briefly, a sterile cotton-tipped swab moistened with sterile saline was inserted into the rat's vaginal opening. The swab was rotated gently against the vaginal wall and removed. The swab immediately was rolled onto a glass slide, and then the smear was sprayed with a fixative (Safetex Cytology Spray Fixative, Andwin Scientific, Woodland Hills, CA). After fixation, the slide was stained (Dip Quick Stain Preparation, JorVet, Loveland, CO) by dipping each slide10 times into each of the 3 solutions, as this procedure was determined to produce optimal staining quality. On the day of experiments, vaginal cytology was performed immediately prior to ischemia–reperfusion experiments to get the most accurate reading of the cycle stage.
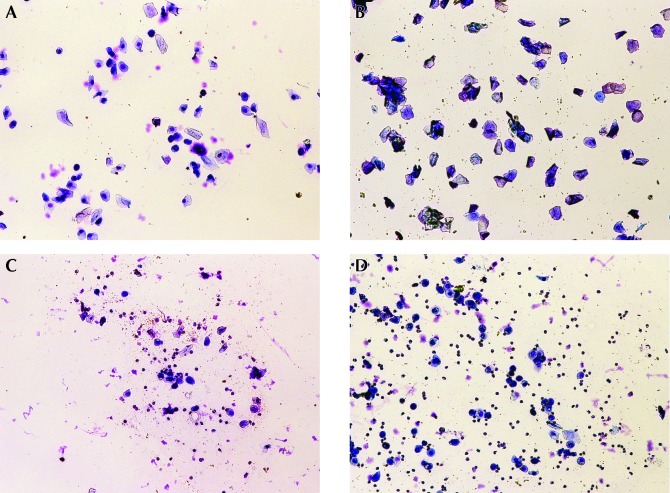
Two independent reviewers examined the smears by counting at least 100 cells per slide in a blinded fashion to determine the stage of the estrous cycle. When the 2 reviewers could not agree, a third independent reviewer was used to determine classification. Estrous stage was determined by using the following cell population criteria (Figure 1): proestrus, mix of basal and squamous (intermediate and superficial) cells accounting for more than 80% of cells; estrus, more than 75% superficial squamous (cornified) cells; metestrus, mix of squamous cells and occasional neutrophils; and diestrus, more than 60% neutrophils with parabasal and basal cells (often vacuolated).
Figure 1.
Representative images of vaginal cytology of rats in (A) proestrus, (B) estrus, (C) metestrus, and (D) diestrus.
In vivo preparation.
In vivo ischemia–reperfusion was performed similar to established protocols.8,39 Rats were anesthetized with ketamine–xylazine (ketamine, 90 mg/kg IP; xylazine, 10 mg/kg IP), and a midline tracheotomy was performed. The rats were intubated with PE90 tubing and mechanically ventilated. A circulating-water heating pad was used to maintain body temperature at 37 °C during the surgery. Two leads were placed on each rat in a configuration similar to that for lead II in a standard 12-lead electrocardiogram.
Rats were given a 10-min equilibration period while ventilated. Then the thorax was opened via a left parasternal incision, the pericardium was removed from the heart, and the left anterior descending coronary artery was ligated by using a reversible snare that was applied 4 mm distal to the origin and between the conus arteriosus and left atrium. Occlusion of the left anterior descending coronary artery was confirmed with the appearance of myocardial cyanosis distal to the occlusion. After 25 min of occlusion, the ligature was released, and reperfusion ensued for 2 h. To minimize desiccation, the chest walls were approximated by using paraffin film.
To determine the area at risk, the left anterior descending coronary artery was religated at the original point of occlusion immediately after reperfusion, and 1% Evans blue solution was infused through the aorta. After Evans blue staining, infarct size was determined via tetrazolium staining as described previously7,36 and expressed as a percentage of the area at risk.
Ex vivo preparation.
Rats were injected with ketamine–xylazine anesthetic as done previously, and once reflexes were absent, hearts were removed via midline thoracotomy. Hearts were retrograde perfused on the cannula of a modified Langendorff apparatus and instrumented for measurement of ventricular pressure development, coronary flow, and electrocardiogram according to our established protocols.11,36 After a 5-min baseline period, global, no-flow ischemia was induced for 25 min, after which flow was reestablished and reperfusion ensued for 2 h. Immediately after reperfusion, the left ventricle was isolated and assessed for infarcts via tetrazolium staining as described in the in vivo preparation.
