Abstract
A-Kinase Anchoring Proteins (AKAPs) direct the flow of cellular information by positioning multiprotein signaling complexes into proximity with effector proteins. However, certain AKAPs are not stationary but can undergo spatiotemporal redistribution in response to stimuli. Gravin, a 300kD AKAP that intersects with a diverse signaling array, is localized to the plasma membrane but has been shown to translocate to the cytosol following the elevation of intracellular calcium ([Ca2+]i). Despite the potential for gravin redistribution to impact multiple signaling pathways, the dynamics of this event remain poorly understood. In this study, quantitative microscopy of cells expressing gravin-EGFP revealed that Ca2+ elevation caused the complete translocation of gravin from the cell cortex to the cytosol in as little as 60 seconds of treatment with ionomycin or thapsigargin. In addition, receptor mediated signaling was also shown to cause gravin redistribution following ATP treatment, and this event required both [Ca2+]i elevation and PKC activation. To understand the mechanism for Ca2+ mediated gravin dynamics, deletion of calmodulin-binding domains revealed that a fourth putative calmodulin binding domain called CB4 (a.a. 670-694) is critical for targeting gravin to the cell cortex despite its location downstream of gravin’s membrane-targeting domains, which include an N-terminal myristoylation site and three polybasic domains. Finally, confocal microscopy of cells co-transfected with gravin-EYFP and PKA RII-ECFP revealed that gravin redistribution mediated by ionomycin, thapsigargin, and ATP each triggered the gravin-dependent loss of PKA localized at the cell cortex. Our results support the hypothesis that gravin redistribution regulates cross-talk between PKA-dependent signaling and receptor-mediated events involving Ca2+ and PKC.
Keywords: AKAP12, Gravin, PKA, Calcium, Purinergic receptor, AKAP, Compartmentalization
1. Introduction
Compartmentalization of intracellular signaling molecules is essential to signal transduction and is achieved in part by scaffold proteins, which have the ability to shape and direct intracellular signaling networks by organizing molecular complexes into distinct subcellular compartments [1]. The ability of scaffold proteins to control the flow of signal transduction in space and time gives them a powerful role in regulating cellular behavior. Conceptually, scaffold proteins help provide a framework for understanding how sophisticated signaling networks can function accurately and efficiently among a widely diverse milieu of intracellular signaling proteins, some with opposing effects. PKA, a critical intracellular enzyme with several hundred phosphorylation substrates [2], relies heavily upon spatial targeting to exert its signaling effects. This occurs largely through A-Kinase Anchoring Proteins (AKAPs), which anchor PKA to various subcellular locations through a conserved amphipathic helical domain and a widely varied subcellular targeting domain. AKAPs are known to localize to a number of cellular compartments including the cytoskeleton, plasma membrane, nucleus, Golgi, and endoplasmic reticulum, positioning multiprotein signaling complexes into proximity with other effector proteins [reviewed in 3]. Numerous reports demonstrate that the loss of PKA compartmentalization significantly disrupts PKA signaling and leads to a many physiological dysfunctions, for example, in memory [4], immune response [5], and cytoskeletal dynamics [6].
The AKAP12 gene encodes a 300kD AKAP known as gravin in humans [7, 8] or SSeCKS [9] in rodents, that binds the RII subunit of PKA and is expressed in a variety of cell types and tissues [10]. Three isoforms of gravin have been described (gravin-α, -β, -γ) which differ in subcellular localization [11]. The canonical α isoform used in this study is expressed in humans and is localized to the cell cortex through an N-myristoylation sequence and by three polybasic domains (PB1-3) which adhere to negatively charged phospholipids [12, 13]. Like many AKAPs, gravin is a multivalent scaffold and interacts not only with PKA, but also with PKC [8, 14, 15], phosphodiesterase 4D [16], Ca2+/calmodulin [12], and the β2-adrenergic receptor [17-20]. Accordingly, gravin has been implicated in a wide range of cellular functions. Gelman and colleagues have published extensively on the role of SSeCKS as a tumor suppressor [21-24]. Along similar lines, Choi et al. [25] demonstrated in human SNU-449 heptaocellular cancer cells that gravin is critical in cytokinesis and interacts with actin and myosin light chain kinase during cell division. More recently, Scott and colleagues showed that gravin recruits Polo-like kinase to the mitotic spindle to regulate cell cycle progression [26]. Malbon and colleagues determined that gravin associates with the β2-adrenergic receptor in A293 cells and is crucial in regulating this receptor’s desensitization and resensitization [17-20]. Willoughby et al. [16] determined that gravin-anchored PKA and PDE4D establish a negative feedback loop for regulating [cAMP] in the submembrane compartment of HEK293 cells. It is clear from these examples that gravin intersects with a broad network of signaling pathways; however the precise molecular dynamics behind these diverse functions remain poorly understood.
Although gravin (α isoform) localizes at the plasma membrane under basal conditions, it is known to translocate to alternative subcellular compartments in response to stimuli. Further knowledge of this may be critical in understanding how gravin regulates the activity of its binding partners. Redistribution of gravin from the cell periphery to the cytosol has been demonstrated in response to PKC activation by phorbol ester treatment [15, 27]. Previous work in our lab demonstrated that PKC activity directs gravin to a vesicular compartment near the nucleus, and that this translocation also causes similar redistribution of PKA [13]. In addition, elevation of intracellular calcium concentration ([Ca2+]i) has been shown to cause gravin redistribution through a presumed mechanism involving Ca2+/calmodulin (CaM) binding to gravin’s membrane-associated polybasic domains (PB1, 2 and 3) [12]. Although CaM binding to PB1-3 has been clearly demonstrated, the notion that this interaction is alone responsible for Ca2+ mediated gravin redistribution may be incomplete in light of our previous findings which show that the myristoylation site is sufficient to target gravin to the plasma membrane even in the absence of PB1-3 [13]. While it is likely that CaM binding to PB1-3 contributes to the dissociation of gravin from the plasma membrane, the role of the myristoylation sequence in the event is yet to be determined. In addition, it is currently unknown whether calcium mediated gravin redistribution also results in the redistribution of PKA, or if these dynamics are linked to receptor-mediated signaling. Given that PKA signaling often relies heavily on spatial compartmentalization, the findings that both [Ca2+] elevation and PKC activation lead to gravin redistribution raise the interesting possibility that signaling cascades involving Ca2+ and/or PKC may engage in cross-talk with PKA-dependent signaling events through the redistribution of gravin. This would be a novel finding particularly with regard to Ca2+/PKA crosstalk, which has been thought to occur primarily through Ca2+ sensitive adenylyl cyclases and phosphodiesterases which regulate cAMP concentrations. From these observations, we hypothesize that gravin redistribution is mediated by receptors which trigger [Ca2+]i elevation and/or PKC activation, and that these dynamics also cause the redistribution of PKA.
