Abstract
Compared to Caucasians, African Americans have lower circulating concentrations of 25-hydroxyvitamin D (25(OH)D), the major storage form of vitamin D, leading to the widespread assumption that African Americans are at higher risk of vitamin D deficiency. However, the finding that African Americans maintain better indices of musculoskeletal health than Caucasians throughout their lifespan despite having lower circulating 25(OH)D concentrations suggests that the relationship between vitamin D deficiency and racial health disparities may not be so straight forward. The fairly recent emergence of fibroblast growth factor 23 (FGF23) may help resolve some of this uncertainty. FGF23 strongly modulates both systemic and local activation of 25(OH)D, playing a potentially important role in the degree to which lower 25(OH)D concentrations impact health outcomes, including differences in the incidence and rate of progression of chronic kidney disease by race. The focus of this review will be to critically assess ongoing controversies surrounding the relationship between vitamin D and racial disparities in chronic kidney disease outcomes, and how FGF23 may help to clarify the picture.
Keywords: vitamin D, FGF23, disparities
INTRODUCTION
It is well established that African Americans have lower circulating 25-hydroxyvitamin D (25(OH)D) concentrations than Caucasians.1–5 The primary reason for this is that melanin efficiently absorbs ultraviolet B light from sun exposure, inhibiting cutaneous synthesis of cholecalciferol (vitamin D3), the metabolic precursor to 25(OH)D.6 On the basis of these findings, African Americans have long been assumed to be at increased risk of vitamin D deficiency, defined by most experts as a circulating 25(OH)D concentration < 20 ng/ml.7 If this assumption is taken at face value, the prevalence of vitamin D deficiency among African Americans could only be described as staggering. Work from our group showed that greater than 80% of African American participants of the 2003–2004 and 2005–2006 National Health and Nutrition Examination Surveys (NHANES) had 25(OH)D concentrations < 20 ng/ml (compared to only 28% of Caucasians), incluing 30% who had 25(OH)D levels < 10 ng/ml (generally defined as severe vitamin D deficiency).8 Prevalence rates of vitamin D deficiency in other populations with high skin pigmentation such as Hispanics and Asian Indians are also higher than their Caucasian counterparts,8, 9 underscoring the disruptive effect of melanin in the cutaneous synthesis of vitamin D. In light of the classical effects of vitamin D on bone and mineral health, not to mention its non-classical effects on cardiovascular function, insulin signaling and immunity, these data support the notion that vitamin D deficiency is at epidemic levels among African Americans and should be a prime target of therapy for improving health disparities, such as the excess risk of end-stage renal disease (ESRD) among African Americans.
However, there is a lingering paradox in the relationship between vitamin D and health outcomes in African Americans that should prompt re-assessment of these conclusions. Despite having lower 25(OH)D concentrations and lower dietary calcium intake than Caucasians, African Americans have more favorable indices of bone structure and function than Caucasians.10–12 Similarly, despite a wealth of data showing positive associations of serum 25(OH)D with muscle mass and strength,13–17 African Americans have far better indices of muscle health than Caucasians across the life span.18 If nothing else, these data strongly suggest that the musculoskeletal consequences of low 25(OH)D are less severe in African Americans than in Caucasians, with important implications for the concept of vitamin D deficiency in multiracial populations. Perhaps more importantly, these data question whether it makes sense to link lower 25(OH)D concentrations (relative to Caucasians) with excess risk of non-musculoskeletal disease (e.g., cardiovascular and kidney disease) in African Americans when no such association has been observed with respect to musculoskeletal disease. In considering this question, it is important to recall that 25(OH)D does not exist in a vacuum—indeed, the relationship between vitamin D and the numerous physiological pathways it interacts with is modified by a host of related factors such as parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23). FGF23 in particular has been shown to regulate the activation of vitamin D in a variety of tissues, modulating both the local and systemic effects of vitamin D. Given that FGF23 plays such a central role in vitamin D metabolism, FGF23 may fundamentally impact the relationship between vitamin D and health outcomes, which in turn, may force us to revise our approach to investigating vitamin D and racial disparities in chronic kidney disease (CKD).
Vitamin D and health in African Americans: is the picture so clear?
Numerous excellent reviews (including those by Essien, Goel and Melamed and by Li in this issue of Seminars in Nephrology) have documented the associations of low circulating 25(OH)D concentrations with cardiovascular and kidney disease, insulin resistance and impaired immune function. On the basis of these data, investigators have hypothesized that lower 25(OH)D concentrations may contribute to a variety of racial health disparities, such as the higher risk of ESRD among African Americans. While the breadth of these data are compelling, in evaluating the strengths of these claims it is instructive to consider racial differences in the association of vitamin D with the physiological system(s) that it is most strongly linked with, namely bone and mineral metabolism. The primary role of vitamin D is to maintain calcium homeostasis (and by extension skeletal health).7 Indeed, vitamin D deficiency as an entity was first identified as one of the primary etiological agents underlying rickets in children and osteomalacia in adults.19 Studies have since documented that the efficiency of calcium absorption from the intestinal lumen is exquisitely sensitive to vitamin D and that in the absence of appropriate amounts of circulating 25(OH)D concentrations, intestinal calcium absorption is markedly impaired.20 When low 25(OH)D is coupled with low dietary calcium intake, this presents the perfect scenario for deficient calcium incorporation into the bone and with it, diminished bone mineralization. Given that African Americans on average have both lower circulating 25(OH)D concentrations and lower dietary calcium intake than Caucasians,8 this would presumably put them at higher risk for bone disease. However, with the notable exception of the higher prevalence of rickets in black as compared to white children,21 quite the opposite has been observed in large, population-based studies.