Assessment of arrhythmias.
Arrhythmias were scored from the electrocardiographic signal as described previously6,11 and in accordance with the Lambeth Conventions41 as follows: score of 0, 0 to 49 premature ventricular beats; 1, 50 to 499 premature ventricular beats; 2, 500 or more premature ventricular beats or 1 episode of spontaneously reverting ventricular tachycardia (VT) or ventricular fibrillation (VF) less than 30 s in total duration; 3, one or more episodes of reverting VT or VF (total duration of less than 60 s); 4, one or more episodes of reverting VT or VF (total duration of 61 to 119 s); 5, VT or VF of more than 119 s in total duration; 6, fatal (nonreverting) VT or VF that began more than 15 min into treatment; 7, fatal VT or VF that began between 4 and 15 min into treatment; 8, fatal VT or VF that began between 1 and 4 min into treatment; and 9, fatal VT or VF that began within the first 59 s of treatment.
Hormone concentrations.
Rat blood was collected from the body cavity immediately after heart excision. Serum levels of 17β-estradiol were determined by using a commercially available ELISA kit (Cayman Chemicals, Ann Arbor, MI) according to the manufacturer's instructions.
Statistical analysis.
Analysis was done by using either GraphPad (Prism, La Jolla, CA) or SPSS (SPSS, Chicago, IL) software. Differences between stages of estrous were tested by using one-way ANOVA with the Tukey post hoc test. All binary variables were analyzed by using χ2 tests. All data are presented as mean ± SEM.
Results
Hemodynamic parameters.
Morphologic data and baseline cardiac functional (ex vivo) data of our rats are presented in Table 1. No significant differences were observed in body weight or heart weight across stages of the estrous cycle. Furthermore, there were no differences in baseline left ventricular developed pressure, coronary flow, or heart rate in ex vivo hearts. In addition, no differences in heart rate were observed across stages of the estrous cycle in vivo (proestrous, 230 ± 8 bpm; estrus, 192 ± 16 bpm; metestrus, 192 ± 14 bpm; and diestrus, 220 ± 16 bpm).
Table 1.
Baseline characteristics of rats used in the ex vivo preparation
| Proestrus | Estrus | Metestrus | Diestrus | |
| Body weight (g) | 204 ± 2 | 200 ± 4 | 205 ± 4 | 201 ± 4 |
| Heart weight (mg) | 930 ± 30 | 990 ± 40 | 950 ± 30 | 1000 ± 30 |
| Left ventricular developed pressure (mm Hg) | 117.6 ± 0.5 | 107.0 ± 0.6 | 124.1 ± 0.4 | 127.7 ± 0.5 |
| Heart rate (bpm) | 233 ± 3 | 187 ± 3 | 193 ± 2 | 209 ± 2 |
| Coronary flow (mL/min/g) | 11.73 ± 0.05 | 10.05 ± 0.07 | 10.48 ± 0.04 | 11.06 ± 0.06 |
Infarct size.
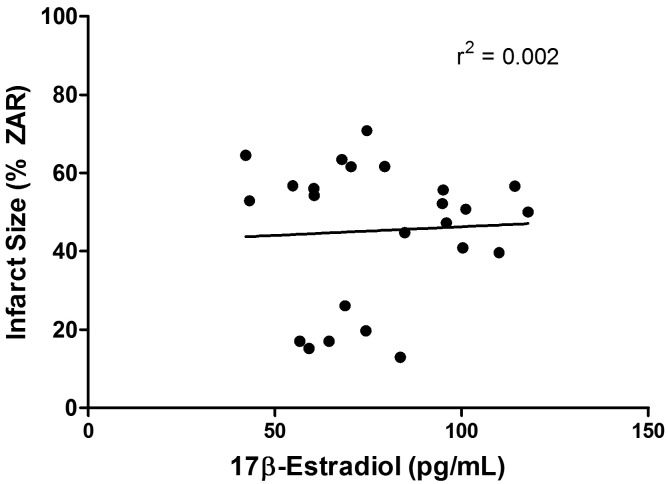
There were no differences observed in the infarcted area (expressed as a percentage of the zone-at-risk) in ex vivo, isolated hearts according to the stage of the estrous cycle (proestrus, 42% ± 6%; estrus, 49% ± 4%; metestrus, 40% ± 9%; diestrus, 47% ± 9%; P = 0.77). In addition, no differences in infarct size were seen between stages for the in vivo, surgical preparation (proestrus, 31% ± 4%; estrus, 29% ± 4%; metestrus, 28% ± 2%; diestrus, 30% ± 4%; P = 0.91). In the in vivo studies, there were no differences in the zone-at-risk between stages (proestrus, 48% ± 4%; estrus, 52% ± 3%; metestrus, 49% ± 5%; diestrus, 53% ± 4%; P = 0.90). Furthermore, there was no correlation observed between the level of circulating 17β-estradiol at the time of heart excision and infarct size in the ex vivo preparation (r2 = 0.002; Figure 2).