To test the hypothesis that calcium-mediated gravin redistribution also triggers PKA redistribution, we used fluorescent constructs of gravin and PKA RII to determine the effect of cytosolic calcium increase on gravin-PKA localization. Our results revealed that both gravin and PKA are redistributed away from the cell periphery following extracellular calcium influx, release of calcium from intracellular stores, or upon activation of purinergic receptors by ATP. In addition, these studies demonstrate that purinergic P2Y receptors utilize both PKC activation and [Ca2+]i increase to trigger gravin-PKA redistribution. Although calcium-mediated gravin redistribution has been proposed to occur through CaM binding to polybasic regions 1-3, our studies additionally demonstrate that the deletion of these regions had no effect on gravin redistribution. A fourth calmodulin binding domain, termed CB4, was also assessed to determine the role of calmodulin binding on gravin redistribution; however deletion of this region dramatically reduced the membrane localization of gravin.
2. Material and Methods
2.1. Cell culture and transfection
AN3 CA cells and HEC-1-A cells (Manassas, VA, ATCC numbers: HTB-111, HTB-112 respectively) were cultured at 37°C with 5% CO2 in low glucose Dubecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 100 units/ml penicillin and 100ug/ml streptomycin. Growth medium was replaced three times each week, and cells were split 1:25 upon reaching confluence.
For experiments involving transfected expression vectors, cells were transfected 2-3 days after placing 2×104 cells/cm2 onto 25mm glass coverslips in an 8-well plate. Cells were incubated in a transfection solution containing 3μl/ml GeneJammer (Agilent) and 1μg/ml plasmid DNA for approx. 12-24 hours prior to microscopy.
2.2. Construction of gravin-EGFP expression vectors
Gravin-EGFP, (ΔPB1-3) gravin-EGFP, and PKA RII ECFP constructs were initially constructed as described in previous work [13]. The current study used four additional gravin constructs which were generated as follows. A gravin-EYFP construct lacking the PKA RII binding domain (Δ4621-4662) termed “(ΔPKA) gravin-EYFP” was generated using a Phusion® site-directed mutagenesis kit (New England Biolabs, product F-541; forward primer 5′ACAGCCGTTGACCAGTTTGTACGTACAGAA; reverse primer 5′TTCCAAAATCCCATTTTCAGGCTCTAAATC). A gravin-EGFP vector lacking the three polybasic domains and the downstream putative calmodulin binding domain (Δ125-2086) termed “(ΔPB1-3, CB4) gravin-EGFP” was generated by inserting a SacII site directly downstream of the CB4 domain, and then removing nucleotides 125-2086 by SacII digestion (Primers: 5′CCAAAGC[CCGCGG]TGGATACCTCAGTATCT; 5′GGTGCGTCAAAGTCGGCTACGGGGGTGC, mutagenic nucleotides underlined, constructed SacII sequence in brackets). A gravin-EGFP vector lacking the polybasic domains and all nucleotides up to the beginning of the CB4 domain (Δ125-2011) termed “(ΔPB1-3+) gravin-EGFP” was similarly generated by inserting a SacII restriction site directly upstream of the CB4 domain, and removing nucleotides 125-2011 by SacII digestion (Primers: 5′GCAAGGAGAAGT[CCGCGG]CTGATGAGGAA; 5′GGTGCGTCAAAGTCGGCTACGGGGGTGC, mutagenic nucleotides underlined, constructed SacII sequence in brackets). Finally, a gravin-EGFP construct lacking only CB4 (Δ2005-2079) termed “(ΔCB4) gravin-EGFP” was generated by site-directed mutagenesis (Phusion®, New England Biolabs, product F-541; forward primer 5′AGGTCGTCTTCTGATGAGGAAGGGGGACCA; reverse primer 5′TGGTTCTTCCGGCTTTGGCTCTTCAACGCT).
2.3. Western blotting
To obtain protein samples for Western blotting, transfected AN3 CA cells in T-25 flasks were harvested by scraping into 2 ml of ice-cold PBS (58 mM Na2HPO4, 17 mM NaH2PO4–H2O, 68 mM NaCl), pelleted by centrifugation, and resuspended in 50 μl of lysis buffer (20 mM Tris base, 150 mM NaCl, 10 mM EDTA, 10 mM Benzamidine HCl, 1% (v/v) Triton X-100, 0.05% (v/v), Tween 20, 1 mM PMSF and 100 μg/ml leupeptin). Cell lysates were then run on a 5% SDS-polyacrylamide gel and transferred to nitrocellulose membranes by standard methods [28]. Blots were probed using an antigravin polyclonal antibody [10, 14] and immunoreactive bands were detected by chemiluminescence.
2.4. Ca2+ imaging and epifluorescence microscopy
Changes in [Ca2+]i in response to agonist treatment were measured using ratiometric fluorescent imaging of Fura-2 fluorescence at excitation wavelengths of 340 and 380 nm. Cells on coverslips were incubated at 37°C with 1 mg/ml Fura-2 AM (Invitrogen/Molecular Probes) for 30 minutes, rinsed and then placed in an Attofluor chamber heated to 37° C containing either SES medium (1.5 mM CaCl2 dihydrate, 145 mM NaCl, 5 mM KCl, 1 mM MgCl2 anhydrous, 10 mM glucose, 10 mM Hepes) or Ca2+ free SES (145 mM NaCl, 5 mM KCl, 1 mM MgCl2 anhydrous, 10 mM glucose, 10 mM Hepes). Images were obtained at 15 or 30 second intervals using a 40× Oil Plan Fluor objective lens (NA=1.3) mounted on a Nikon TE300 inverted microscope that was equipped with a Hamamatsu CCD camera, an excitation filter wheel (Ludl Electronics) with 340 and 380 nm excitation filters, and filter sets for imaging both Fura-2 and EGFP fluorescence. For measurement of [Ca2+]i, the Fura-2 fluorescence images were background subtracted and the ratio of Fura-2 fluorescence intensity at 340 and 380 nm excitation wavelengths was determined for 10-20 cells per coverslip at each time interval using ImageJ plugins for background subtraction and ratiometric analysis (compiled by Tony Collins, McMaster Biophotonics Facility, Department of Biochemistry and Biomedical Sciences, McMaster University, Hamilton, ON).