The vast majority of these studies in fact have shown that African Americans maintain higher bone mineral density (BMD) than Caucasians in both the appendicular and axial skeleton starting in adolescence and continuing throughout adulthood.8, 10–12, 22–30 It has been argued that this is because African Americans compensate for lower 25(OH)D by increasing the secretion of PTH, the hormone required to convert 25(OH)D to its activated metabolite, 1,25-dihydroxyvitamin D (1,25(OH)2D).31, 32 While this helps to maintain calcium homeostasis in the short-term, so the argument goes, this compensation becomes maladaptive in the long-term because it comes at the cost of chronically higher PTH concentrations, substantiating the belief that having lower 25(OH)D concentrations (relative to Caucasians) is pathologic in African Americans. It is certainly true that African Americans have higher average PTH concentrations than Caucasians and that they tend to have higher 1,25(OH)2D concentrations as a result.33–36 However, if anything, one would assume that higher PTH and 1,25(OH)2D concentrations would help to keep bone mass similar or only slightly lower, not higher, in African Americans than Caucasians if it were solely a maladaptive compensation for lower 25(OH)D concentrations. Furthermore, differences in BMD by race have been observed even in the absence of differences in PTH concentrations.30 Finally, it is unclear that higher average PTH concentrations are necessarily harmful in African Americans. For example, studies have shown that African Americans manifest skeletal resistance to the bone-resorbing effects of PTH,37, 38 suggesting that higher PTH levels may not adversely affect bone strength in African Americans, at least in comparison to Caucasians.39 Consistent with this, African Americans have a lower risk of skeletal fractures than Caucasians despite their higher PTH concentrations. For example, in a prospective study of black and white women from four US centers participating in the Study of Osteoporosis Fractures, the incidence rate of non-spinal fracture was significantly higher in white women as compared to black women irrespective of baseline bone mineral content or density.40 Further, the relative risk of fracture remained substantially lower in black compared to white women after adjustment for potential confounders (0.43, 95% confidence interval 0.32– 0.57), in line with the findings of other studies.41–45
A number of other explanations have been offered for the paradoxical association of higher BMD in African Americans as compared to Caucasians. These include more efficient dietary calcium utilization, better renal calcium conservation (perhaps due to higher basal PTH concentrations), and lower bone turnover in African Americans than Caucasians.18, 46 It may be hypothesized that these differences evolved in Africans, or perhaps more precisely individuals with high skin pigmentation, to compensate for their lower 25(OH)D concentrations. However, given that humans originated in Africa and subsequently migrated to areas of the world with much less direct ultraviolet B exposure, it is likely more accurate to say that Caucasians evolved mechanisms to compensate for their less efficient calcium economy, including higher requirements for 25(OH)D concentrations to maintain bone health and with it, lower skin pigmentation.47 Whatever the exact sequence of events, it seems reasonable to conclude that requirements for 25(OH)D may be lower in African Americans than Caucasians, at least with respect to optimizing skeletal health.
In support of this, a number of studies have shown that the relationship between 25(OH)D and bone parameters differs by race. Analyzing NHANES data, Gutiérrez et al. showed that whereas whole-body BMD as measured by dual-energy X-ray absorptiometry (DXA) declined with decreasing serum concentrations of 25(OH)D among white and Mexican American participants, no such relationship was observed among African American participants.8 In addition, there were significant racial differences in the association of 25(OH)D with PTH—whereas serum PTH concentrations continuously declined as 25(OH)D concentrations increased in whites and Mexican Americans (consistent with the negative feedback loop between these hormones), PTH concentrations were maximally suppressed at a 25(OH)D concentration of ∼20 ng/ml among African Americans, above which the slope of the relationship between PTH and 25(OH)D was essentially flat. Similarly, an analysis of black and white participants of the Osteoporotic Fractures in Men (MrOs) study showed that whereas serum 25(OH)D concentrations were linearly associated with indices of bone mineral density and strength in white men, no such findings were observed in black men.48 Other studies have shown similar findings (Table 1).
Table 1.
List of studies showing differences in bone and mineral parameters by race.
