Abstract
We report a case of a generalized seizure in an adult female rhesus macaque (Macaca mulatta) undergoing a urodynamic evaluation while she was anesthetized with continuous-infusion ketamine. The seizure presented with generalized tonic–clonic activity during bladder infusion with saline. The tonic–clonic phase was self-limited and was followed by focal facial twitching, which was interrupted by bolus administration of intravenous diazepam. The ictal event was documented as pressure oscillations during cystometrogram recordings and a period of external urethral sphincter muscle activation, which was detectable by electromyography. An acute decrease in urethral pressure was demonstrated at the end of the generalized seizures. Ketamine anesthesia combined with relatively rapid infusion of saline into the bladder may have contributed to the onset of seizures. In addition, this case highlights the value of having a fast-acting benzodiazepine agent available to stop continuous or residual seizure activity during diagnostic or experimental procedures in anesthetized nonhuman primates.
Anesthetic agents are used to sedate and immobilize nonhuman primates for veterinary health care and experimental studies. For these purposes, ketamine, a dissociative general anesthetic, is the agent used most commonly in macaques. Several protocols have been developed for the safe administration of ketamine, either as a single agent or as part of a balanced anesthetic approach administered orally, per rectum, intramuscularly, or intravascularly.9,16,18 Although ketamine and other anesthetic agents are known to suppress physiologic responses during experimental studies, reflexive micturition may still be evoked, as demonstrated by urodynamic recordings in anesthetized rats.1,2,10 In addition, urodynamic studies have demonstrated that bladder reflexes are suppressed by anesthesia in nonhuman primates, although comparative studies on the effects of different anesthetic agents have suggested that ketamine may be a suitable anesthetic for cystometrograms in rhesus macaques.5
Here, parenteral administration of ketamine was used to sedate and immobilize adult rhesus macaques for comprehensive urodynamic studies. In one subject, the infusion of saline into the urinary bladder was associated with the acute onset of a generalized seizure, characterized by a self-limited period of tonic–clonic activity, followed by focal facial twitching, which was relieved by prompt intravenous administration of diazepam, and voiding. The tonic–clonic phase of the event was documented by cystometrography and electromyography recordings. We suggest the administration of ketamine anesthesia and the relatively rapid infusion of saline into the bladder as possible factors that may have contributed to the ictal event in this rare case of a clinical seizure during a urodynamic study in a nonhuman primate.
Materials and Methods
All animals were housed in AAALAC-accredited facilities, and all procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals.8 All animal research procedures were approved by the IACUC at University of California at Davis and by the Department of Defense. Comprehensive urodynamic studies were performed in a total of 12 female rhesus macaques. All subjects were neurologically intact, had no history of any prior neurosurgical procedures, and showed no behavioral abnormalities. The case subject, a female rhesus macaque (Macaca mulatta), was housed indoor at the California National Primate Research Center in a stainless steel cage (Lab Product, Seaford, DE) at 22 °C, and ambient humidity varied between 30% to 70%. The room had a 12:12-h light:dark cycle. Air changes in the room were 10 to 15 each hour. Tap water was available ad libitum, and the animal was fed commercial chow (High-protein diet, Ralston Purina, St Louis, MO) and fruit. Although all attempts are made at pair-housing animals at the California National Primate Research Center, including subjects on research protocols, this macaque was single-housed at the time of the urodynamic evaluation due to 3 previous unsuccessful pairing attempts. However, after the ictal event, this female macaque has been paired successfully for more than 1 y.
All macaques initially were immobilized by an intramuscular injection of ketamine (10 mg/kg) followed by constant-rate infusion of ketamine (12 mg/kg/h) or propofol (20 mg/kg/h). A constant-rate infusion protocol was chosen to provide a stable plane of anesthesia. For combined cystometry and urethral pressure measurements, a 7-French triple-lumen catheter (Life-Tech, Stafford, TX) was inserted into the urinary bladder. The bladder and urethral ports of the catheter then were connected to separate pressure transducers (Biopac Systems, Goleta, CA). For electromyographic studies, bipolar electrodes were inserted into the left and right sides of the external urethral sphincter muscle by using wire-electrode sets equipped with a 1.0-in., 27-gauge needle (1512A-F, Life-Tech). The external end of each wire electrode was connected to an amplifier (EMG100C, Biopac Systems). A ground electrode was placed in the left hindlimb. Reflex bladder contractions were evoked by rapid manual infusion of saline into the urinary bladder by using the third port of the catheter. A total of 52 micturition reflex cycles were attempted. Of these functional assessments of the lower urinary tract, 49 bladder-infusion cycles were performed under ketamine anesthesia, and the remaining 3 bladder-infusion studies were in animals receiving propofol.
