Abstract
The key role of IL-23 in the pathogenesis of autoimmune and chronic inflammatory disorders is supported by the identification of IL-23R susceptibility alleles associated with IBD, psoriasis and ankylosing spondylitis. IL-23 driven inflammation has primarily been linked to the actions of Th17 cells1. Somewhat overlooked, IL-23 also has inflammatory effects on innate immune cells2 and can drive T cell- independent colitis. However the downstream cellular and molecular pathways involved in this innate intestinal inflammatory response are poorly characterized. Here we show that bacteria-driven innate colitis is associated with increased IL-17 and IFN-γ production in the colon. Stimulation of colonic leukocytes with IL-23 induced IL-17 and IFN-γ production exclusively by innate lymphoid cells expressing Thy1, SCA-1, RORγt and IL-23R and these cells markedly accumulated in the inflamed colon. Importantly, IL-23 responsive innate intestinal cells are also a feature of T-cell dependent models of colitis. The transcription factor RORγt, which controls IL-23R expression, plays a functional role as Ror−/−Rag−/− mice failed to develop innate colitis. Lastly, depletion of Thy1+ innate lymphoid cells completely abrogated acute and chronic innate colitis. These results identify a novel IL-23 responsive innate lymphoid population that mediates intestinal immune pathology and may therefore represent a target in IBD.
Th17 cells produce a variety of inflammatory cytokines including interleukin-17A (IL-17), IL-17F, IL-22, IL-6 and TNFα, and are implicated in both host defence against extracellular pathogens and the pathogenesis of several inflammatory disorders1. Recently Th17 cells have been shown to exhibit flexibility of function and acquisition of IFN-γ production has been linked to their pathogenicity in vivo3,4. Transforming growth factor-β (TGFβ) and IL-6 or IL-21 drive Th17 cell differentiation5 and this process is orchestrated by the transcription factor, retinoic acid related orphan receptor γt (RORγt) (ref 6). RORγt promotes IL-23R expression, allowing IL-23 to control the expansion and maintenance of Th17 cells7. Interestingly, RORγt is also expressed by innate lymphoid cells such as intestinal cells that express NK markers, fetal LTi (lymphoid tissue inducer) cells and adult LTi-like cells8. Various innate tissue leukocytes, such as CD11c+ myeloid cells, LTi-like cells and mucosal NKp46+ cells, have been shown to produce IL-22 and/or IL-17 upon IL-23 stimulation, but the contribution of these tissue resident innate immune cells to pathology in the intestine is not known2, 9-11.