| Name of Study |
Objective | Imaging technique |
Results of Study | Comments |
|---|---|---|---|---|
| Pollock et al., 201173 | Compare trabecular and cortical bone parameters at the radius and tibia between adolescent white and black females |
Peripheral quantitative computed tomography (QCT) |
Adolescent black females have a stronger bone profile at the tibia compared to their white counterparts. |
Bone parameters were similar at the radius |
| Leonard et al., 201074 | Effects of sex, puberty, and race on cortical bone development |
QCT | Cortical bone parameters were greater in blacks than whites in Tanner stage 1–4 |
|
| Barbour et al., 201148 | Association of Serum 25 hydroxyvitamin D with bone parameters in Caucasian and African men |
QCT | Positive linear trend noted between increasing 25 hydroxyvitamin D and bone parameters in Caucasian but not in men of African ancestry |
In men of African ancestry there was threshold effect for serum 25 hydroxyvitamin D at approximately 18 ng/ml on cortical thickness in distal tibia. |
| Gutierrez et al., 20118 | Racial differences in bone mineral density, parathyroid hormone (PTH) and vitamin D |
Dual energy X- ray Absorptiometry (Dxa) |
Bone mineral density decreased with decline in 25 hydroxyvitamin D in Caucasians and Mexican Americans but not in African Americans |
Data from study suggests that PTH secretion is suppressed at a lower vitamin D level in African Americans than in Caucasians. |
| Hannan et al., 200875 |
Relation between Serum 25 hydroxyvitamin D and bone mineral density by race and ethnic group amongst men |
DXA | Positive correlation noted between 25 hydroxyvitamin D and bone mineral density in white men but not in black or Hispanic men |
|
| Cauley et al., 201149 | Studying association of 25 hydroxyvitamin D to risk of fracture in multiethnic women |
Not applicable | Low 25 hydroxyvitamin D levels were associated with higher risk of fracture in white women but not in black women |
Study implicated that the ideal level of 25 hydroxyvitamin D for skeletal health may be different for white and black women. |
| Wetzsteon et al, 200976 | Ethnic differences in bone parameters in childhood |
QCT | African American and Hispanic children have greater bone strength than white children |
Study suggested that ethnic differences in bone strength emerge in childhood |
| Peacock et al., 200977 | Racial and sexual differences of bone parameters at the femur |
CT, DXA | American blacks have higher bone density than American whites at the femur |
|
| Chen et al, 201178 | Relationship between African admixture and hip geometry amongst post-menopausal women |
DXA | Greater African admixture is correlated with better bone density and hip structure in black than white post-menopausal women |
Even when considering fracture outcomes instead of surrogate measures of bone health such as DXA scans, the story remains much the same. Cauley and colleagues examined the association of 25(OH)D concentrations with fracture risk in participants of the Women’s Health Initiative Observational Study.49 Among white women in this study, 25(OH)D concentrations in the highest tertile of 25(OH)D (≥ 30 ng/mL) were associated with 44% lower risk of fracture as compared to the lowest tertile of 25(OH)D (< 20 ng/mL) in multivariable models adjusted for clinical factors, physical activity, calcium intake, previous history of fracture and PTH. In contrast, the highest tertile of 25(OH)D was associated with higher risk of fracture as compared to the lowest tertile of 25(OH)D among black women in both unadjusted and fully-adjusted models. Perhaps most compelling are the results of a large randomized, controlled study of the effects of vitamin D supplementation on bone health and fracture outcomes in post-menopausal African American women.50 In this study, there were no differences in the rate of decline in BMD after three years of supplementation with cholecalciferol in treated compared to control patients, in contrast to what has been observed in studies of white women.51, 52
In the aggregate, the results of these studies suggest that optimal levels of 25(OH)D may not be the same in white individuals as compared to black individuals, at least with respect to bone health. Indeed, it is entirely reasonable to conclude from these data that individuals of African descent require lower 25(OH)D concentrations to optimize bone and mineral metabolism as compared to their counterparts of European descent. This in turn begs the question of whether the same is true with respect to the relationships between 25(OH)D and non-skeletal outcomes such as cardiovascular health, glucose metabolism, immune function and, of greatest relevance to this issue of Seminars, kidney disease in African Americans. Though supporting evidence is scarce, important clues may be gleaned from available studies. In a study of 2,766 non-Hispanic white, 1,736 non-Hispanic black and 1,726 Mexican American participants of NHANES, higher serum concentrations of 25(OH)D were associated with lower odds of diabetes in non-Hispanic whites and Mexican Americans.53 In contrast, no associations were noted between serum concentrations of 25(OH)D and odds of diabetes among African Americans overall—in fact, in some models, higher 25(OH)D was associated with higher odds of diabetes, though these results should be interpreted cautiously because of the low sample size of African Americans in higher categories of 25(OH)D. Similarly, despite the observation that 25(OH)D is inversely associated with calcified plaque in population-based studies of European Americans,54, 55 a study showed that 25(OH)D was positively associated with calcified plaque in African Americans with type 2 diabetes, suggesting that higher 25(OH)D may have adverse instead of beneficial effects on calcified atherosclerotic lesions in African Americans.56
The results of these “negative” studies should be interpreted in the context of other studies that showed that lower 25(OH)D concentrations in African Americans at least partially explained racial disparities in hypertension, albuminuria and ESRD prevalence (reviewed in the manuscript by Essien, Goel and Melamed), supporting the potential mediating role of low vitamin D in disparities in CKD outcomes by race. Nevertheless, while it appears biologically plausible that lower 25(OH)D concentrations (relative to Caucasians) are “bad” for African Americans and should be treated (appealing to the very strong sense of wanting to “treat” something among health professionals), it remains very much unclear whether this is truly the case. Moreover, even if it were, it is entirely unclear at what level of 25(OH)D the excess risk for chronic disease among African Americans begins to manifest. To state it a different way, while African Americans can and do become vitamin D deficient at some critical level of 25(OH)D, whether that level is the same as for Caucasians remains an open question. As the design of studies meant to test the efficacy of vitamin D supplementation in African Americans with CKD continue to move forward, these are some of the most important questions to resolve prior to study initiation.
Fibroblast Growth Factor 23—how might it impact vitamin D and racial disparities in CKD?