Case Report
The subject of this case is a female rhesus macaque that was 11 y 2 mo old and weighed 9.05 kg at the time of the experimental procedures. Before the urodynamic studies, an assessment of lean muscle mass and fat was performed and demonstrated an optimal body condition (3.0).19 The subject was born at the California National Primate Research Center and was the product of an uncomplicated pregnancy via vaginal birth. This female macaque subsequently was raised in captivity as a member of the facility breeding program. She has been in excellent general physical health and has no history of any prior seizures.
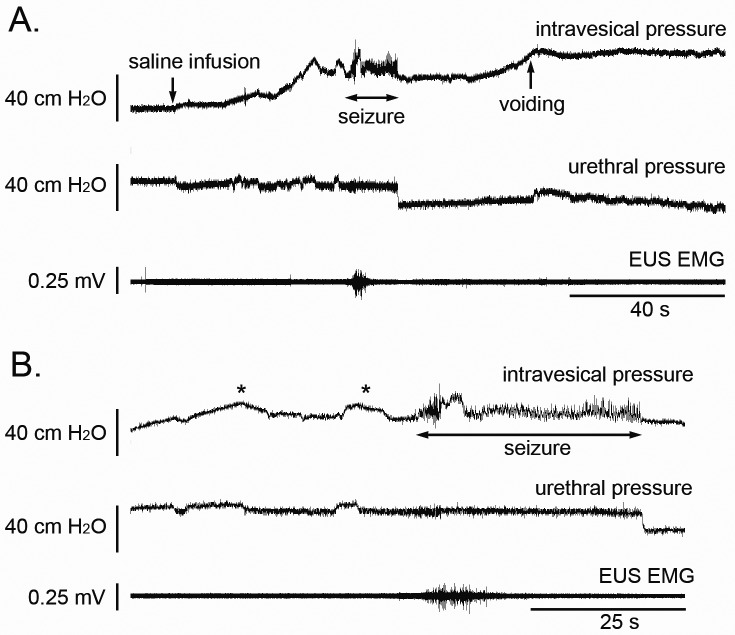
For urodynamic recordings, the macaque was immobilized by using an initial dose of ketamine (10 mg/kg IM) followed by continuous-rate infusion of ketamine, which was titrated to a dose of 12 mg/kg/h to maintain sedation. A triple-lumen bladder catheter was placed for concurrent bladder and urethral pressure recordings, and the bladder was emptied. Surface electrodes were attached to the abdominal wall over the bilateral external oblique muscles for electromyography. Next, by using one port of the triple-lumen bladder catheter, the bladder was infused with 130 mL of saline over 45 s. However, after the completion of the saline infusion, the subject suddenly developed generalized tonic–clonic seizures. The generalized seizure lasted 36 s and stopped spontaneously. The seizure was associated with voiding both during and after the phase of active tonic–clonic activity. The total voided volume was 115 mL, with a residual bladder volume of 20 mL; the calculated voiding efficiency was 88%. In addition to the clinical presentation of a seizure, the muscle fasciculations were detectable by our continuous urodynamic recordings (Figure 1). Specifically, oscillations of the bladder pressure recordings occurred concurrently with the clinical seizure. In addition, the urethral pressure was elevated for the duration of the generalized tonic–clonic activity. Both the bladder pressure oscillations and urethral pressure elevation resolved promptly when the tonic–clonic phase stopped. However, residual left-sided facial twitching was detected but halted instantly with the administration of diazepam (10 mg IV). Ketamine administration was discontinued immediately, and the macaque subsequently was monitored closely. Over the next several hours, the animal recovered and was awake and active later that same day. On recovery, the macaque showed no clinical signs of any recurrent seizures or any other residual neurologic sequelae.
Figure 1.
