Abstract
Bimetallic Pt-Pd/ZrO2 catalysts with different Pt/Pd atomic ratio and homogeneous dispersion of the metal nanoparticles were prepared in a single step by flame-spray pyrolysis. The catalysts show high activity and tuneable product selectivity for the solvent-free hydrodeoxygenation of the bio-oil model compounds cyclopentanone and acetophenone.
Increased worldwide demand for energy andimpending depletion of fossil-based energy resources, along with CO2 emissions from their utilization cause two serious challenges for global sustainable development: Energy and environment.1 These challenges spur research towards renewable and environmentally benign energy resources.2 Biomass could not only supplement petroleum as the main feedstock for fuel and chemical production, but also recycle CO2 during natural photo-synthesis by green plants. While bio-oil production from pyrolysis and hydrothermal treatment of biomass is commercially practiced, the crude bio-oil cannot be directly used as a transportation fuel due to low heating value, high viscosity and inadequate volatility.3 Many efforts have been focused on the development of suitable upgrading techniques to improve the technical viability of bio-oil.
Bio-oil is a mixture of 400 or more compounds with large concentrations of oxygen containing ones. Currently, there are two main approaches to upgrade bio-oil: i) hydrodeoxygenation (HDO) with typical hydrotreating catalysts;4-8 and ii) deoxygenation via acidic reactions on zeolites.9-16 Due to highly reactive oxygen containing compounds in bio-oil, relatively fast deactivation of zeolites occurs due to heavy coke generation during acidic upgrading.10, 11 For the HDO process, utilization of aqueous or acidic solvents leads to challenges in product separation and environmental issues.4, 5, 7 Therefore, it is necessary to develop solvent-free HDO processes for efficient bio-oil upgrading or other clean fine chemical processes. To realize the solvent-free HDO of complex bio-oils, it is of fundamental interest to understand how bio-oil model compounds react without solvent. Ketones such as acetophenone and cyclopentanone are listed as the main compounds in bio-oil, and acetophenone is the simplest aromatic ketone for testing the chemoselective hydrogenation of phenyl and carbonyl groups.3, 17-20
Supported noble metal catalysts, such as Pt and/or Pd, have been used in the liquid phase bio-oil HDO reactions with various solvents.4, 5, 7 Here we targeted solvent-free HDO reactions. A major challenge is the development of suitable highly active and selective catalysts. It has been reported that the activity and selectivity of bimetallic Pt-Pd catalysts can be enhanced by creating electron deficient Pt sites when Pd is in close vicinity to Pt.21 It is well known that the support properties play a crucial role for optimizing the catalytic properties of metal nanoparticles. Possessing intrinsic structural defects for enhanced interaction with metal particles, zirconia has been used as a support in many catalytic reactions.22 Recently, we developed a simple method of flame spray pyrolysis (FSP) to prepare novel catalysts in one step combining synthesis and calcination.23-26 This promising technique not only offers a very efficient production of solid catalysts, but also good control of the distribution of various atoms on the surface.27 Here we report on the remarkable properties of flame-derived bimetallic Pt-Pd/ZrO2 catalysts for the solvent-free HDO reaction of the bio-oil model compounds, cyclopentanone and acetophenone.
For FSP synthesis, appropriate amounts of platinum acetylacetonate and palladium acetylacetonate were added to zirconium (IV) isopropoxide and diluted 1:2 by volume with toluene. Five l/min of this clear precursor solution were sprayed and dispersed with 5 L/min oxygen and ignited with a flame of premixed CH4 and O2 of 1 and 2 L/min, respectively. The as-prepared 3 wt% Pt-Pd/ZrO2 catalysts with different Pt and Pd atomic ratios (Table 1) are denoted as PtxPdy/ZrO2, where x and y correspond to the atomic ratios of Pt and Pd, respectively. The specific surface areas of these FSP derived catalysts were in the range of 66.7 and 85.8 m2/g, as determined by N2 adsorption using the BET method at 77 K (Table 1).
Table 1.
Structural properties of 3 wt% Pt-Pd/ZrO2 catalysts.
| Catalyst | SSA, m2/g | dXRD_ZrO2, nm | Dispersion, % |
|---|---|---|---|
| Pt/ZrO2 | 70.1 | 21.5 | 66 |
| Pt75Pd25/ZrO2 | 68.8 | 21.8 | 56 |
| Pt50Pd50/ZrO2 | 66.7 | 22.9 | 42 |
| Pt25Pd75/ZrO2 | 82.1 | 18.4 | 44 |
| Pd/ZrO2 | 85.8 | 17.5 | 32 |
Powder XRD patterns showed only reflections of tetragonal ZrO2 and no reflections due to Pt or Pd (Fig. S1 in the Supporting Information) consistent with the low loading (3 wt%) and high dispersion of the noble metals. However, the XRD patterns revealed that the different loadings of the metal constituents had a slight effect on the crystallite size of ZrO2, which varied between 17.5 and 22.9 nm (Table 1). The ZrO2 particle sizes correlate with the surface areas of the catalysts.