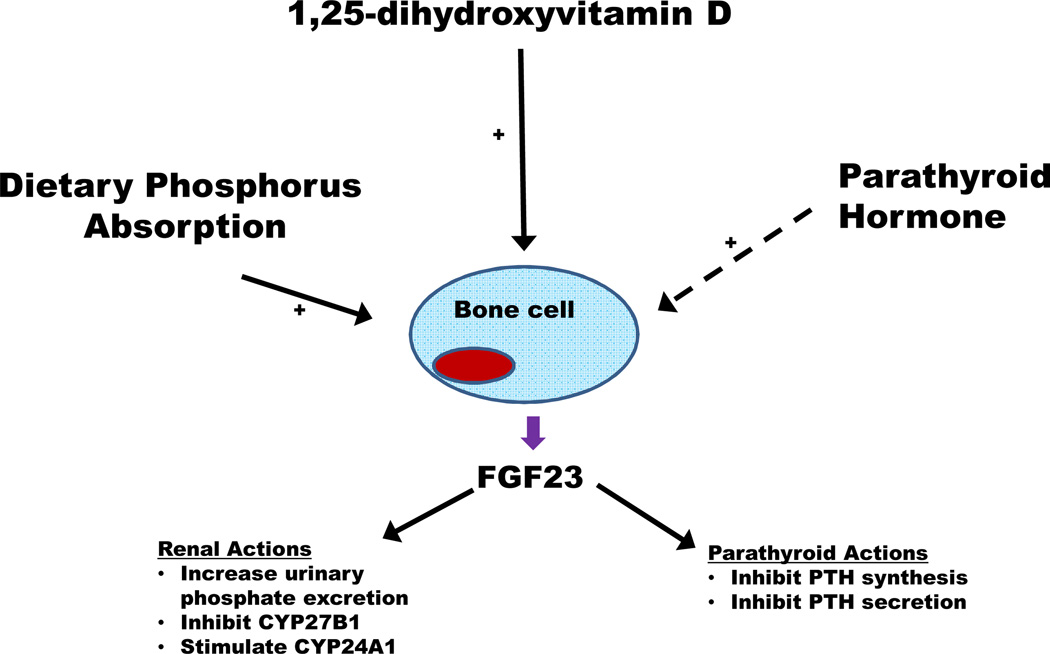
Unfortunately, vitamin D does not operate within a vacuum, adding to the complexity of the relationship between vitamin D and racial disparities in CKD. Among the most important factors that likely modulate this relationship is FGF23. Primarily synthesized and secreted by bone cells, FGF23 regulates phosphorus metabolism through actions in both the kidneys and parathyroid glands (Figure 1).57 In the kidneys, FGF23 stimulates urinary phosphate excretion by inducing the endocytosis of sodium-phosphate co-transporters from the apical membrane of renal proximal tubular cells.58 FGF23 also regulates systemic phosphorus homeostasis via modulation of vitamin D metabolism through two pathways.59, 60 First, FGF23 inhibits the synthesis of CYP27B1, the enzyme required to convert 25(OH)D to 1,25(OH)2D. In addition, FGF23 up-regulates the activity of CYP24A1, the enzyme that represents the major catabolic pathway for both 25(OH)D and 1,25(OH)2D. The importance of FGF23 in the regulation of vitamin D was highlighted by experimental data showing that elevations in serum FGF23 concentrations early in CKD decreased systemic 1,25(OH)2D concentrations,61, 62 even in the setting of elevated PTH concentrations, indicating that excess FGF23 can impose a powerful physiological block on 1,25(OH)2D synthesis in the kidney.
Figure 1. Broad overview of fibroblast growth factor 23 (FGF23).
Bone cells are the primary cells that synthesize and secrete FGF23. There are a number of systemic stimuli of FGF23 secretion—increased dietary phosphorus absorption and increased 1,25-dihydroxyvitamin D concentrations. The effects of parathyroid hormone on FGF23 are more controversial, with some studies suggesting that parathyroid hormone directly stimulates FGF23 while others do not. FGF23 acts primarily in the kidneys and parathyroid glands. In the kidneys, FGF23 augments urinary phosphate excretion and modulates vitamin D metabolism by inhibiting the synthesis of CYP27B1 and up-regulating CYP24A1, both of which serve to decrease circulating 1,25-dihydroxyvitamin D concentrations. In the parathyroid glands, FGF23 inhibits both the synthesis and secretion of parathyroid hormone.
FGF23-induced inhibition of 1,25(OH)2D synthesis has been identified as a key factor underlying the initiation and progression of secondary hyperparathyroidism in individuals with CKD.63 Since activated vitamin D analogs are commonly used to treat secondary hyperparathyroidism in CKD, this likely impacts racial differences in CKD outcomes in at least one way. Studies have shown that African Americans with ESRD are more likely to be treated with activated vitamin D analogs than Caucasians with ESRD because African Americans have higher average PTH concentrations than Caucasians, particularly at the initiation of dialysis.64, 65 Greater exposure to activated vitamin D analogs, in turn, was shown to partially explain the higher survival rate of African Americans as compared to Caucasians on dialysis.64, 65 Thus, to the extent to which higher FGF23 exacerbates secondary hyperparathyroidism in CKD, this may have an appreciable impact on the utilization of vitamin D analogs in African Americans as compared to Caucasians and by extension, the link between vitamin D and racial differences in survival in ESRD.
The actions of FGF23 on CYP27B1 activity in peripheral tissues constitute another way in which FGF23 may impact vitamin D-related racial differences in CKD outcomes. CYP27B1 is expressed in extra-renal sites such as pancreatic islet cells, neural cells, vascular endothelial cells, cardiomyocytes, and cells involved in innate immunity.66 Importantly, like in the kidney, FGF23 can inhibit extra-renal CYP27B1 synthesis, blocking activation of vitamin D required for autocrine/paracrine actions. As an example, in a recent study by Bacchetta and colleagues, FGF23 strongly inhibited the expression of CYP27B1 in peripheral blood mononuclear cells and peritoneal dialysate monocytes, resulting in lower intracellular conversion of 25(OH)D to 1,25(OH)2D.67 Though similar effects have yet to be demonstrated in other extra-renal sites, there is no reason to believe a priori that FGF23 would not be able to suppress extra-renal CYP27B1 activity in tissues such as vascular endothelial cells, which have been shown to express both FGF receptors and Klotho,68 the co-receptor needed for FGF23 to bind to FGF receptors with high affinity. The potential importance of FGF23 inhibition of extra-renal CYP27B1 in CKD is strongly supported by the finding that the autocrine/paracrine actions of 1,25(OH)2D synthesized in macrophage foam cells may play an important role in limiting atherogenesis and maintaining proper vascular integrity (as thoroughly reviewed by Li in this issue of Seminars). Thus, in addition to reducing systemic 1,25(OH)2D concentrations, FGF23 may block local actions of 1,25(OH)2D in key peripheral tissues involved in maintaining kidney health. Given that FGF23 concentrations were shown to be higher in black as compared to white individuals with CKD,69 this may play a key role in mediating vitamin D-related disparities in CKD outcomes by race.