Figure 2.
There was no correlation between infarct size and serum levels of estradiol in isolated rat hearts.
Arrhythmias.
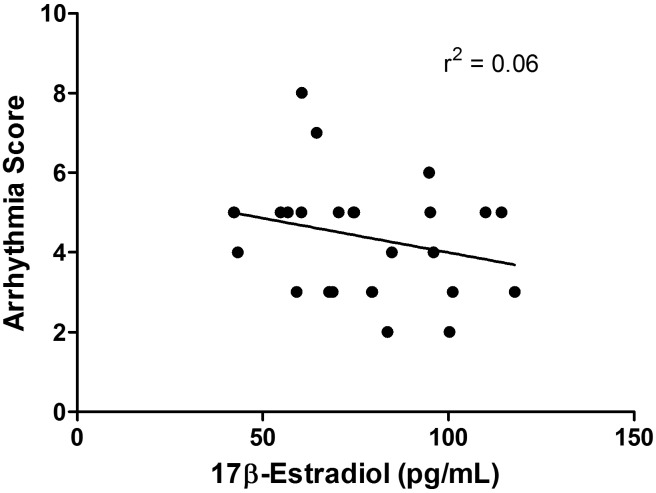
Arrhythmias were scored for the first 15 min of reperfusion in isolated rat heart (ex vivo) experiments. There were no differences observed in the severity of arrhythmias (that is, arrhythmia score; P = 0.32) or the incidence of VF (P = 0.21). The severity of these arrhythmias was not correlated with the levels of estradiol (r2 = 0.06; Figure 3).
Figure 3.
There was no correlation between the arrhythmia score and serum levels of estradiol in isolated rat hearts.
Discussion
It has been widely demonstrated in animal models that females exhibit a cardioprotective phenotype during their reproductive years.7,9,17,21,22,42 This characteristic has become an extensively studied area, with several different hypotheses regarding the mechanism. However, inherent variability in the data collected in these studies may have arisen due to animals in different stages of the estrous cycle. The goal of the current study was to investigate whether the stage of estrous cycle affected the susceptibility of rats to cardiac ischemia–reperfusion injury. We found that estrous stage has no direct effect on the level of myocardial infarction in either in vivo or ex vivo rat models. The level of myocardial infarction was not correlated with serum levels of 17β-estradiol, supporting our conclusion that estrous cycle stage has no discernible bearing on the extent of ischemia–reperfusion injury. No differences in the severity of arrhythmias or incidence of VF according to cycle stage were apparent. Our current study helps to establish that the rat estrous cycle does not influence ischemia–reperfusion injury in studies where estrous has not, or cannot, be controlled for.
The cardioprotective phenotype of premenopausal female animals has been demonstrated in mice, rats, rabbits and dogs.7,9,17,21,22,42 Although several mechanisms have been suggested for the possible underlying mechanism, none of the cited studies delved into the potential changes to cardiac tissue during specific stages of the estrous cycle. Specifically, the influence of fluctuating estrous cycle hormones on cardiac tissue remains largely unknown, because studies typically have focused specifically on vascular function.40 In a recent study, rats receiving ketamine and medetomidine combination as an injectable anesthetic for echocardiographic assessment demonstrated a reduction in left ventricle systolic function as serum levels of estradiol increased.32
Another recent study found that cardiovascular events in menstruating women account for only 1% of all events.26 However, the stage of the menstrual cycle can have profound effects on cardiovascular function in humans. For example, blood pressure and heart rate can vary between stages.25 In addition, women early in the menstrual cycle are at increased risk for angina,19supraventricular tachycardia,27,30 and exercise-induced ST depression.23 Furthermore, episodes of supraventricular tachycardia have been linked to decreased levels of circulating estradiol and increased levels of progesterone,30 and women who have irregular cycles are more susceptible to future coronary heart disease3,37 that can be extended to their postmenopausal years.1 Because animal models are powerful tools for investigating cardiovascular disease, these data provide valuable insight regarding the estrous cycle and cardiovascular health.