To quantify the subcellular redistribution of wild type or mutant gravin-EGFP following agonist treatment, the ratio of membrane to cytosolic fluorescence intensity was calculated from images for each time point. Following background subtraction in ImageJ, this ratio was generated by measuring the fluorescence intensity profile along a line 1 pixel wide by 36 pixels long drawn at several points across the plasma membrane of cells expressing gravin-EGFP (Fig. 1A), calculating the mean fluorescence intensity of the four brightest pixels at the peak of the intensity profile and dividing that by the mean fluorescence intensity of the profile corresponding to the cytosolic portion of the cell (Fig. 1B,C). Preliminary analysis of cells in which the plasma membrane was labeled with FM4-64 revealed that the plasma membrane region of the line profile was marked by a sharp elevation in intensity roughly 4 pixels in width, while the region of the line profile on the cytosolic side of these peak values provided a reasonable representation of the cytosolic fluorescence intensity. Points of measurement across the plasma membrane were carefully selected to avoid non-fluorescent intracellular regions (e.g. nucleus) and brightly fluorescent puncta within the cytosol. Loss of gravin-EGFP at the cell cortex resulted in a decrease in membrane fluorescence intensity, an increase in cytosolic fluorescence intensity, and a decrease in the ratio of membrane fluorescence against cytolic fluorescence (Fig 1D). Ratios were normalized by dividing each ratio in the time series by the mean ratio value prior to agonist treatment. All analysis and quantification was done on images in their native format (16-bit, 72 pixels/inch). For figures, cropped greyscale images of gravin-EGFP were converted to 8-bit and resized using Adobe Photoshop.
Fig. 1.
Quantification of gravin-EGFP redistribution. This was accomplished by measuring fluorescence intensity along a line transect across the plasma membrane using ImageJ (A). When gravin-EGFP was localized to the cell cortex, the fluorescence intensity along this line yielded a sharp peak in intensity at the membrane with cytosolic intensity following the peak. However, as gravin-EGFP underwent redistribution away from the membrane and into the cytosol, the membrane intensity decreased while the cytosolic intensity increased (B). The average fluorescence intensity at the membrane and at the cytosol was determined for each time point (C) and plotted as a ratio of membrane to cytosolic intensity over time (D). Ratios of membrane to cytosolic intensity (Mem/Cyt) were normalized by dividing each ratio in the time series by the average ratio value prior to treatment.
2.5. Confocal Microscopy
Live-cell imaging of cells co-transfected with gravin-EYFP and PKA RII-ECFP were obtained using a Zeiss 510 META laser scanning confocal microscope, with a Zeiss 100× Plan-Fluar oil objective lens (NA 1.45). Cells were incubated in regular SES medium and maintained at 37°C throughout each experiment. Two color imaging was performed to simultaneously capture EYFP and ECFP fluorescence: EYFP excitation light was generated with a 514 nm argon laser operated at 10% power, and ECFP was excited by a 458 nm argon laser at 80% power. Fluorescence emissions were passed through a NFT 515 beam splitter; 475 nm ECFP longpass filter and 530 nm EYFP longpass filter. Because control images of cells expressing gravin-EYFP or PKA RII-ECFP alone were critical in demonstrating minimal signal crossover, detector gain and amplifier offset settings were changed only slightly (EYFP channel: 825-900 gain, −0.18 to 0.01 offset; ECFP channel: 1150-1250 gain, −0.13 to −0.01 offset). Post acquisition adjustments to image brightness and contrast were applied equally across corresponding images from different experimental treatments.
3. Results
3.1. Ionomycin and thapsigargin cause the calcium-dependent redistribution of gravin
Treatment of cells expressing gravin-EGFP with the calcium ionophore ionomycin (1μM) resulted in the complete translocation of gravin-EGFP from the cell cortex to the cytosol (Fig. 2A images). Plots of the ratio of membrane to cytosolic fluorescence over time revealed that gravin redistribution began immediately after ionomycin treatment and was complete within 60 seconds, at which time [Ca2+]i elevation reached its plateau (Fig. 2B,C).
Fig. 2.
Ionomycin treatment induces gravin redistribution. (A) Fluorescent micrographs illustrating that gravin-EGFP undergoes redistribution immediately following the addition of 1 μM ionomycin. (B) Plot of membrane:cytosol fluorescence intensity ratio over time illustrating the time course of gravin redistribution. (C) Corresponding plot of the Fura2 fluorescence ratio illustrating the rapid increase in [Ca2+]i upon ionomycin treatment. Note that gravin translocation is complete at t = 60 seconds, which corresponds to the time at which maximal cytosolic calcium increase is observed.
To determine if ionomycin mediated redistribution of gravin-EGFP was indeed calcium-dependent, inhibition studies were performed in regular and Ca2+ free SES medium by treating cells with BAPTA-AM (10 μM), a high-affinity intracellular calcium chelator, for 30 min prior to ionomycin treatment. In regular SES medium, control ionomycin treatments with no BAPTA-AM resulted in the complete redistribution of gravin and an immediate, sustained increase in [Ca2+]i (Fig. 3A,B). In Ca2+ free SES, ionomycin caused both the increase of [Ca2+]i and the redistribution of gravin, although the magnitude and duration of calcium increase were substantially reduced (Fig. 3E,F). This finding is consistent with other studies which have demonstrated that ionomycin targets the release of calcium from intracellular stores in addition to triggering extracellular calcium influx [29, 30]. Ionomycin mediated gravin redistribution and cytosolic calcium increase were both fully prevented in Ca2+ free SES when BAPTA-AM was present (Fig. 3G,H), confirming that ionomycin mediated gravin redistribution is calcium dependent. Surprisingly, pre-treatment of cells with BAPTA-AM in regular SES also showed ionomycin-mediated gravin redistribution and a sustained [Ca2+]i increase; however there was a 60 second delay in gravin redistribution and [Ca2+]i increase (Fig. 3C,D). Even at BAPTA-AM concentrations as high as 500 μM, ionomycin caused gravin redistribution in regular SES (not shown) and suggests that the intracellular BAPTA-AM concentration required to prevent [Ca2+] elevation in these cells could not be achieved. Nonetheless, our finding that BAPTA-AM prevented gravin redistribution in Ca2+ free SES demonstrated that ionomycin-mediated gravin redistribution was calcium-dependent.
Fig. 3.