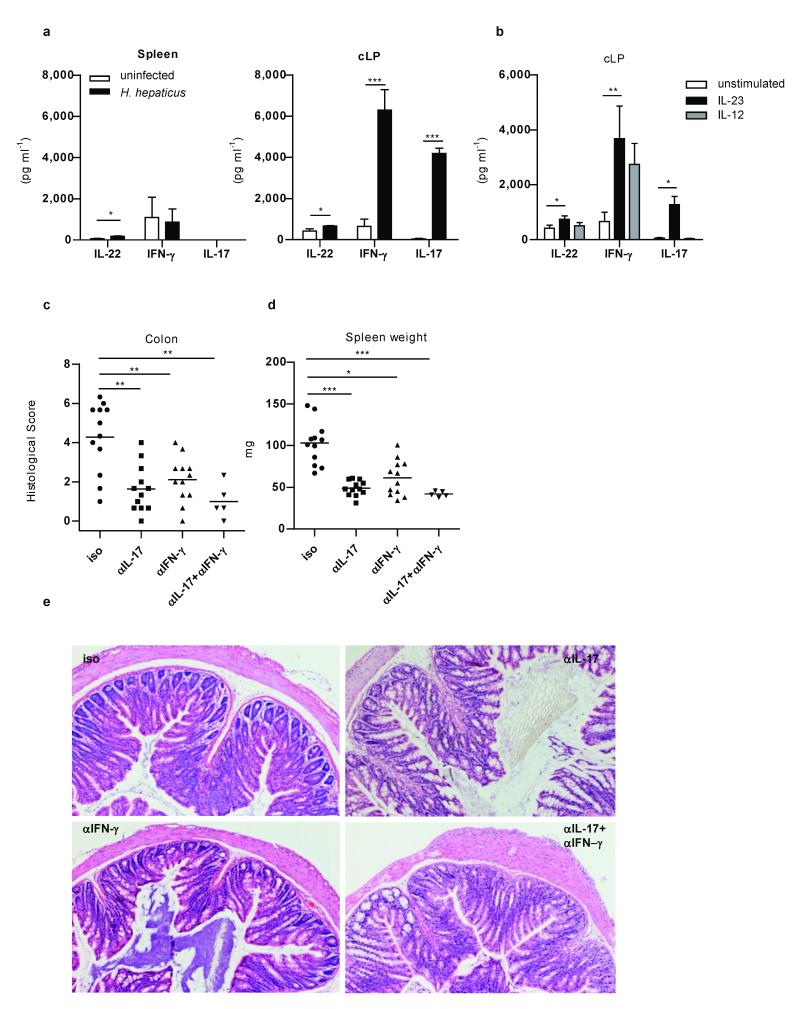
We have shown that innate immune colitis in Rag−/− mice following infection with Helicobacter hepaticus is IL-23 dependent12. To identify the cellular and molecular pathways involved, we first analysed the expression of inflammatory cytokines in this model. Consistent with selective upregulation of IL-23 in the intestine12, we observed significant increases in the expression of Th17 and Th1 signature cytokines including IL-17, IL-22 and IFN-γ by colonic lamina propria cells (cLP) from H. hepaticus infected Rag−/− mice but not from spleen cells (Fig. 1a). IL-23 mediated pathology was not associated with an increase of IL-6 (Supplementary Fig. 1). To determine whether IL-23 acts directly on innate cells to induce Th1 and Th17 cytokines, cLP cells were isolated from healthy colons of Rag−/−mice and stimulated with IL-12 or IL-23. Addition of IL-23 induced secretion of IL-17, IL-22 and IFN-γ (Fig. 1b), whereas IL-12 induced IFN-γ only. To determine whether IL-17 and IFN-γ played a functional role in innate colitis, H. hepaticus infected Rag−/− mice were treated with neutralising α-IL-17 or α-IFN-γ mAbs. Blockade of either IL-17 or IFN-γ was sufficient to significantly reduce colitis (Fig. 1c and e), without affecting colonisation with H. hepaticus (Supplementary Fig. 2). Similarly, systemic immune activation, assessed by splenomegaly, was also abrogated by IL-17 or IFN-γ blockade (Fig. 1d). Collectively, these results indicate that H. hepaticus induced IL-23 regulates the innate expression of effector cytokines such as IL-17 and IFN-γ that play functional roles in the intestinal innate inflammatory response.
Figure 1.
IL-23 induced IL-17 and IFN-γ are required for H. hepaticus-mediated innate colitis.
a, Cytokine secretion following overnight culture of splenocytes or cLP cells from control or H. hepaticus–infected 129SvEvRag−/− mice (n=6 per group). b, Cytokine secretion by cLP cells from control 129SvEvRag−/− mice following overnight culture with IL-12 or IL-23 (n=6). Data represents mean ± s.e.m. Colitis scores (c), splenomegaly (d), and representative colon photomicrographs (×50) (e) from H. hepaticus infected 129SvEvRag−/− mice treated with blocking α-IL-17 and/or α-IFNγ or isotype (Iso) control mAbs. Data represents two pooled experiments (n=5-12 per group). *P<0.05; **P<0.01; ***P<0.001.