Our findings demonstrate that endogenous fluctuations in reproductive hormones do not exert a measurable influence on cardiac injury. Several others have found that exogenous estradiol administration can affect ischemia–reperfusion injury, with several distinct mechanisms promulgated (for review, see references 4 and 10). For example, estradiol augments the activity of the PI3K/Akt pathway, and inhibition of PI3K and PKC has been shown to diminish cardiac function and increase infarct size in female, but not male, rats.2 Using isolated hearts, other investigators found that female mice lacking estrogen receptor β exhibit diminished protection from myocardial infarction, inferring that increased activation of PI3K/Akt and decreased apoptosis may be responsible for this cardioprotective phenotype.42 Further demonstrating the possible antiapoptotic effects of estrogen, activation of a G-protein–coupled receptor that binds directly to estrogen decreases mitochondrial permeability transition.5 Each of these studies demonstrates the cardioprotective effects of estrogen-dependent signaling as a mechanism in young women. However, during the estrous stage in young female rats, endogenous changes in reproductive hormones do not appear to be a confounding variable in ischemia–reperfusion studies.
Acute pharmacologic doses of exogenous estradiol have been shown to be cardioprotective in the isolated heart models and lower, more physiologic levels of estradiol have no acute effect.38 The fact that we saw no estrous-stage–associated difference in infarct size nor correlation between infarct size and estradiol levels substantiates this observation. Debates among investigators regarding the protective effects of exogenous estradiol administration may reflect the use of supraphysiologic compared with physiologic doses of estradiol in these studies.
Our current study is important in establishing that estrous cycle does not influence myocardial infarction in young, healthy female rats. However, our results cannot rule out an effect of the estrous cycle on the extent of infarction in animal models where the coronary vasculature is compromised (such as atherosclerosis).
The benefits of exogenous hormone therapy to human health may also be limited, given that studies on hormone replacement therapy and morbidity have yielded conflicting results (for review and discussion, please see references 28 and 29). Initially, findings from the World Health Initiative study led investigators to believe that the risks of hormone replacement therapy outweighed its benefits. However, others investigators have challenged this belief, and current debate exists regarding whether the efficacy of hormone replacement therapy depends on the timing of administration.13,20,31 Interestingly, there is an overall decrease in the risk of cardiovascular events with no increase in the adverse risks (that is breast cancer, deep vein thrombosis, stroke) if treatment begins early in menopause.34
Although several studies have shown that the levels of female sex hormones can have profound effects on cellular function, no one previously had investigated whether circulating hormones affect ischemia–reperfusion injury in rats. Our current study revealed that circulating hormone levels during various estrous- cycle stages do not influence cardiac ischemia–reperfusion injury in rats. In addition, the serum estradiol level of rats is not correlated with infarct size or arrhythmia. These findings were confirmed by using both in vivo and ex vivo models, demonstrating that the effects of circulating hormones do not markedly influence ischemia–reperfusion injury in rat hearts. Our study is important in establishing the validity of data obtained from female rats in studies where estrous cycle stage was not determined.
Acknowledgment
This work was supported by an American Heart Association Predoctoral Fellowship (AHA no. 11PRE7590086 to CRF).