Representative fluorescent images of EGFP-gravin and corresponding plots of Fura2 fluorescence ratios illustrating that ionomycin-mediated gravin distribution is calcium dependent. Note that ionomycin treatment in both the presence and absence of extracellular calcium resulted in elevation of intracellular calcium and redistribution of gravin. Ionomycin treatment in the presence of BAPTA-AM (10 μM) and extracellular calcium also resulted in a sustained increase in cytosolic calcium and gravin redistribution. However, when BAPTA-AM was present in Ca2+ free medium, ionomycin-mediated gravin redistribution was fully prevented. Scale bar = 10 μM.
To examine the effect of [Ca2+]i increase through the release of Ca2+ from intracellular stores on gravin distribution, cells expressing gravin-EGFP were treated with thapsigargin, an agent which rapidly elevates cytosolic calcium by inhibiting SERCA-mediated Ca2+ reuptake into the endoplasmic reticulum [31]. Upon treatment with thapsigargin (0.4 μM), gravin-EGFP underwent redistribution from the cell periphery to the cytosol in both the presence and absence of extracellular Ca2+. Thapsigargin mediated calcium increase peaked within 60 seconds and then gradually decreased to basal levels over the course of several minutes (Fig. 4A-B,E-F). Pre-treatment of cells with BAPTA-AM (10 μM) 30 minutes prior to thapsigargin treatment prevented gravin redistribution and cytosolic calcium increase in both regular and Ca2+ free SES (Fig. 4C-D,G-H). These results confirm that thapsigargin elicited gravin redistribution in a calcium-dependent manner, raising the possibility that receptor-mediated signaling events which target the release intracellular Ca2+ stores into the cytosol would also cause the redistribution of gravin.
Fig. 4.
Representative fluorescent images of EGFP-gravin and corresponding plots of Fura2 fluorescence ratios illustrating that thapsigargin treatment (0.4 μM) causes gravin redistribution through the release of Ca2+ from intracellular stores. Note that thapsigargin treatment in the presence and absence of extracellular calcium caused redistribution of gravin concurrent with a transient increase in cytosolic calcium. Addition of BAPTA-AM (10 μM) prior to thapsigargin treatment fully prevented gravin redistribution in either the presence or absence of extracellular Ca2+. Scale bar = 10 μM.
3.2. Role of putative calmodulin binding domains in Ca2+ mediated gravin redistribution
Currently, the mechanism underlying redistribution of gravin in response to elevated [Ca2+]i is poorly understood. However, a study by Tao et al. [12] proposed that calcium mediated gravin redistribution occurs as a result of Ca2+/calmodulin binding to three polybasic domains located in the N-terminal half of gravin (PB1-3) and inhibiting their association with the plasma membrane [12], Based on this, we predicted that deletion of PB1-3 would alter the rate of gravin redistribution in response to ionomycin treatment. However, fluorescence microscopy revealed that a gravin-EGFP construct lacking the three polybasic domains (ΔPB1-3) localized to the cell cortex prior to treatment and underwent redistribution from the cell cortex to the cytosol at an identical rate to the wild type gravin construct after ionomycin treatment (Fig. 5A). This suggests that the polybasic domains PB1-3 are not required for calcium-mediated redistribution of gravin.
Fig. 5.
Role of four calmodulin binding domains (PB1-3, CB4) in gravin redistribution and subcellular localization. (A, B) Comparison of the rate of redistribution from cell membrane to cytosol for either WT gravin-EGFP and (ΔPB1-3) gravin-EGFP (A) or WT gravin-EGFP and (ΔPB1-3+) gravin-EGFP (B) after ionomycin treatment. The deleted regions are illustrated below the graphs. No difference was observed between WT gravin and (ΔPB1-3) gravin, but the rate of (ΔPB1-3+) gravin translocation from membrane to cytosol was significantly reduced compared to WT gravin. (Comparison at each time point revealed significant differences between constructs as indicated by the horizontal bar; Mann-Whitney Rank Sum Test, p < 0.05). (C) Graph illustrating the effect of deleting the CB4 region on localization of gravin at the cell periphery in transfected cells. Note that localization of the (ΔPB1-3, CB4) mutant at the cell cortex was significantly reduced compared to WT gravin. Reconstitution of the CB4 domain (ΔPB1-3+) restored membrane localization, while deletion of the CB4 domain (ΔCB4) alone resulted in a decrease in membrane localization similar to that of ΔPB1-3,CB4. Astericks indicate significant differences from WT gravin-EGFP (one-way ANOVA followed by a Holm-Sidak post-hoc test; p < 0.05). (D) A Western blot demonstrating the expression of full-length gravin-EGFP and its deletion mutants in AN3CA cells. Sixty micrograms total protein was loaded in all lanes. The number of amino acids comprising each construct is marked at the top of the blot.
The study by Tao et al. [12] also revealed the location of a possible fourth calmodulin binding domain on gravin between amino acids 670-694 which, unlike polybasic domains 1-3, did not bind to phospholipid vesicles. This fourth putative calmodulin binding domain (CB4) conforms to a 1-5-10 consensus sequence for Ca2+/calmodulin binding [32] and corresponds exactly to the SSeCKS-3 domain, which was shown to bind calmodulin in SSeCKS, the murine orthologue of gravin [33]. To further understand the role of all four calmodulin binding domains of gravin in Ca2+ mediated redistribution, additional mutant gravin constructs were generated with the intent of comparing their rates of redistribution to that of the full length (WT) gravin. A gravin-EGFP construct lacking the polybasic domains and everything up to but not including the CB4 domain (ΔPB1-3+) localized to the cell cortex prior to treatment, but in response to ionomycin (1 μM) underwent translocation of away from the cell cortex at a significantly delayed rate compared to WT gravin (Mann-Whitney Rank Sum Test, horizontal bar denotes time points at which p < 0.05) (Fig. 5B). Surprisingly, a gravin-EGFP construct lacking the three polybasic domains and the fourth putative calmodulin binding domain (ΔPB1-3, CB4) did not localize at the cell cortex in a manner which was sufficient to measure its rate of redistribution. As illustrated in Fig. 5C, 75.7% (± 14.8%, 231 cells) of cells transfected with full-length (WT) gravin-EGFP, displayed cortically localized fluorescence, while only 17.8% (± 11.4%, 215 cells) of cells expressing (ΔPB1-3,CB4) gravin-EGFP displayed any appreciable cortical fluorescence. Normal membrane localization was observed in (ΔPB1-3+) gravin-EGFP (81.7% ±10.2%, 125 cells) compared to WT gravin, but a gravin-EGFP construct lacking only the CB4 domain (ΔCB4) localized to the cell cortex in significantly fewer transfected cells (19.7% ± 6.2%, 90 cells) (ANOVA followed by Holm-Sidok post-hoc, significant differences from WT gravin denoted by asterisks, p < 0.05).