Importantly, a distinctive difference in the activity of CYP27B1 in extra-renal sites is that it is much less tightly regulated than renal CYP27B1.70 Thus, whereas circulating 1,25(OH)2D concentrations do not change substantially in response to changes in circulating 25(OH)D concentrations (within the normal physiological range of 25(OH)D), extra-renal production of 1,25(OH)2D is exquisitely sensitive to changes in the availability of 25(OH)D substrate, making 25(OH)D deficiency a more pressing dilemma for local (extra-renal) 1,25(OH)2D production and utilization than for systemic 1,25(OH)2D activity. The importance of this was demonstrated in a now classic study showing the integral role of 25(OH)D in the anti-microbial actions of macrophages.71 In this study, activation of toll-like receptors up-regulated the expression of vitamin D receptors and CYP27B1 in macrophages, allowing for increased intracellular synthesis of 1,25(OH)2D and subsequent induction of the anti-microbial peptide cathelicidin. Importantly, in the absence of sufficient 25(OH)D substrate to allow for intracellular synthesis of 1,25(OH)2D, this pathway was largely shut down, resulting in ineffectual production of cathelicidin in response to toll-like receptor activation and decreased effectiveness of macrophages in destroying invading pathogens.
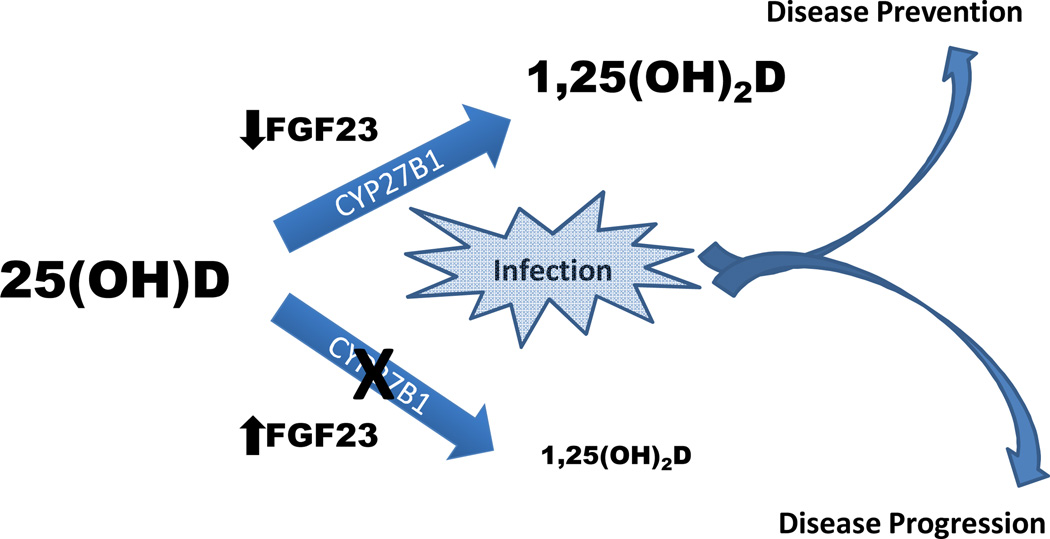
This observation—namely, that synthesis of 1,25(OH)2D (and by extension the production of cathelicidin) in macrophages is strongly dependent on adequate 25(OH)D substrate—has been proposed to explain the higher risk and severity of tuberculosis infection among African Americans,71 supporting the notion that lower 25(OH)D concentrations in African Americans (relative to Caucasians) are inimical to maintaining optimal health. Given the role of FGF23 in the expression of CYP27B1 in peripheral tissues, however, the impact of vitamin D deficiency on health outcomes such as infection risk may also heavily depend on concurrent circulating concentrations of FGF23. Indeed, excess FGF23 may potentiate the adverse effects of low 25(OH)D concentrations by accelerating 25(OH)D catabolism (via stimulation of CYP24A1) and perhaps more importantly, by blocking intracellular conversion of 25(OH)D to 1,25(OH)2D. In this conceptual framework, the adverse effects of low 25(OH)D concentrations would synergize with those of elevated FGF23 (Figure 2), making it vital to examine both hormones in tandem when assessing the association of vitamin D with adverse outcomes. In support of this, the study by Bacchetta and colleagues referenced above showed that FGF23-induced inhibition of CYP27B1 in mononuclear cells resulted in the down-stream inhibition of cathelicidin synthesis, even in the setting of adequate 25(OH)D substrate.67 Further, a large, population-based study of Japanese patients with pre-dialysis CKD showed that having both low 25(OH)D and high FGF23 concentrations was associated with substantially higher risk of all-cause mortality than having either one alone.72 Thus, in addition to a lack of adequate 25(OH)D substrate, a secondary or complementary mechanism by which vitamin D-related health can be hampered is via excess circulating concentrations of FGF23. Whether this is also the case in the setting of adequate circulating 25(OH)D stores (≥ 30 ng/ml) is unclear and merits further study. If so, it would suggest that the physiological effects of 25(OH)D on cardiovascular and renal health depend as much on the concomitant level of FGF23 as it does on the actual level of 25(OH)D itself.
Figure 2. Paradigm for how fibroblast growth factor 23 (FGF23) may impact vitamin D-related health outcomes.