References
- 1.Azevedo GD, Duarte JM, Souza MO, Costa ESTD, Soares EM, Maranhao TM.2006. [Menstrual cycle irregularity as a marker of cardiovascular risk factors at postmenopausal years] Arq Bras Endocrinol Metabol 50:876–883. [Article in Portuguese]
- 2.Bae S, Zhang L. 2005. Gender differences in cardioprotection against ischemia–reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther 315:1125–1135 [DOI] [PubMed] [Google Scholar]
- 3.Bertuccio P, Tavani A, Gallus S, Negri E, La Vecchia C. 2007. Menstrual and reproductive factors and risk of nonfatal acute myocardial infarction in Italy. Eur J Obstet Gynecol Reprod Biol 134:67–72 [DOI] [PubMed] [Google Scholar]
- 4.Booth EA, Lucchesi BR. 2008. Estrogen-mediated protection in myocardial ischemia–reperfusion injury. Cardiovasc Toxicol 8:101–113 [DOI] [PubMed] [Google Scholar]
- 5.Bopassa JC, Eghbali M, Toro L, Stefani E. 2010. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol 298:H16–H23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown DA, Aon MA, Frasier CR, Sloan RC, Maloney AH, Anderson EJ, O'Rourke B. 2010. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol 48:673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DA, Lynch JM, Armstrong CJ, Caruso NM, Ehlers LB, Johnson MS, Moore RL. 2005. Susceptibility of the heart to ischaemia–reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. J Physiol 564:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cozzi E, Hazarika S, Stallings HW, 3rd, Cascio WE, Devlin RB, Lust RM, Wingard CJ, Van Scott MR. 2006. Ultrafine particulate matter exposure augments ischemia–reperfusion injury in mice. Am J Physiol Heart Circ Physiol 291:H894–H903 [DOI] [PubMed] [Google Scholar]
- 9.Das B, Sarkar C. 2006. Similarities between ischemic preconditioning and 17β-estradiol mediated cardiomyocyte KATP channel activation leading to cardioprotective and antiarrhythmic effects during ischemia–reperfusion in the intact rabbit heart. J Cardiovasc Pharmacol 47:277–286 [DOI] [PubMed] [Google Scholar]
- 10.Deschamps AM, Murphy E, Sun J. 2010. Estrogen receptor activation and cardioprotection in ischemia–reperfusion injury. Trends Cardiovasc Med 20:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frasier CR, Sloan RC, Bostian PA, Gonzon MD, Kurowicki J, Lopresto SJ, Anderson EJ, Brown DA. 2011. Short-term exercise preserves myocardial glutathione and decreases arrhythmias after thiol oxidation and ischemia in isolated rat hearts. J Appl Physiol 111:1751–1759 [DOI] [PubMed] [Google Scholar]
- 12.Goldman JM, Murr AS, Cooper RL. 2007. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80:84–97 [DOI] [PubMed] [Google Scholar]
- 13.Guallar E, Manson JE, Laine C, Mulrow C. 2013. Postmenopausal hormone therapy: the heart of the matter. Ann Intern Med 158:69–70 [DOI] [PubMed] [Google Scholar]
- 14.Hale SL, Birnbaum Y, Kloner RA. 1996. β-Estradiol, but not α-estradiol, reduced myocardial necrosis in rabbits after ischemia and reperfusion. Am Heart J 132:258–262 [DOI] [PubMed] [Google Scholar]
- 15.Hale SL, Birnbaum Y, Kloner RA. 1997. Estradiol, administered acutely, protects ischemic myocardium in both female and male rabbits. J Cardiovasc Pharmacol Ther 2:47–52 [DOI] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 17.Johnson MS, Moore RL, Brown DA. 2006. Sex differences in myocardial infarct size are abolished by sarcolemmal KATP channel blockade in rat. Am J Physiol Heart Circ Physiol 290:H2644–H2647 [DOI] [PubMed] [Google Scholar]
- 18.Karim BO, Landolfi JA, Christian A, Ricart-Arbona R, Qiu W, McAlonis M, Eyabi PO, Khan KA, Dicello JF, Mann JF, Huso DL. 2003. Estrous cycle and ovarian changes in a rat mammary carcinogenesis model after irradiation, tamoxifen chemoprevention, and aging. Comp Med 53:532–538 [PubMed] [Google Scholar]
- 19.Kawano H, Motoyama T, Ohgushi M, Kugiyama K, Ogawa H, Yasue H. 2001. Menstrual cyclic variation of myocardial ischemia in premenopausal women with variant angina. Ann Intern Med 135:977–981 [DOI] [PubMed] [Google Scholar]
- 20.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J. 2011. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA 305:1305–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. 2010. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res 106:1681–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee TM, Su SF, Tsai CC, Lee YT, Tsai CH. 2000. Cardioprotective effects of 17β-estradiol produced by activation of mitochondrial ATP-sensitive K+ channels in canine hearts. J Mol Cell Cardiol 32:1147–1158 [DOI] [PubMed] [Google Scholar]