The expression of the mutant gravin–EGFP vectors was confirmed in AN3 CA cells by Western blotting using an anti-gravin antibody. As seen in Fig. 5D, the lanes loaded with lysates from different gravin–EGFP transfectants showed gravin expression at the positions that matched the size of the desired gravin–EGFP mutants. No gravin expression was detected in control untransfected cells. These findings demonstrate that the presence of the CB4 domain is critical in targeting gravin to the cell cortex, and that this domain seems to regulate the membrane-binding activity of upstream domains (myr, PB1-3) which have been well-characterized as necessary for membrane localization. These results also suggest that activity at this domain may in fact regulate the redistribution of gravin. While this activity is likely to involve Ca2+/calmodulin binding, future studies will be required to understand the precise function of Ca2+/calmodulin at this region.
3.3. ATP mediated gravin redistribution involves both Ca2+ and PKC
Since gravin was demonstrated to undergo redistribution from the cell membrane to the cytosol after ionomycin and thapsigargin treatment, we sought to determine if gravin redistribution would occur through receptor signaling linked to [Ca2+]i elevation. ATP is well known to induce an increase in [Ca2+]i through both ionotropic (P2X) and metabotropic (P2Y) purinergic receptors. While P2X receptors trigger the influx of extracellular calcium, P2Y receptors stimulate cytosolic calcium increases primarily through their association with Gq/11, which activates PLCβ to stimulate both PKC activation and InsP3 mediated release of calcium from intracellular stores [34, 35]. Treatment of HEC-1A cells with 10 mM ATP resulted in a change in gravin-EGFP distribution from the cell cortex to the cytosol. This change was also accompanied by an immediate increase in [Ca2+]i followed by a subsequent decrease back to basal levels (Fig. 6A,B). ATP-mediated gravin redistribution and [Ca2+]i increase also occurred in Ca2+ free SES (Fig. 6C,D), a result consistent with P2Y receptor activation.
Fig. 6.
Fluorescence micrographs illustrating the effect of_ATP treatment on gravin redistribution and changes in intracellular calcium in the presence and absence of extracellular calcium. Gravin redistribution was observed following ATP treatment (10 mM) in both regular SES and Ca2+ free SES. Moreover, cytosolic [Ca2+] increased to the same extent (an approximately 1.7 fold increase) in cells treated with ATP in regular and Ca2+ free SES. Scale bar = 10 μM.
To determine the mechanism of ATP-mediated gravin redistribution in HEC-1A cells, inhibition studies were performed using bisindolylmaleamide (BIM, 1 μM), a PKC inhibitor, and/or BAPTA-AM (50 μM). Treatment with 10mM ATP caused complete redistribution of gravin from the cell membrane to the cytosol, compared to cells with no ATP added (Fig. 7A-D). ATP treatment in the presence of either BIM or BAPTA-AM (Fig 7E-H) caused partial redistribution of gravin, while ATP treatment in the presence of both inhibitors showed no gravin redistribution (Fig. 7I-J). Measurement of the ratio of membrane to cytosolic fluorescence at each time point revealed that BIM and BAPTA-AM significantly attenuated ATP-mediated gravin redistribution (Fig. 7K,L). However, treatment with both BAPTA-AM and BIM together was necessary to completely prevent gravin redistribution in cells treated with ATP (Fig. 7M), suggesting that both Ca2+ and PKC are required for ATP-mediated gravin dynamics. Statistical analyses were performed at each time point using a Mann-Whitney Rank Sum Test (horizontal bars denote time points at which p < 0.05). Analysis of the maximum change in membrane to cytosolic ratio for each of the treatments further revealed three statistically significant subsets: ATP in the presence of no inhibitors showed the greatest change in gravin distribution; ATP in the presence of either BIM or BAPTA-AM showed a significantly reduced change in gravin distribution; and ATP in the presence of both BIM and BAPTA-AM showed no difference in gravin distribution from untreated cells (ANOVA followed by Holm-Sidok post-hoc, significantly different subsets denoted by asterisks, p < 0.05) (Fig. 7N).
Fig. 7.
Regulation of gravin redistribution by ATP treatment. (A-J) Fluorescence micrographs illustrating the effect of ATP treatment (10 mM) on gravin distribution in the presence of bisindolylmaleimide (BIM), BAPTA-AM, or BIM and BAPTA-AM together. ATP treatment induced complete redistribution of gravin (C,D) compared to untreated cells (A,B). Gravin redistribution was partially attenuated when ATP was administered in the presence of 2 μM bisindolylmaleimide (BIM) or 50 μM BAPTA-AM (G,H). However, gravin redistribution was fully prevented when ATP was administered in the presence of both BIM and BAPTA-AM (I,J). (K-M) Quantitative analysis of gravin-EGFP dynamics in response to ATP in the presence of BIM and BAPTA-AM. BIM (K) or BAPTA-AM (L) caused a significant attenuation in the redistribution of gravin-EGFP. However, ATP treatment in the presence of both BIM and BAPTA-AM completely inhibited redistribution of gravin-EGFP (M). (N) Histogram illustrating the extent to which gravin distribution changed under the different treatment conditions. The height of the bars corresponds to the difference between the membrane/cytosol ratio at t=0 and the minimum membrane/cytosol ratio reached during each run. One-way ANOVA followed by Holm-Sidak post hoc tests revealed three significantly distinct responses to the treatments, which are denoted by asterisks (p < 0.05). Scale bar = 10 μM.