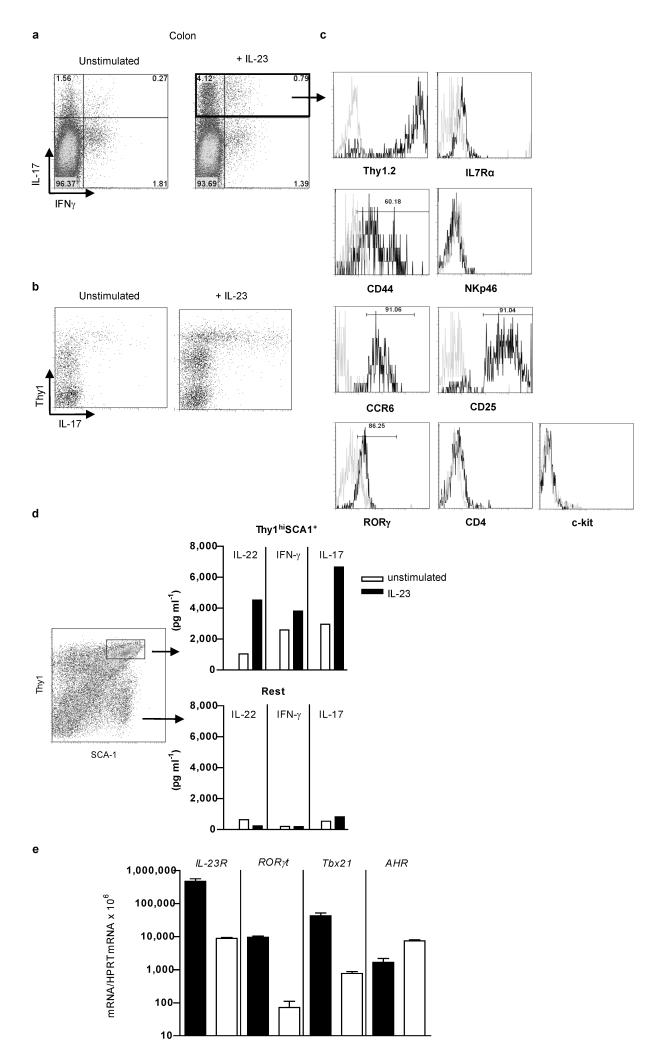
To identify IL-23 responsive innate immune cells present in the inflamed intestine, we used a cell sorting approach. Using leukocyte lineage (Lin) markers CD11b, GR1 and B220, we found that cytokine expressing cells were CD45+Lin− and distinct from common innate cell populations (Supplementary Fig. 3). To identify these cells, we performed intracellular cytokine staining in combination with cell surface marker expression on IL-23 stimulated cLP cells from colitic mice. We found that IL-23 not only enhanced the frequency of IL-17+IFN-γ− cells but also increased the frequency of IL-17+IFN-γ+ cLP cells whereas the frequency of IL-17−IFN-γ+ cells did not increase (Fig. 2a). Analysis of surface markers showed that the vast majority of the cLP IL-17 secreting cells expressed high levels of Thy1 (Fig 2b). Lin−Thy1+ cells in Rag−/− mice include a population of cells required for secondary lymphoid organ (SLOs) organogenesis, termed LTi/LTi-like cells13. Similar to classical LTi-like cells, IL-17 expressing cells were found to be IL-7R+CD44+NKp46−CCR6+CD25+RORγ+ (Fig. 2c). They also expressed LTi related genes such as LTα and β, TRANCE and CXCR5, recently found to be important for the recruitment of LTi-like cells during inflammation (Supplementary Fig 4) (refs 13, 14). However, IL-17- expressing innate lymphoid cells were also phenotypically distinct from LTi-like cells as they were CD4−c-kit− and also expressed SCA-1 (Fig. 2c and d) suggesting heterogeneity amongst Thy1+ innate lymphoid cells in the intestine.
Figure 2.