25-hydroxyvitamin D (25(OH)D) requires activation by CYP27B1 to 1,25-dihydroxyvitamin D (1,25(OH)2D) for vitamin D to exert is full physiological effects. In settings in which this activation may need to be up-regulated (for example infection), excess circulating concentrations of FGF23 may modulate this system by inhibiting central (renal) and peripheral (extra-renal) synthesis of 1,25(OH)2D, reducing the efficacy of vitamin D in both systemic and autocrine/paracrine actions, and thereby helping to promote disease progression. When FGF23 concentrations are normal to low, this may service as a permissive factor in allowing vitamin D to be activated and thus, to play a role in disease prevention, such as in fighting infection as depicted in the figure.
When taken together, the complex interactions between 25(OH)D and FGF23 reviewed above may force us to revise our understanding of vitamin D deficiency and its role in racial disparities in health outcomes in general and CKD outcomes in particular. This is because while lower 25(OH)D concentrations (relative to Caucasians) do not seem to have a negative effect on musculoskeletal outcomes among African Americans in health, it is possible that low 25(OH)D may hamper normal physiological responses to disease, especially in the setting of excess concentrations of FGF23. Indeed, when confronted with the need to rapidly increase 25(OH)D activation in response to new or ongoing physiological threats (e.g., infection, kidney injury, endothelial damage), African Americans may be at a relative disadvantage due to having lower 25(OH)D substrate to support autocrine/paracrine actions of vitamin D in peripheral tissues. The ability of the vitamin D system to respond to emerging physiological needs may also depend heavily on concurrent circulating FGF23 concentrations, particularly in individuals with CKD who have constitutively elevated FGF23 concentrations. As such, while low average 25(OH)D concentrations may not be pathologic for African Americans per se (at least with respect to musculoskeletal health), when confronted by new environmental pressures unleashed by the African diaspora such as increased salt intake, excess calorie consumption, and exposure to new infectious agents, low average 25(OH)D concentrations and high FGF23 concentrations may act together to impede physiological responses meant to adapt to these changes, especially in the setting of CKD.
The interactions between 25(OH)D and FGF23 also raise several intriguing questions about how to define vitamin D deficiency, not only in African Americans but other populations as well. For example, if the impact of 25(OH)D concentrations on vitamin D-related health is fundamentally modulated by FGF23, is it possible to assess the potential health consequences of lower 25(OH)D concentrations without knowing anything about concurrent circulating FGF23? Put more provocatively, can we even establish whether someone is truly vitamin D deficient without knowing their blood FGF23 concentration (much like we generally need to assess his or her blood PTH concentration to determine the necessity of treating low 25(OH)D)? By extension, do elevated FGF23 concentrations in CKD have a disproportionately deleterious effect in African Americans since it exacerbates their already low average 25(OH)D concentrations in the face of myriad physiological threats such as changes in nutrition, insulin sensitivity, and blood pressure control related to kidney injury? As the role of vitamin D in racial disparities continues to be studied, placing 25(OH)D within the broader context of factors that mediate mineral metabolism will be crucial to answering these questions.
Conclusions
Vitamin D deficiency has captured a great deal of attention in the ongoing quest to understand well-known but poorly understood discrepancies in ESRD risk by race. The basis for this enthusiasm is well-founded in plausible biological pathways linking lower 25(OH)D concentrations with cardiovascular and renal pathology. However, the lingering paradox between lower 25(OH)D concentrations (relative to Caucasians) in African Americans and musculoskeletal health should provide a measure of caution in rushing to the conclusion that low average 25(OH)D concentrations are truly detrimental to the overall health of African Americans, especially with respect to CKD outcomes. Importantly, determining the answer to this issue needs to done in the broader context of other mediators of vitamin D metabolism such as FGF23, which appears to have important effects on the activity of vitamin D both systemically and also within extra-renal sites (Table 2). Given potential racial differences in FGF23 in CKD, future investigations of racial disparities in vitamin D will need to take into account the potential complementary or mediating role of excess FGF23 concentrations in CKD and ultimately determine whether a focus on FGF23 excess needs to be added to that of vitamin D deficiency.
Table 2.