3.4. Loss of cortical PKA compartmentalization following gravin redistribution
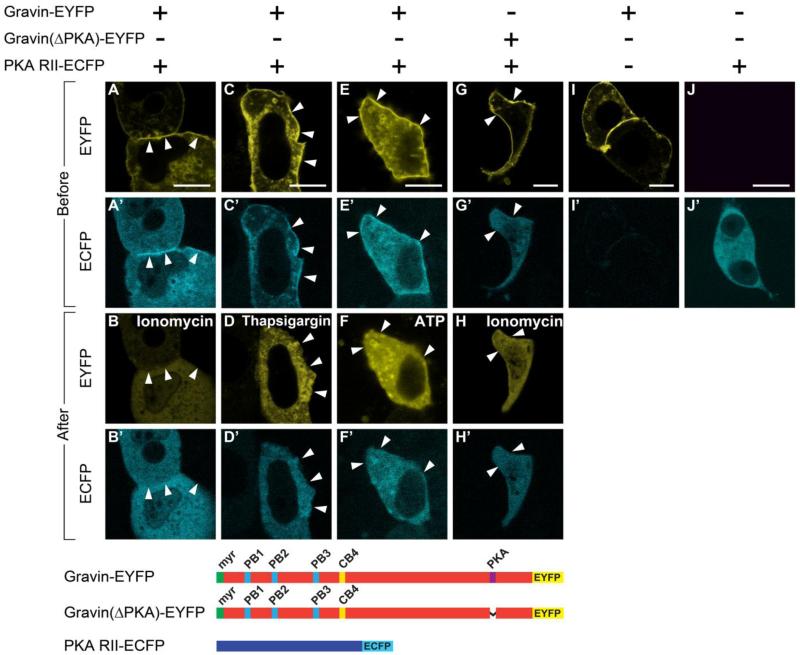
Since a major function of AKAPs is to direct PKA to specific subcellular compartments, we sought to examine whether Ca2+-mediated or purinergic receptor-mediated gravin redistribution also causes the redistribution of PKA. We used confocal microscopy to simultaneously visualize the subcellular distribution of gravin-EYFP and PKA RII-ECFP before and after treatment with ionomycin, thapsigargin, or ATP. As seen in Fig. 8, expression of PKA RII-ECFP was distributed throughout the cytosol in cells transfected with PKA RII-ECFP alone or when co-transfected with a gravin construct lacking the PKA-binding domain, (ΔPKA) gravin-EYFP, but PKA RII-ECFP was concentrated at the cell periphery in cells co-expressing WT gravin-EYFP. Treatment of cells with ionomycin, thapsigargin, or ATP resulted in in the loss of PKA RII-ECFP localization at the cell cortex, concurrent with the redistribution of gravin-EYFP (Fig. 8A-F’). These studies demonstrate that signaling events that trigger translocation of gravin also alter the subcellular distribution of PKA.
Fig. 8.
Confocal micrographs of HEC1A cells transfected with gravin-EYFP and PKA RII-ECFP. PKA co-distributes with gravin at the cell cortex in cells expressing both PKA and full-length gravin (A,A’; C,C’, E,E’), However, redistribution of gravin following treatment with ionomycin (B, 1 μM), thapsigargin (D, 0.4 μM), or ATP (F, 10mM) triggers the loss of PKA localization at the cell periphery (B’, D’, F’) in concert with redistribution of gravin. PKA RII-ECFP did not localize at the cell cortex nor change distribution after ionomycin treatment in cells co-expressing PKA RII-ECFP and a gravin-EYFP construct lacking the PKA RII binding domain (Δ-PKA gravin; G,G’; H,H’), although the gravin construct underwent redistribution to the cytosol. Controls transfected with either gravin-EYFP or PKA RII-ECFP alone confirmed that the observed codistribution of gravin and PKA was not due to cross-over of the EYFP signal into the ECFP channel, or vice versa. Scale bar = 10 μM.
4. Discussion
The aim of the current study was to characterize the redistribution of gravin following an increase in [Ca2+]i and determine its effect on subcellular PKA localization. This study demonstrated that gravin undergoes subcellular redistribution following treatment with ionomycin or thapsigargin, both from extracellular Ca2+ influx and from intracellular store release. Calcium mediated redistribution of gravin does not require the presence of polybasic domains 1-3, three regions rich in basic amino acids which bind Ca2+/calmodulin and are involved in targeting gravin to the plasma membrane. Interestingly, deletion of a fourth calmodulin binding domain (amino acids 670-694) which is reported to not associate with phospholipid vesicles [12] resulted in a dramatic decrease in the localization of gravin at the cell cortex. This study also demonstrated that purinergic receptor mediated elevation of [Ca2+]i and activation of PKC can also induce gravin redistribution. Finally, ionomycin, thapsigargin, and ATP mediated gravin redistribution also triggered the loss of PKA localization at the cell cortex. Our results support the hypothesis that receptor mediated signaling events involving calcium and/or PKC can influence cAMP-dependent signaling through the spatial regulation of gravin.
Calcium mediated redistribution of gravin was first reported by Tao et al. [12] to occur in response to A23187, a calcium ionophore. The current study adds to this finding in several important ways. First, our approach revealed that gravin redistribution occurs immediately following treatment with the calcium elevating agents ionomycin, thapsigargin, or ATP, and that these agents caused the complete redistribution of gravin from the cell periphery to the cytosol in as little as 60 seconds. Second, our findings with ionomycin and thapsigargin demonstrated that gravin redistribution can be mediated by both influx of extracellular calcium and the release of calcium from intracellular stores. Third, our approach demonstrated that both sustained and transient increases in cytosolic calcium following ionomycin or thapsigargin treatment were sufficient to mediate the complete redistribution of gravin. Finally, the current study demonstrated that Ca2+ signaling is involved in receptor-mediated gravin redistribution following ATP treatment. In these experiments ATP-generated Ca2+ transients were sufficient to cause partial gravin redistribution, in contrast to experiments involving ionomycin and thapsigargin, which caused complete gravin redistribution. This apparent contrast suggests that while Ca2+ alone may be sufficient for complete gravin redistribution under certain conditions, the mechanisms involved in receptor-mediated gravin redistribution may be more complex. Indeed, our findings demonstrate that ATP-mediated gravin redistribution also acted through PKC activity in addition to [Ca2+]i elevation. Previous reports have shown that PKC activation causes gravin redistribution and complements our finding that PKC is involved in ATP-mediated gravin redistribution [13, 15, 27]. In sum, the results of the current study further our understanding of the real-time dynamics behind Ca2+ mediated gravin redistribution, and provide additional insight into its biological context and molecular mechanism.