IL-23-responsive innate lymphoid cells in inflamed colon are Thy1hiSCA-1+ RORγt+.
a,b IL-17, IFN-γ and Thy1 expression in Lin− cLP cells from H. hepaticus–infected 129SvEvRag−/− mice following overnight culture with or without IL-23 c, Phenotypic analysis of Lin−IL-17+ cLP cells from H. hepaticus-infected 129SvEvRag−/− mice, using specific antibodies (black line) and isotype controls (grey line). d, Cytokine secretion and e, IL-23R, RORγt,AHR and Tbx21 mRNA expression, by sorted Thy1hiSCA-1+ or the remaining cLP cells (Rest) from H. hepaticus–infected 129SvEvRag−/− mice following overnight culture with or without IL-23. Results are representative of ≥ 2 independent experiments.
To further characterize Thy1hiSCA-1+ innate lymphoid cells, this population was sorted from the inflamed colon of H. hepaticus infected Rag−/− mice. Even in the absence of cytokine stimulation, the Thy1hiSCA-1+ population produced IL-17, IL-22 and IFN-γ ex vivo, in contrast to the majority of cLP cells (Fig. 2d). In addition, cytokine secretion was enhanced upon stimulation with IL-23 (Fig. 2d). Consistent with their production of Th17 signature cytokines and IFN-γ in response to IL-23 stimulation, Thy1hiSCA-1+ cells expressed higher levels of IL-23R, RORγt and Tbx21 mRNA but not of AHR mRNA (Fig. 2e).
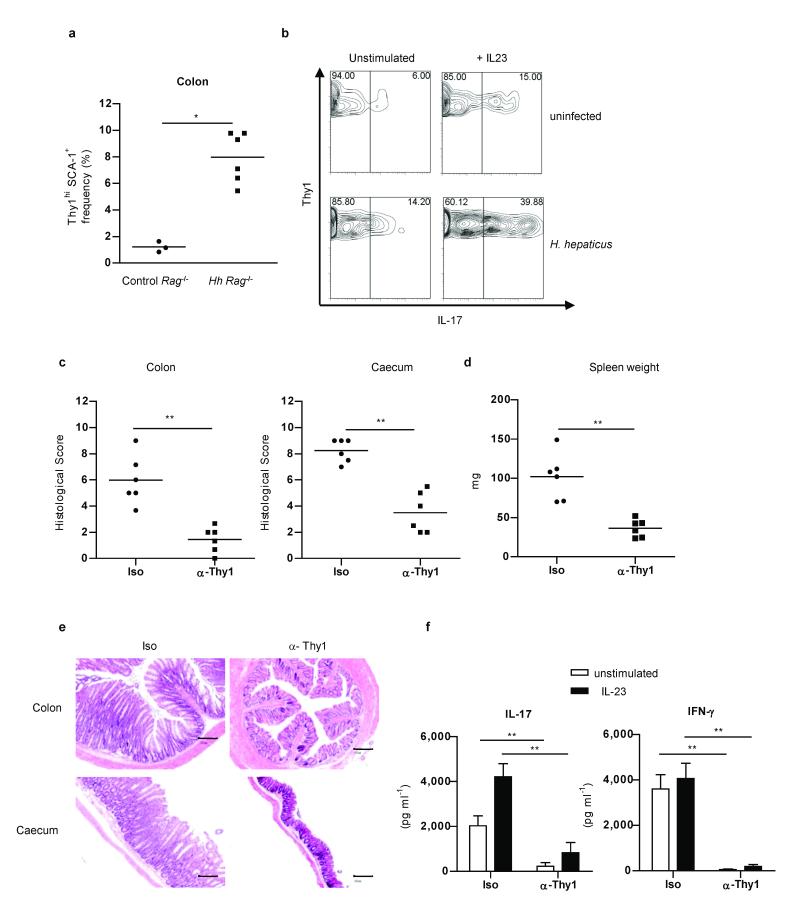
Thy1hiSCA-1+ innate lymphoid cells were present at low frequency in the Rag−/− colon but increased significantly during intestinal inflammation (Fig. 3a). As there is around a 10-fold increase in leukocytes in inflamed colons compared to controls15 this represents about a 100-fold increase in total number of Thy1hiSCA-1+ cells. There was also a marked increase in the proportion of Thy1hiSCA-1+ cells that produced IL-17 de novo from H. hepaticus infected mice and following IL-23 stimulation suggesting not only accumulation, but also activation of this population in the inflamed intestine (Fig 3b). The presence of Thy1hiSCA-1+ innate lymphoid cells was not restricted to the colon, as these cells were also observed in the small intestine (Supplementary Fig. 5a). Furthermore, they were also present in other tissues where H. hepaticus has been shown to mediate immune pathology such as the liver15 where