- 23.Lloyd GW, Patel NR, McGing E, Cooper AF, Brennand-Roper D, Jackson G. 2000. Does angina vary with the menstrual cycle in women with premenopausal coronary artery disease? Heart 84:189–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long JA, Evans HM. 1922. The oestrus cycle in the rat and its associated phenomena. Berkeley (CA): University of California Press [Google Scholar]
- 25.Moran VH, Leathard HL, Coley J. 2000. Cardiovascular functioning during the menstrual cycle. Clin Physiol 20:496–504 [DOI] [PubMed] [Google Scholar]
- 26.Mukamal KJ, Muller JE, Maclure M, Sherwood JB, Mittleman MA. 2002. Variation in the risk of onset of acute myocardial infarction during the menstrual cycle. Am J Cardiol 90:49–51 [DOI] [PubMed] [Google Scholar]
- 27.Myerburg RJ, Cox MM, Interian A, Jr, Mitrani R, Girgis I, Dylewski J, Castellanos A. 1999. Cycling of inducibility of paroxysmal supraventricular tachycardia in women and its implications for timing of electrophysiologic procedures. Am J Cardiol 83:1049–1054 [DOI] [PubMed] [Google Scholar]
- 28.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. 2002. Postmenopausal hormone replacement therapy: scientific review. JAMA 288:872–881 [DOI] [PubMed] [Google Scholar]
- 29.North American Menopause Society 2012. The 2012 hormone therapy position statement of The North American Menopause Society. Menopause 19:257–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosano GM, Leonardo F, Sarrel PM, Beale CM, De Luca F, Collins P. 1996. Cyclical variation in paroxysmal supraventricular tachycardia in women. Lancet 347:786–788 [DOI] [PubMed] [Google Scholar]
- 31.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. 2007. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297:1465–1477 [DOI] [PubMed] [Google Scholar]
- 32.Sabatini CF, O'Sullivan M, Valcour J, Sears W, Johnson R. 2013. Effects of injectable anesthetic combinations on left ventricular function and cardiac morphology in Sprague–Dawley rats. J Am Assoc Lab Anim Sci 52:34–43 [PMC free article] [PubMed] [Google Scholar]
- 33.Schedin P, Mitrenga T, Kaeck M. 2000. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague–Dawley rat: a model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia 5:211–225 [DOI] [PubMed] [Google Scholar]
- 34.Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, Kober L, Jensen JE. 2012. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 345:e6409. [DOI] [PubMed] [Google Scholar]
- 35.Singletary SJ, Kirsch AJ, Watson J, Karim BO, Huso DL, Hurn PD, Murphy SJ. 2005. Lack of correlation of vaginal impedance measurements with hormone levels in the rat. Contemp Top Lab Anim Sci 44:37–42 [PMC free article] [PubMed] [Google Scholar]
- 36.Sloan RC, Rosenbaum M, O'Rourke D, Oppelt K, Frasier CR, Waston CA, Allan AG, Brown DA. 2011. High doses of ketamine–xylazine anesthesia reduce cardiac ischemia–reperfusion injury in guinea pigs. J Am Assoc Lab Anim Sci 50:349–354 [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. 2002. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab 87:2013–2017 [DOI] [PubMed] [Google Scholar]
- 38.Sovershaev MA, Egorina EM, Andreasen TV, Jonassen AK, Ytrehus K. 2006. Preconditioning by 17β-estradiol in isolated rat heart depends on PI3K/PKB pathway, PKC, and ROS. Am J Physiol Heart Circ Physiol 291:H1554–H1562 [DOI] [PubMed] [Google Scholar]
- 39.Urankar RN, Lust RM, Mann E, Katwa P, Wang X, Podila R, Hilderbrand SC, Harrison BS, Chen P, Ke PC, Rao AM, Brown JM, Wingard CJ. 2012. Expansion of cardiac ischemia–reperfusion injury after instillation of 3 forms of multiwalled carbon nanotubes. Part Fibre Toxicol 9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A, Koller A, Marzilli M, Pries A, Bugiardini R. 2011. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position Paper from the Working Group on Coronary Pathophysiology and Microcirculation of the European Society of Cardiology. Cardiovasc Res 90:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, Cobbe SM, Coker SJ, Harness JB, Harron DW, Higgins AJ, Julian DG, Lab MJ, Manning AS, Northover BJ, Parratt JR, Riemersma RA, Riva E, Russell DC, Sheridan DJ, Winslow E, Woodward B. 1988. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc Res 22:447–455 [DOI] [PubMed] [Google Scholar]
- 42.Wang M, Wang Y, Weil B, Abarbanell A, Herrmann J, Tan J, Kelly M, Meldrum DR. 2009. Estrogen receptor β mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol 296:R972–R978 [DOI] [PMC free article] [PubMed] [Google Scholar]