Proposed pathways by which fibroblast growth factor 23 (FGF23) may modulate the relationship between vitamin D and health disparities by race
| Mechanisms | Comments |
|---|---|
| FGF23-induced decreases in systemic 1,25- dihydroxyvitamin D concentrations |
Low 1,25-dihydroxyvitamin D concentrations play an important role in the initiation and progression of secondary hyperparathyroidism; since parathyroid hormone concentrations largely determine the use of activated vitamin D analogs, this may impact the use of vitamin D analogs in African Americans as compared to Caucasians |
| FGF23-induced block in the activation of 25- hydroxyvitamin D in peripheral tissue |
Local conversion of 25-hydroxyvitamin D to1,25- dihydroxyvitamin D is essential for a variety of key autocrine/paracrine actions; inhibition of this conversion could block these pathways, leading to atherosclerosis, endothelial dysfunction, proteinuria, and higher infection risk |
| FGF23-induced catabolism of 25-hydroxyvitamin D | FGF23 stimulates catabolism of 25-hydroxyvitamin D; excess FGF23 concentrations could exacerbate low 25-hydroxyvitamin concentrations by inducing faster catabolism and/or blocking activation of in peripheral tissues, which may be particularly deleterious among African Americans who have lower average 25-hydroxyvitamin D concentrations than Caucasians |
Acknowledgments
Financial support
OMG was supported by grants K23DK081673, R03DK095005, and R01NS080850 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None
REFERENCES
- 1.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67:1232–1236. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 2.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85:4125–4130. doi: 10.1210/jcem.85.11.6962. [DOI] [PubMed] [Google Scholar]
- 3.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-Hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 4.Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 5.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15 S5-97-101. [PubMed] [Google Scholar]
- 6.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22:1745–1753. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awumey EM, Mitra DA, Hollis BW, Kumar R, Bell NH. Vitamin D metabolism is altered in Asian Indians in the southern United States: a clinical research center study. J Clin Endocrinol Metab. 1998;83:169–173. doi: 10.1210/jcem.83.1.4514. [DOI] [PubMed] [Google Scholar]
- 10.Aloia JF, Vaswani A, Ma R, Flaster E. Comparison of body composition in black and white premenopausal women. J Lab Clin Med. 1997;129:294–299. doi: 10.1016/s0022-2143(97)90177-3. [DOI] [PubMed] [Google Scholar]
- 11.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 12.Nelson DA, Jacobsen G, Barondess DA, Parfitt AM. Ethnic differences in regional bone density, hip axis length, and lifestyle variables among healthy black and white men. J Bone Miner Res. 1995;10:782–787. doi: 10.1002/jbmr.5650100515. [DOI] [PubMed] [Google Scholar]
- 13.Glerup H, Mikkelsen K, Poulsen L, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66:419–424. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 14.Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet. 1976;1:626–629. doi: 10.1016/s0140-6736(76)90428-1. [DOI] [PubMed] [Google Scholar]
- 15.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 16.Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 17.Ensrud KE, Ewing SK, Fredman L, et al. Circulating 25-hydroxyvitamin D levels and frailty status in older women. J Clin Endocrinol Metab. 2010;95:5266–5273. doi: 10.1210/jc.2010-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008;88:545S–550S. doi: 10.1093/ajcn/88.2.545S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajakumar K. Vitamin D, cod-liver oil, sunlight, and rickets: a historical perspective. Pediatrics. 2003;112:e132–e135. doi: 10.1542/peds.112.2.e132. [DOI] [PubMed] [Google Scholar]
- 20.Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol. 2011;347:25–29. doi: 10.1016/j.mce.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aloia JF, Vaswani A, Delerme-Pagan C, Flaster E. Discordance between ultrasound of the calcaneus and bone mineral density in black and white women. Calcif Tissue Int. 1998;62:481–485. doi: 10.1007/s002239900465. [DOI] [PubMed] [Google Scholar]
- 23.Aloia JF, Vaswani A, Yeh JK, Flaster E. Risk for osteoporosis in black women. Calcif Tissue Int. 1996;59:415–423. doi: 10.1007/BF00369203. [DOI] [PubMed] [Google Scholar]
- 24.Bell NH, Shary J, Stevens J, Garza M, Gordon L, Edwards J. Demonstration that bone mass is greater in black than in white children. J Bone Miner Res. 1991;6:719–723. doi: 10.1002/jbmr.5650060709. [DOI] [PubMed] [Google Scholar]
- 25.Harris SS, Wood MJ, Dawson-Hughes B. Bone mineral density of the total body and forearm in premenopausal black and white women. Bone. 1995;16:311S–315S. doi: 10.1016/8756-3282(95)00050-n. [DOI] [PubMed] [Google Scholar]
- 26.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 27.Luckey MM, Meier DE, Mandeli JP, DaCosta MC, Hubbard ML, Goldsmith SJ. Radial and vertebral bone density in white and black women: evidence for racial differences in premenopausal bone homeostasis. J Clin Endocrinol Metab. 1989;69:762–770. doi: 10.1210/jcem-69-4-762. [DOI] [PubMed] [Google Scholar]
- 28.Pollitzer WS, Anderson JJ. Ethnic and genetic differences in bone mass: a review with a hereditary vs environmental perspective. Am J Clin Nutr. 1989;50:1244–1259. doi: 10.1093/ajcn/50.6.1244. [DOI] [PubMed] [Google Scholar]
- 29.Cauley JA, Gutai JP, Kuller LH, Scott J, Nevitt MC. Black-white differences in serum sex hormones and bone mineral density. Am J Epidemiol. 1994;139:1035–1046. doi: 10.1093/oxfordjournals.aje.a116943. [DOI] [PubMed] [Google Scholar]
- 30.Meier DE, Luckey MM, Wallenstein S, Clemens TL, Orwoll ES, Waslien CI. Calcium, vitamin D, and parathyroid hormone status in young white and black women: association with racial differences in bone mass. J Clin Endocrinol Metab. 1991;72:703–710. doi: 10.1210/jcem-72-3-703. [DOI] [PubMed] [Google Scholar]
- 31.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136:1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 32.Dawson-Hughes B. Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. Am J Clin Nutr. 2004;80:1763S–1766S. doi: 10.1093/ajcn/80.6.1763S. [DOI] [PubMed] [Google Scholar]
- 33.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76:470–473. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawson-Hughes B, Harris S, Kramich C, Dallal G, Rasmussen HM. Calcium retention and hormone levels in black and white women on high- and low-calcium diets. J Bone Miner Res. 1993;8:779–787. doi: 10.1002/jbmr.5650080702. [DOI] [PubMed] [Google Scholar]