they also responded to IL-23 by secreting IL-22, IFN-γ and IL-17 (Supplementary Fig. 5b). A similar population of Lin−CD3ε−Thy1hi cells was also present in the colon of immunocompetent mice both at steady state and during intestinal inflammation induced by infection with H. hepaticus plus concomitant blockade of IL-10R (ref 16) (Supplementary Fig. 6a). Lin−CD3ε−Thy1+ cells again expressed IL-23R, RORγt, and Tbx21 (Supplementary Figure 6b) and secreted IL-17, IFN-γ and IL-22 in response to IL-23 stimulation (Supplementary Fig. 6c). Immunohistological analyses demonstrated that CD3ε−Thy1+ cells were mainly localized within leucocytic clusters and were often present in close association with CD3+ T cell infiltrates (Supplementary Fig. 7). However, some CD3ε−Thy1+ cells were also observed scattered throughout the lamina propria (Supplementary Fig. 7).
Figure 3.
Thy1hi innate lymphoid cells drive H. hepaticus-induced innate intestinal inflammation.
a, Frequency of Thy1hiSCA-1+ cells (n=3-6) and b, IL-17 expression among Thy1hi cells following culture with or without IL-23 in cLP cells from control or H. hepaticus–infected 129SvEvRag−/− mice. Data are representative of 2 independent experiments. Colitis and typhlitis scores (c), splenomegaly (d), and representative photomicrographs (×50; scale bars 200μm) (e) from H. hepaticus infected mice treated with α-Thy1 or isotype (Iso) control mAbs (n=6 per group). f, IL-17 and IFN-γ secretion by cLP cells from the mice described above, following stimulation with or without IL-23. Data represents mean ± s.e.m. (n=6). *P<0.05; ** P<0.01.
To determine whether Thy1hiSCA-1+ innate lymphoid cells played a functional role in H. hepaticus innate immune driven typhlocolitis, we depleted these cells by injection of a α-Thy1 mAb during the course of infection (Supplementary Fig. 8). Efficient depletion of Thy1+ cells led to abrogation of both colitis and typhlitis (Fig 3c and e). IL-23 dependent systemic immune activation was also ablated as shown by the significantly reduced splenomegaly (Fig. 3d). Absence of intestinal inflammation following depletion of Thy1+SCA-1+ correlated with abrogation of both spontaneous and IL-23-induced IL-17 and IFN-γ production by cLP cells (Fig. 3f).