(A) Comprehensive urodynamic studies were performed in an adult female rhesus macaque with concurrent cystometrographic recordings of bladder pressures (upper trace), urethral pressure monitoring (middle trace), and external urethral sphincter electromyography (lower trace). Note the increase in bladder pressure in response to bladder filling with saline solution and the following onset of a generalized seizure. The tonic–clonic activity is associated with oscillating pressure recordings from the bladder and brief electromyographically visible activation of the external urethral sphincter. In addition, there is an acute reduction in urethral pressure at the end of clinical seizure activity. The tonic–clonic activity was followed by an additional rise in bladder pressure, corresponding to active bladder contractions, and voiding. The * indicates peaks of bladder pressures associated with successive infusions of saline by using handheld syringes. (B) The recording of convulsive seizure activity is shown at an increased rate to highlight the pressure oscillations demonstrated by cystometrographic recordings and external urethral electromyographic activity during the period of active tonic–clonic movement.
Discussion
Here, we report the association of generalized tonic–clonic seizure activity with ketamine anesthesia and infusion of saline into the bladder to evoke a micturition reflex in a female rhesus macaque. The generalized motor features of the seizure were self-limited, and subsequent partial motor activity of facial twitching was interrupted readily by intravenous administration of diazepam.
Focal rhythmic muscle twitching and generalized convulsions, clinically suggestive or suspicious for seizures, have been documented in humans under anesthesia induced by various of agents, including injectable agents such as ketamine and propofol.11,12,21 However, the overall risk of anesthesia-induced epileptic seizures and relative frequency of occurrences for individual agents remain less precisely defined and a source of controversy, because most involuntary motor activity during anesthesia takes place without simultaneous electroencephalographic monitoring. Regarding ketamine, both pro- and anticonvulsant effects have been reported.21 For instance, ketamine may induce electroconvulsive activity in the human limbic and thalamic regions.3 Ketamine also has been associated with the activation of epileptogenic foci in patients with known seizure disorders as well as myoclonic seizure and seizure-like motor activity in nonepileptic children and adults.12,14 In contrast to these proconvulsive effects, ketamine has been shown to terminate refractory seizures and status epilepticus.15,17 A potential rational and mechanistic explanation for the use of ketamine for treating refractory seizes come from experimental studies, which have shown that ongoing seizure activity can result in a concurrent reduction of γ-aminobutyric acid A receptor subunits and an increase in N-methyl-d-aspartate receptors in the brain.6,13 Because ketamine has an antagonistic action on N-methyl-d-aspartate receptors, the drug has been suggested as a potential candidate for inclusion in polytherapy options for the treatment of both acute seizures and status epilepticus.22 The mechanisms of action for the pro- and anticonvulsant effects of ketamine are not well understood. However, dose-dependent activity may contribute, given that lower doses of ketamine may suppress seizure activity and higher doses potentiate seizures in rat epilepsy models.20
In the case we present, the generalized seizure activity occurred in close association with the infusion of saline into the bladder while we attempted to evoke a reflex bladder contraction. The generalized tonic–clonic activity began after a relatively rapid rise in bladder pressure in response to the bladder infusion. The seizure onset took place when the bladder pressure exceeded baseline levels and within the range of bladder pressures normally associated with the onset of bladder contractions and reflex voiding. In this experimental model, visceral primary afferents are activated by infusion of saline into the bladder and provide sensory information to the CNS, with extensive connections and feedback systems present between the spinal cord and brain for micturition control.4,7 We speculate that a relatively rapid infusion of saline into the bladder provided an excessively strong stimulus to the nervous system, compared with the slower physiologic filling of the bladder provided by renal function alone, and thereby contributed to seizure onset. However, the other 11 subjects that underwent similar urodynamic studies as part of this series did not show any seizure activity, and reports of seizures associated with urodynamic studies are absent from the literature.
Cystometrographic recordings typically are performed in awake human subjects; anesthesia usually is reserved for urodynamic evaluations in young children and patients who are unable to tolerate functional micturition studies without sedation. Regardless, diagnostic evaluations of the urinary tract are common outpatient procedures and generally are well-tolerated in the clinical setting.
We conclude that seizures with generalized tonic–clonic activity may occur in association with urodynamic recordings under ketamine anesthesia in nonhuman primates. The anesthetic agent, the diagnostic procedure itself, or a combination of the 2 may have contributed to the ictal event. In addition, our case presentation also highlights the value of having available a quick-acting benzodiazepine agent (for example, diazepam or lorazepam) to interrupt any prolonged or residual seizure activity.
Acknowledgments
The studies were supported by a Translational Research Partnership Grant from the Department of Defense Congressionally Directed Medical Research Program on Spinal Cord Injury (SC090273), the Dr Miriam and Sheldon G Adelson Medical Research Foundation, and the National Center for Research Resources (RR000169) of the NIH.