Although the mechanism behind calcium mediated redistribution of gravin has yet to be fully elucidated, previous work indicates the involvement of Ca2+/calmodulin. Tao et al. [12] reported compelling evidence that Ca2+/calmodulin interacts with the membrane-binding polybasic domains (PB1-3) of gravin to cause the dissociation of these domains from phospholipid vesicles, but this work used gravin constructs lacking the N-terminal myristoylation sequence. More recently, work in our lab demonstrated that the myristoylation sequence is sufficient to target gravin to the plasma membrane, even in the absence of the polybasic domains (13). We therefore postulated that a myristoylated gravin construct lacking the polybasic domains would not undergo redistribution in response to elevated [Ca2+]. Surprisingly, such a construct underwent redistribution from the plasma membrane to the cytosol at the same rate as full-length gravin. This finding indicates that Ca2+/calmodulin binding to the polybasic domains does not fully explain the mechanism for Ca2+ mediated redistribution of myristoylated gravin. Indeed, the membrane-binding activity of the myristoylation site must also be altered by [Ca2+]i elevation. Our results suggest that Ca2+/calmodulin interaction with an additional calmodulin binding domain, termed CB4, may regulate the membrane targeting of gravin. This CB4 domain conforms to the 1-5-10 consensus sequence for Ca2+/calmodulin binding (669KRKVDTSVSWEALICV) and is identical to the SSeCKS-3 domain found in the rat orthologue of gravin, reported by Lin and Gelman to bind Ca2+/calmodulin in vitro [33]. Deletion of the four known gravin calmodulin binding domains (ΔPB1-3, CB4) resulted in a dramatic loss of membrane localization of gravin, while reconstitution of only the CB4 domain fully restored membrane localization to that of full-length gravin. Moreover, deletion of CB4 alone caused a similar loss of membrane localization to that of (ΔPB1-3,CB4) gravin-EGFP. These results are the first to demonstrate that the CB4 domain is critical in the subcellular localization of gravin. Although the precise effect of CB4 deletion is unclear, one possibility is that CB4 deletion mimics Ca2+/calmodulin binding to gravin. Nonetheless, our results support the notion that calcium-mediated gravin redistribution operates through a mechanism which involves the interaction of Ca2+/calmodulin with gravin.
Our findings that gravin redistribution occurs from both intracellular calcium influx and from the release of calcium from intracellular stores suggest that gravin redistribution occurs across a variety of physiological contexts. These modes of calcium signaling are central to cellular homeostasis and exist not only in G protein coupled receptor (GPCR) systems, but also in the form of plasma membrane channels such as ionotropic receptors, cyclic-nucleotide gated channels, L-type channels, and membrane components of store operated calcium entry (SOCE). Thus, signaling through any one of a number of pathways that induce an increase in cytosolic [Ca2+] may result in the redistribution of gravin and affect the signaling dynamics of molecules associated with gravin. Our finding that ATP triggered redistribution of gravin through a pathway that involved both [Ca2+]i increase and PKC activation supports this notion and indicates that GPCR signaling through the canonical Gq/11 pathway may be a major pathway for gravin redistribution. Given the widespread occurrence of signaling pathways involving these modes of signaling, gravin redistribution is likely to be a widespread response to signaling events and could serve as an important mediator for cross-talk over the signaling dynamics of molecules bound to the gravin scaffold. Since gravin interacts with a diverse array of signaling molecules including PKA, PKC, PDE4, β2-adrenergic receptor (β2AR), cyclin D and others [16, 20, 36, 37], subcellular translocation of this AKAP would likely affect signaling events involving these binding partners. PKA, for instance, is known to require spatial compartmentalization by AKAPs [38]. Thus, loss of cortically-localized gravin/PKA would likely affect PKA signaling by reducing activity at the plasma membrane or directing PKA signaling to another subcellular compartment. β2AR signaling is known to be regulated by gravin expression and redistribution in a variety of contexts [12, 17-20] and thus receptor mediated events leading to gravin redistribution would most certainly impact a wide range of β2AR dependent physiological activities known to be linked to gravin. Finally, reports that PDE4 binds to gravin and that this complex regulates cortical cAMP levels suggest that receptor mediated relocalization of gravin could impact cAMP dependent signaling broadly by altering dynamic control of [cAMP].
5. Conclusions
We report that gravin undergoes rapid redistribution from the cell periphery to the cytosol following treatment with the calcium-elevating agents ionomycin, thapsigargin, or ATP, and that this redistribution is accompanied by a change in subcellular PKA localization. The effect of ionomycin and thapsigargin on gravin distribution was calcium dependent, whereas ATP’s effect on gravin distribution involved both calcium and PKC. Surprisingly, calcium mediated redistribution of myristoylated gravin did not require polybasic regions 1-3, but deletion of a 1-5-10 consensus sequence for calmodulin binding downstream of the polybasic regions seems to regulate the targeting of gravin to the cell cortex. Our data supports the hypothesis that receptor mediated signaling events involving calcium and/or PKC can alter cAMP-dependent signaling through the spatial regulation of gravin and anchored PKA. This finding suggests that gravin facilitates a novel cross-talk mechanism in which cAMP-dependent signaling pathways are altered by calcium and PKC, and lays the groundwork for future studies of gravin spatiotemporal dynamics in regulating cAMP-dependent signaling events.
Highlights.
A novel Ca2+/cAMP crosstalk mechanism mediated by the AKAP gravin is investigated.
Ca2+ elevation and purinergic receptor activation induces gravin relocalization.
Deletion of a critical CaM binding domain abolishes gravin localization at the cell cortex.
Gravin translocation is associated with PKA redistribution away from the cell cortex.
Acknowledgements
This work was supported by funds from NIH grant P30GM103329. In addition, the authors acknowledge use of the Edward C. Carlson Imaging and Image Analysis Core Facility which is also supported in part by NIH grant P30GM103329. We thank Faith Gonowolo for her assistance in the construction of the (ΔCB4) gravin-EGFP construct, and Sarah Abrahamson for general training in imaging and image analysis.