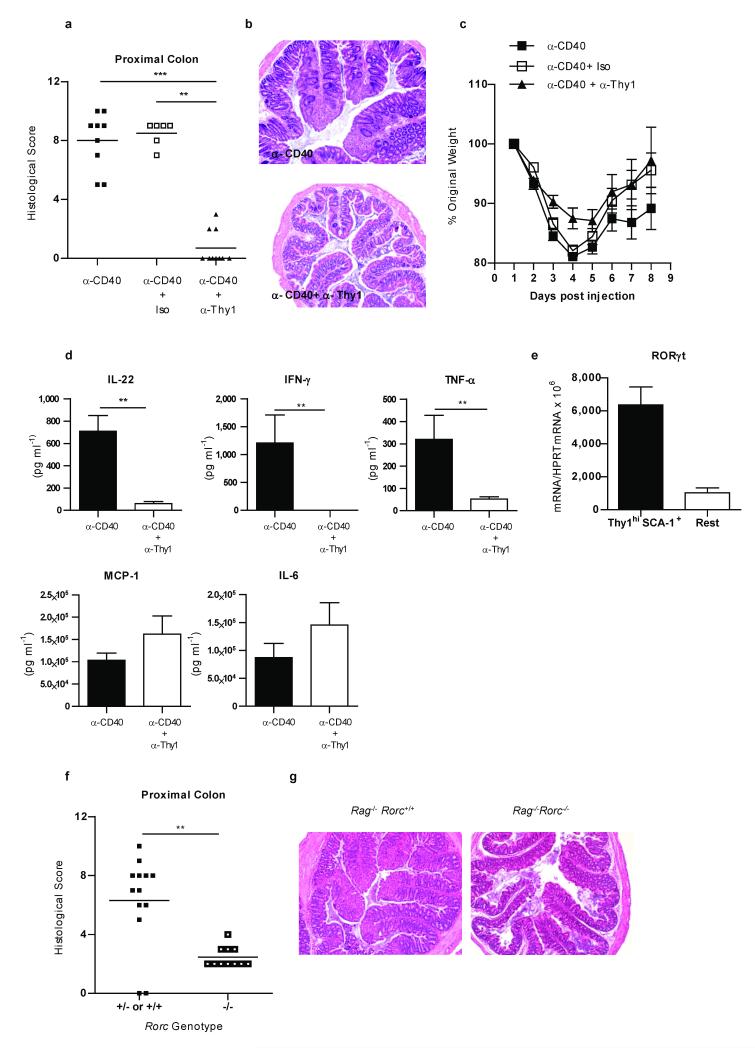
We next assessed the role of Thy1hiSCA-1+ cells in another distinct model of IL-23 driven innate intestinal inflammation. Injection of agonistic α-CD40 mAb into Rag−/− mice induces an IL-23 dependent acute innate immune colitis that is accompanied by an IL-23 independent systemic inflammatory response17. We found that depletion of Thy1+ cells completely abrogated α-CD40 induced innate colitis (Fig. 4a,b), but did not affect the IL-23-independent systemic wasting disease (Fig. 4c). In this acute innate colitis model there was no detectable IL-17 production by Thy1+ cells from the inflamed colon even after IL-23 stimulation (Supplementary Fig. 9a) and IL-17 was not required for colitis development (Supplementary Fig. 9b and c). By contrast, intestinal Thy1hiSCA-1+ cells were found to be the major source of IFN-γ during α-CD40 innate colitis, with IL-23 further enhancing their IFN-γ production (Supplementary Fig. 9d). Furthermore, depletion of Thy1+SCA-1+ cells was associated with a significant decrease in production of IFN-γ, IL-22, TNF-α but not MCP-1 or IL-6 in the colon (Fig. 4d). In this innate model, activation of Thy1hiSCA-1+ by the agonistic α-CD40 mAb appears to be indirect, since no CD40 expression was observed on these cells (Supplementary Fig. 10).
Figure 4.
RORγt-expressing Thy1hi innate lymphoid cells are required for α-CD40-induced innate intestinal inflammation.
Colitis scores (a), colon photomicrographs (×50) (b) weight loss (c) and cLP cytokine secretion following overnight culture (d) in α-CD40 treated C57BL/6 Rag−/− mice injected with or without α-Thy1 or isotype control mAb. e, RORγt mRNA expression by sorted Thy1hi SCA-1+ or the remaining cLP cells (Rest) from α-CD40 treated mice. Results are representative of 2 independent experiments. Colitis scores (f) and photomicrographs (×50) (g) from α-CD40 treated C57BL/6 Rag−/− or C57BL/6 Rag−/−Rorc−/− mice. (a-c) Data represent pooled results from two experiments (n=6-10 ) and (f,g) (n=11-13). (d) Data represents mean ± s.e.m. (n=5).**P<0.01; ***P<0.001.