References
- 1.Cannon TW, Damaser MS. 2001. Effects of anesthesia on cystometry and leak-point pressure of the female rat. Life Sci 69:1193–1202 [DOI] [PubMed] [Google Scholar]
- 2.Chang HY, Havton LA. 2008. Differential effects of urethane and isoflurane on external urethral sphincter electromyography and cystometry in rats. Am J Physiol Renal Physiol 295:F1248–F1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrer-Allado T, Brechner VL, Dymond A, Cozen H, Crandall P. 1973. Ketamine-induced electroconvulsive phenomena in the human limbic and thalamic regions. Anesthesiology 38:333–344 [DOI] [PubMed] [Google Scholar]
- 4.Fowler CJ, Griffiths D, de Groat WC. 2008. The neural control of micturition. Nat Rev Neurosci 9:453–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghoniem GM, Shoukry MS, Monga M. 1996. Effects of anesthesia on urodynamic studies in the primate model. J Urol 156:233–236 [PubMed] [Google Scholar]
- 6.Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. 2008. Subunit-specific trafficking of GABAA receptors during status epilepticus. J Neurosci 28:2527–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holstege G. 2005. Micturition and the soul. J Comp Neurol 493:15–20 [DOI] [PubMed] [Google Scholar]
- 8.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 9.Lee VK, Flynt KS, Haag LM, Taylor DK. 2010. Comparison of the effects of ketamine, ketamine–medetomidine, and ketamine–midazolam on physiologic parameters and anesthesia-induced stress in rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques. J Am Assoc Lab Anim Sci 49:57–63 [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuura S, Downie JW. 2000. 2000 Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol Urodyn 19:87–99 [DOI] [PubMed] [Google Scholar]
- 11.Modica PA, Tempelhoff R, White PF. 1990. Pro- and anticonvulsant effects of anesthetics (part I). Anesth Analg 70:303–315 [DOI] [PubMed] [Google Scholar]
- 12.Modica PA, Tempelhoff R, White PF. 1990. Pro- and anticonvulsant effects of anesthetics (part II). Anesth Analg 70:433–444 [DOI] [PubMed] [Google Scholar]
- 13.Naylor DE, Liu H, Wasterlain CG. 2005. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 25:7724–7733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plumb DC. 2002. Veterinary drug handbook, 4th ed. Ames (IA): Iowa State Press. [Google Scholar]
- 15.Prüss H, Holtkamp M. 2008. Ketamine successfully terminates malignant status epilepticus. Epilepsy Res 82:219–222 [DOI] [PubMed] [Google Scholar]
- 16.Pulley AC, Roberts JA, Lerche NW. 2004. Four preanesthetic oral sedation protocols for rhesus macaques (Macaca mulatta). J Zoo Wildl Med 35:497–502 [DOI] [PubMed] [Google Scholar]
- 17.Sheth RD, Gidal BE. 1998. Refractory status epilepticus: response to ketamine. Neurology 51:1765–1766 [DOI] [PubMed] [Google Scholar]
- 18.Steelman R, Seale NS, Bellinger L, Harris M, Wagner M, Williams F. 1991. Conscious sedation and analgesia with rectal ketamine in the Macata fuscata monkey. Anesth Prog 38:50–56 [PMC free article] [PubMed] [Google Scholar]
- 19.Summers L, Clingerman KJ, Yang X. 2012. Validation of a body condition scoring system in rhesus macaques (Macaca mulatta): assessment of body composition using dual-energy X-ray absorptiometry. J Am Assoc Lab Anim Sci 51:88–93 [PMC free article] [PubMed] [Google Scholar]
- 20.Velísek L, Vondricková R, Mares P. 1993. Models of simple partial and absence seizures in freely moving rats: action of ketamine. Pharmacol Biochem Behav 45:889–896 [DOI] [PubMed] [Google Scholar]
- 21.Voss LJ, Sleigh JW, Barnard JP, Kirsch HE. 2008. The howling cortex: seizures and general anesthetic drugs. Anesth Analg 107:1689–1703 [DOI] [PubMed] [Google Scholar]
- 22.Wasterlain CG, Baldwin R, Naylor DE, Thompson KW, Suchomelova L, Niquet J. 2011. Rational polytherapy in the treatment of acute seizures and status epilepticus. Epilepsia 52:70–71 [DOI] [PMC free article] [PubMed] [Google Scholar]