Abbreviations
- AKAP
A-Kinase Anchoring Protein
- ATP
adenosine triphosphate
- β2AR
β2-adrenergic receptor
- BAPTA-AM
1,2-Bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid tetrakis(acetoxymethyl ester)
- BIM
bisindolylmaleimide
- [Ca2+]i
intracellular calcium concentration
- CaM
calcium-calmodulin
- CB4
calmodulin binding domain 4
- ECFP
enhanced cyan fluorescent protein
- EGFP
enhanced green fluorescent protein
- EYFP
enhanced yellow fluorescent protein
- GPCR
G protein coupled receptor
- IM
ionomycin
- InsP3
inositol triphosphate
- PB1-3
polybasic domains 1 through 3
- PDE4
phosphodiesterase type 4
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- RII
regulatory subunit of type II PKA
- SERCA
sarco/endoplasmic reticulum calcium ATPase
- SES
standard extracellular solution
- SOCE
store-operated calcium entry
- SSeCKS
Src-Suppressed C-Kinase Substrate
- Tg
thapsigargin
- WT
wild-type/full-length
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Good MC, Zalatan JG, Lim WA. Science. 2011;332:680–686. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gao X, Jin C, Ren J, Yao X, Xue Y. Genomics. 2008;92:457–463. doi: 10.1016/j.ygeno.2008.08.013. [DOI] [PubMed] [Google Scholar]
- [3].Welch EJ, Jones BW, Scott JD. Mol Interv. 2010;10:86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nijholt IM, Ostroveanu A, Scheper WA, Penke B, Luiten PG, Van der Zee EA, Eisel UL. Neurobiol Learn Mem. 2008;90:223–229. doi: 10.1016/j.nlm.2008.03.008. [DOI] [PubMed] [Google Scholar]
- [5].Schillace RV, Miller CL, Pisenti N, Grotzke JE, Swarbrick GM, Lewinsohn DM, Carr DW. PLoS One. 2009;4:e4807. doi: 10.1371/journal.pone.0004807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang Y, Chen Y, Chen M, Xu W. Oncol Rep. 2006;16:755–761. [PubMed] [Google Scholar]
- [7].Gordon T, Grove B, Loftus JC, O’Toole T, McMillan R, Lindstrom J, Ginsberg MH. J Clin Invest. 1992;90:992–999. doi: 10.1172/JCI115976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nauert JB, Klauck TM, Langeberg LK, Scott JD. Curr Biol. 1997;7:52–62. doi: 10.1016/s0960-9822(06)00027-3. [DOI] [PubMed] [Google Scholar]
- [9].Lin X, Tombler E, Nelson PJ, Ross M, Gelman IH. J Biol Chem. 1996;271:28430–28438. doi: 10.1074/jbc.271.45.28430. [DOI] [PubMed] [Google Scholar]
- [10].Grove BD, Bowditch R, Gordon T, del Zoppo G, Ginsberg MH. Anat Rec. 1994;239:231–242. doi: 10.1002/ar.1092390302. [DOI] [PubMed] [Google Scholar]
- [11].Streb JW, Kitchen CM, Gelman IH, Miano JM. J Biol Chem. 2004;279:56014–56023. doi: 10.1074/jbc.M408828200. [DOI] [PubMed] [Google Scholar]
- [12].Tao J, Shumay E, McLaughlin S, Wang HY, Malbon CC. J Biol Chem. 2006;281:23932–23944. doi: 10.1074/jbc.M601813200. [DOI] [PubMed] [Google Scholar]
- [13].Yan X, Walkiewicz M, Carlson J, Leiphon L, Grove B. Experimental Cell Research. 2009;315:1247–1259. doi: 10.1016/j.yexcr.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grove BD, Bruchey AK. J Vasc Res. 2001;38:163–175. doi: 10.1159/000051043. [DOI] [PubMed] [Google Scholar]
- [15].Piontek J, Brandt R. J Biol Chem. 2003;278:38970–38979. doi: 10.1074/jbc.M306749200. [DOI] [PubMed] [Google Scholar]
- [16].Willoughby D, Wong W, Schaack J, Scott JD, Cooper DM. EMBO J. 2006;25:2051–2061. doi: 10.1038/sj.emboj.7601113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fan G, Shumay E, Wang H, Malbon CC. J Biol Chem. 2001;276:24005–24014. doi: 10.1074/jbc.M011199200. [DOI] [PubMed] [Google Scholar]
- [18].Lin F, Wang H, Malbon CC. J Biol Chem. 2000;275:19025–19034. doi: 10.1074/jbc.275.25.19025. [DOI] [PubMed] [Google Scholar]
- [19].Shih M, Lin F, Scott JD, Wang HY, Malbon CC. J Biol Chem. 1999;274:1588–1595. doi: 10.1074/jbc.274.3.1588. [DOI] [PubMed] [Google Scholar]
- [20].Tao J, Wang HY, Malbon CC. EMBO J. 2003;22:6419–6429. doi: 10.1093/emboj/cdg628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gelman IH, Lee K, Tombler E, Gordon R, Lin X. Cell Motil Cytoskeleton. 1998;41:1–17. doi: 10.1002/(SICI)1097-0169(1998)41:1<1::AID-CM1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- [22].Gelman IH, Tombler E, Vargas J., Jr. Histochem J. 2000;32:13–26. doi: 10.1023/a:1003950027529. [DOI] [PubMed] [Google Scholar]
- [23].Akakura S, Huang C, Nelson PJ, Foster B, Gelman IH. Cancer Res. 2008;68:5096–5103. doi: 10.1158/0008-5472.CAN-07-5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gelman IH. Genes Cancer. 2010;1:1147–1156. doi: 10.1177/1947601910392984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Choi MC, Lee YU, Kim SH, Park JH, Kim HA, Oh DY, Im SA, Kim TY, Jong HS, Bang YJ. Biochem Biophys Res Commun. 2008;373:85–89. doi: 10.1016/j.bbrc.2008.05.184. [DOI] [PubMed] [Google Scholar]
- [26].Canton DA, Keene CD, Swinney K, Langeberg LK, Nguyen V, Pelletier L, Pawson T, Wordeman L, Stella N, Scott JD. Mol Cell. 2012;48:547–559. doi: 10.1016/j.molcel.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nelson PJ, Moissoglu K, Vargas J, Jr., Klotman PE, Gelman IH. J Cell Sci. 1999;112(Pt 3):361–370. doi: 10.1242/jcs.112.3.361. [DOI] [PubMed] [Google Scholar]
- [28].Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yoshida S, Plant S. J Physiol. 1992;458:307–318. doi: 10.1113/jphysiol.1992.sp019419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Morgan AJ, Jacob R. Biochem J. 1994;300(Pt 3):665–672. doi: 10.1042/bj3000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lytton J, Westlin M, Hanley MR. J Biol Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- [32].Rhoads AR, Friedberg F. FASEB J. 1997;11:331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- [33].Lin X, Gelman IH. Biochem Biophys Res Commun. 2002;290:1368–1375. doi: 10.1006/bbrc.2002.6357. [DOI] [PubMed] [Google Scholar]
- [34].Erb L, Liao Z, Seye CI, Weisman GA. Pflugers Arch. 2006;452:552–562. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- [35].Weisman GA, Yu N, Liao Z, Gonzalez F, Erb L, Seye CI. Biotechnol Genet Eng Rev. 2006;22:171–195. doi: 10.1080/02648725.2006.10648070. [DOI] [PubMed] [Google Scholar]
- [36].Gelman IH. Front Biosci. 2002;7:d1782–1797. doi: 10.2741/A879. [DOI] [PubMed] [Google Scholar]
- [37].Gelman IH, Gao L. Mol Cancer Res. 2006;4:151–158. doi: 10.1158/1541-7786.MCR-05-0252. [DOI] [PubMed] [Google Scholar]
- [38].Skroblin P, Grossmann S, Schafer G, Rosenthal W, Klussmann E. Int Rev Cell Mol Biol. 2010;283:235–330. doi: 10.1016/S1937-6448(10)83005-9. [DOI] [PubMed] [Google Scholar]