RORγt is required for development of LTi and IL-22-producing LTi-like cells8,18,19. Indeed, we found high expression of RORγt by Thy1hiSCA-1+ cells isolated from the colons of α-CD40 treated mice (Fig. 4e). As RORγt is important for IL-23R expression7 we next assessed whether it was required for the α-CD40 induced innate colitis. Although treatment of Rag−/−Rorc−/− mice with α-CD40 mAb elicited potent systemic inflammation and wasting disease comparable to that observed in Rag−/− mice (data not shown), Rag−/−Rorc−/− mice developed only mild intestinal inflammation (Fig. 4f and g). Attenuated colitis was associated with reduced production of IL-22, IFN-γ and TNF-α by cLP cells in Rag−/−Rorc−/− mice (Supplementary Fig. 11a). Strikingly, although we found no differences in the intestinal expression of the IL-23p19 subunit between Rag−/− and Rag−/−Rorc−/− mice following α-CD40 treatment, Rag−/−Rorc−/− mice exhibited significantly reduced colonic expression of IL-23R (Supplementary Fig. 11b). Thus, RORγt may be important in the control of IL-23R and inflammatory cytokine expression in innate lymphoid cells.
Together our results identify a novel innate lymphoid cell population that accumulates in the inflamed colon and directly mediates IL-23-dependent acute and chronic innate immune mediated colitis through production of inflammatory cytokines. Strikingly, this innate lymphoid population bears many hallmarks of the colitogenic T cell response including expression of RORγt and production of IL-17 and IFN-γ. Notably, IL-17 production by innate lymphoid cells was a feature of chronic bacteria-induced colitis but not acute α-CD40 disease suggesting that the tissue IL-23 driven innate lymphoid response exhibits flexibility of function and is shaped by environmental factors. The remarkable conservation in function between IL-23 driven αβ and γδ T cell responses20,21 and IL-23 driven innate lymphoid responses indicates that Th17-associated effector functions can be generated in the complete absence of TCR-mediated signals. Indeed, IL-17 and IFN-γ producing innate lymphoid cells may represent a primitive tissue inflammatory response that is activated in response to microbe induced cytokines such as IL-23 (Supplementary Fig. 12). The tissue innate lymphoid response may extend beyond IL-23 driven responses as non-B/non-T Lin− and Lin− c-kit+SCA-1+ cells have been shown to be a source of Th2-type cytokines in response to IL-25 and IL-33 respectively in parasitic helminth infection models22,23. Intestinal inflammatory innate lymphoid cells share some features of Rorc-dependent LTi cells that mediate organogenesis in the fetus as well as adult LTi-like cells that produce IL-22 or IL-17 in response to IL-23 or zymosan challenge2. Whether these represent distinct tissue innate lymphoid populations or different functional states of the same population is not known. It is notable that hyperplastic lymphoid aggregates have been observed in the colon of IBD patients24,25. Furthermore we have found a similar IL-23 responsive IL-17 producing ILC population in the inflamed intestine of IBD patients (unpublished data). Intestinal innate lymphoid populations may therefore contribute to chronic intestinal inflammation through effects on organogenesis as well as inflammatory cytokine production. Further characterisation of IL-23 responsive ILC in the human intestine is required to establish a role in the pathogenesis of IBD.