- 35.Engelman CD, Fingerlin TE, Langefeld CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93:3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosman F, Nieves J, Dempster D, Lindsay R. Vitamin D economy in blacks. J Bone Miner Res. 2007;22(Suppl 2):V34–V38. doi: 10.1359/jbmr.07s220. [DOI] [PubMed] [Google Scholar]
- 37.Cosman F, Morgan DC, Nieves JW, et al. Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res. 1997;12:958–966. doi: 10.1359/jbmr.1997.12.6.958. [DOI] [PubMed] [Google Scholar]
- 38.Fuleihan GE, Gundberg CM, Gleason R, et al. Racial differences in parathyroid hormone dynamics. J Clin Endocrinol Metab. 1994;79:1642–1647. doi: 10.1210/jcem.79.6.7989469. [DOI] [PubMed] [Google Scholar]
- 39.Perry HM, 3rd, Horowitz M, Morley JE, et al. Aging and bone metabolism in African American and Caucasian women. J Clin Endocrinol Metab. 1996;81:1108–1117. doi: 10.1210/jcem.81.3.8772584. [DOI] [PubMed] [Google Scholar]
- 40.Cauley JA, Lui LY, Ensrud KE, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293:2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 41.Baron JA, Barrett J, Malenka D, et al. Racial differences in fracture risk. Epidemiology. 1994;5:42–47. doi: 10.1097/00001648-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 43.Farmer ME, White LR, Brody JA, Bailey KR. Race and sex differences in hip fracture incidence. Am J Public Health. 1984;74:1374–1380. doi: 10.2105/ajph.74.12.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moldawer M, Zimmerman SJ, Collins LC. Incidence of osteoporosis in elderly whites and elderly Negroes. JAMA. 1965;194:859–862. [PubMed] [Google Scholar]
- 45.Silverman SL, Madison RE. Decrased incidence of hip fracture in Hispanics, Asians, and blacks: California Hospital Discharge Data. Am J Public Health. 1988;78:1482–1483. doi: 10.2105/ajph.78.11.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heaney RP. The importance of calcium intake for lifelong skeletal health. Calcif Tissue Int. 2002;70:70–73. doi: 10.1007/s00223-001-0032-3. [DOI] [PubMed] [Google Scholar]
- 47.Omenn GS. Evolution in health and medicine Sackler colloquium: Evolution and public health. Proc Natl Acad Sci U S A. 2010;107(Suppl 1):1702–1709. doi: 10.1073/pnas.0906198106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbour KE, Zmuda JM, Horwitz MJ, et al. The association of serum 25-hydroxyvitamin D with indicators of bone quality in men of Caucasian and African ancestry. Osteoporos Int. 2010 doi: 10.1007/s00198-010-1481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cauley JA, Danielson ME, Boudreau R, et al. Serum 25 hydroxyvitamin (OH)D and clinical fracture risk in a multiethnic Cohort of women: The Women's health initiative (WHI) J Bone Miner Res. 2011 doi: 10.1002/jbmr.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165:1618–1623. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 52.Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med. 1991;115:505–512. doi: 10.7326/0003-4819-115-7-505. [DOI] [PubMed] [Google Scholar]
- 53.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 54.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–1812. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Targher G, Bertolini L, Padovani R, et al. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 2006;65:593–597. doi: 10.1111/j.1365-2265.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 56.Freedman BI, Wagenknecht LE, Hairston KG, et al. Vitamin d, adiposity, and calcified atherosclerotic plaque in african-americans. J Clin Endocrinol Metab. 2010;95:1076–1083. doi: 10.1210/jc.2009-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 58.Saito H, Kusano K, Kinosaki M, et al. Human fibroblast growth factor-23 mutants suppress Na+ -dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278:2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 59.Bowe AE, Finnegan R, Jan de Beur SM, et al. FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun. 2001;284:977–981. doi: 10.1006/bbrc.2001.5084. [DOI] [PubMed] [Google Scholar]
- 60.Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 61.Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–980. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 62.Shalhoub V, Shatzen EM, Ward SC, et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122:2543–2553. doi: 10.1172/JCI61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 64.Wolf M, Betancourt J, Chang Y, et al. Impact of activated vitamin D and race on survival among hemodialysis patients. J Am Soc Nephrol. 2008;19:1379–1388. doi: 10.1681/ASN.2007091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalantar-Zadeh K, Miller JE, Kovesdy CP, et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25:2724–2734. doi: 10.1002/jbmr.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 67.Bacchetta J, Sea JL, Chun RF, et al. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res. 2013;28:46–55. doi: 10.1002/jbmr.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim K, Lu TS, Molostvov G, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 69.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bell NH. Renal and nonrenal 25-hydroxyvitamin D-1alpha-hydroxylases and their clinical significance. J Bone Miner Res. 1998;13:350–353. doi: 10.1359/jbmr.1998.13.3.350. [DOI] [PubMed] [Google Scholar]
- 71.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 72.Nakano C, Hamano T, Fujii N, et al. Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin J Am Soc Nephrol. 2012;7:810–819. doi: 10.2215/CJN.08680811. [DOI] [PubMed] [Google Scholar]
- 73.Pollock NK, Laing EM, Taylor RG, et al. Comparisons of trabecular and cortical bone in late adolescent black and white females. J Bone Miner Metab. 2011;29:44–53. doi: 10.1007/s00774-010-0186-z. [DOI] [PubMed] [Google Scholar]
- 74.Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hannan MT, Litman HJ, Araujo AB, et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93:40–46. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wetzsteon RJ, Hughes JM, Kaufman BC, et al. Ethnic differences in bone geometry and strength are apparent in childhood. Bone. 2009;44:970–975. doi: 10.1016/j.bone.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 77.Peacock M, Buckwalter KA, Persohn S, Hangartner TN, Econs MJ, Hui S. Race and sex differences in bone mineral density and geometry at the femur. Bone. 2009;45:218–225. doi: 10.1016/j.bone.2009.04.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Z, Qi L, Beck T, et al. Stronger bone correlates with African admixture in African American women. J Bone Miner Res. 2011 doi: 10.1002/jbmr.430. [DOI] [PMC free article] [PubMed] [Google Scholar]