METHOD SUMMARY
Isolation of murine cells
LP cells were purified as described26. In brief, colon tissue was cut into 0.5 cm pieces and incubated in RPMI containing 10% heat-inactivated FCS (GIBCO-BRL) and 5 mM EDTA to remove epithelial cells. The remaining tissue was further digested with complete RPMI media containing 15mM HEPES and 100U/ml of Collagenase VIII (100 U/ml; Sigma Chemical Co.). For liver cell isolation, livers were perfused at a rate of 2–3 ml/min, first with PBS then with PBS containing 100U/ml of type VIII collagenase. Livers were then cut in small pieces and digested for and extra hour at 37°C with complete RPMI media containing 15mM HEPES and 100U/ml of Collagenase VIII. Liver cells and LP cells were then layered on a 30%/40%/75% Percoll gradient (Amersham Pharmacia Biotech). Cells were recovered after centrifugation (600 × g, 20 min) at the 40%/75% Percoll interface. Cells were stained with a combination of α-CD45.2, α-CD11b, α-Ly6C/G (GR1), α-B220, α-Thy1.2, α-Ly6A/E (SCA-1) and CD3ε antibodies (all from BD Pharmingen) and sorted on a Moflo sorter (DakoCytomation).
Reagents
Cytokine detection in culture supernatants were quantified using Flow Cytomix Cytokine Bead Assay from Bender MedSystems. Recombinant mouse IL-12 was obtained by transfecting COS7 with the cDNA encoding the p35 and p40 as described previously27. Recombinant mouse IL-23 was purchased from R&D. Both recombinant cytokines were used at 10 ng/ml for overnight (ON) stimulation of cells. α-IFN-γ and α-IL-17 antibodies for intracellular staining were obtained from BD Pharmingen, while α-RORγ antibody came from eBiosciences. Surface antibodies CD90.2, CD3ε, CD45.2, CD11b, Ly6C/G, B220, Ly6A/E (SCA-1), CCR6, CD25, CD44, CD117 (c-kit), CD11c and CD4 were purchased from BD Pharmingen. Antibodies against NKp46 and CD127 (IL7Rα) were obtained from R&D and eBiosciences respectively.
Supplementary Material
Acknowledgements
We thank N.Rust for cell sorting; R.Stillion and M. Ziegler for histology; J.Langhorne for providing the AN18 hybridoma and S.Cobbold and H.Waldmann for the generous gift of the depleting α-Thy1 and isotype control antibodies; UCB Celltech for providing the blocking α-IL-17 and isotype control antibodies; O. Boulard and S. Kirchberger for technical assistance and C. Arancibia for critically reading the manuscript. This work was supported by the Wellcome Trust (F.P and K.J.M), the Marie-Curie Network fellowship (IMDEMI, MRTN-CT2004-006532) (S.B, P.A), Philippe Wiener-Maurice Anspach foundation (S.B), European Crohn’s & Colitis Organisation (ECCO) (S.B), the European Commission research program INFLA-CARE (EC contract number 223151) (S.B) and a Wellcome Trust and Howard Hughes Medical Institute Exchange Programme grant (P.A, F.P and D.R.L).
Footnotes
Supplementary information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Takatori H, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bending D, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 8.Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 9.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh-Takayama N, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Awasthi A, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hue S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finke D. Fate and function of lymphoid tissue inducer cells. Curr Opin Immunol. 2005;17:144–150. doi: 10.1016/j.coi.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Marchesi F, et al. CXCL13 expression in the gut promotes accumulation of IL-22-producing lymphoid tissue-inducer cells, and formation of isolated lymphoid follicles. Mucosal Immunol. 2009 doi: 10.1038/mi.2009.113. [DOI] [PubMed] [Google Scholar]
- 15.Maloy KJ, et al. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kullberg MC, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlig HH, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Sanos SL, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luci C, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 20.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2009 doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 24.Kaiserling E. Newly-formed lymph nodes in the submucosa in chronic inflammatory bowel disease. Lymphology. 2001;34:22–29. [PubMed] [Google Scholar]
- 25.Yeung MM, et al. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-gammadelta expression. Gut. 2000;47:215–227. doi: 10.1136/gut.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powrie F, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 27.Schoenhaut DS, et al. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.