Typical STEM and TEM images of the catalysts are shown in Fig. 1. The crystalline ZrO2 support of all catalysts showed a ball-like morphology. The majority of Pt particles (bright spots in Fig. 1a) in the Pt/ZrO2 sample were below 1 nm indicating a narrow size distribution. The TEM image of this catalyst showed spherical Pt particles (Fig. 1b). When adding Pd precursor to the FSP feed, the resulting bimetallic catalysts showed slightly increased particle size, as emerges from the STEM image of Pt50Pd50/ZrO2 (Fig. 1c). The average particle size of the Pt-Pd nanoparticles was around 1 nm, which is considerably smaller than those normally obtained by classical incipient wetness impregnation (2 nm).28 As the TEM picture (Fig. 1d) indicates, the bimetallic particles were well-dispersed on the ZrO2 surface which showed a similar morphology as the monometallic Pt/ZrO2. EDX investigations (one representative example is shown in Fig. 1f) confirmed that the ratio of the noble metal constituents (Pt and Pd) was close to the nominal ratio of the precursors used in the synthesis. The dispersion of bimetallic Pt-Pd particles (Table 1), as determined by CO chemisorption decreased with increasing Pd loading, it was highest for the monometallic Pt/ZrO2 and lowest for Pd/ZrO2.
Fig. 1.
STEM images of Pt/ZrO2 (a), Pt50Pd50/ZrO2 (c), and Pd/ZrO2 (e). TEM images of Pt/ZrO2 (b) and Pt50Pd50/ZrO2 (d). EDX spectrum of Pt50Pd50/ZrO2 (f).
CO adsorption combined with DRIFT spectroscopy was applied to gain some information about the structural and electronic properties of the supported noble metal nanoparticles. Mainly two regions of bands can be distinguished for adsorbed CO: a band at 2000-2100 cm−1 due to linearly coordinated CO and a band at 1770-2000 cm−1 assigned to CO adsorbed in bridged coordination. Pt/ZrO2 (spectrum a) of Fig. 2) shows a narrow signal at 2085 cm−1 and a broad band at ca. 2010 cm−1 assigned to linearly coordinated CO on (111) and (100) terraces and on low coordinated Pt sites at step-edges, corners, and defects.29, 30 Spectra b-d show that the formation of bimetallic Pt-Pd particles results in a new band at ca. 2055 cm−1, indicating that a new type of site was created. Unlike the spectrum for Pt/ZrO2 (a), the DRIFT spectrum of Pd/ZrO2 (e) showed that a band at 2085 cm−1 is dominant in the high wave-number range attributed to linearly coordinated CO on (111) and (100) terraces of highly coordinated Pd atoms.31 In the low wave-number range, the broad band at 1925 cm−1 and the narrow one at 1980 cm−1 are ascribed to bridged CO on Pd(111) and Pd(100) facets, respectively.32 Obviously, the spectra in Figs. 2 cannot be represented by a simple superposition of the spectra of CO adsorbed on monometallic Pt/ZrO2 and Pd/ZrO2, confirming that bimetallic Pt-Pd particles were formed on the ZrO2 support, typical for flame-made noble metal on ceramic support catalysts26-27. In addition, the broad band of bridged CO shifted to high wave-number from 1775 to 1925 cm−1 correlating well with the increasing Pd content from 0 to 100 at%.
Fig. 2.
DRIFT spectra of CO adsorption on reduced Pt/ZrO2 (a), Pt75Pd25/ZrO2 (b), Pt50Pd50/ZrO2 (c), Pt25Pd75/ZrO2 (d), and Pd/ZrO2 (e).
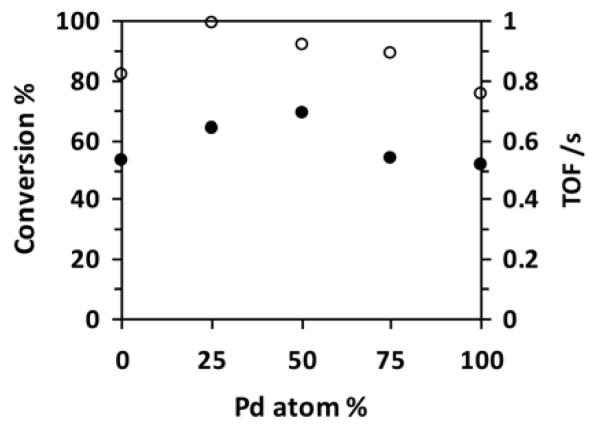
The catalysts were tested in the solvent-free HDO of cyclopentanone and acetophenone. Reactions were carried out in an autoclave at a hydrogen pressure of 60 bar and a temperature of 160°C. Fig. 3 shows the conversions of cyclopentanone and corresponding turnover frequencies (TOFs) as a function of Pd content of the Pt-Pd/ZrO2 catalysts. The conversion of cyclopentanone was 82% and 75% on monometallic Pt/ZrO2 and Pd/ZrO2, respectively, while it reached 99%, 92%, and 89% with Pt75Pd25/ZrO2, Pt50Pd50/ZrO2, and Pt25Pd75/ZrO2, respectively. The bimetallic catalysts clearly showed enhanced activity: The TOF (at 160°C) increased from 0.53 s−1 to 0.64 s−1 for bimetallic catalysts with 25 at% Pd and reached a maximum of 0.69 s−1 for Pt50Pd50/ZrO2. Further increase of the Pd content, resulted in a TOF reduction to 0.54 and 0.52 s−1, for Pt25Pd75/ZrO2 and Pd/ZrO2, respectively.
Fig. 3.
Influence of Pd content of Pt-Pd/ZrO2 catalysts on the conversion (open circles) and TOF (full circles) of cyclopentanone HDO. Conditions: 160°C, 60 bar hydrogen pressure, 3 h reaction time.
During the hydrogenation of cyclopentanone, cyclopentanol was the only product, and no cyclopentane was detected. There are currently two ways to directly convert stable ketones to alkanes: i) introduction of liquid acids to promote the hydrogenation of the carbonyl group; and ii) using bifunctional solid catalysts for gas-phase hydrogenation. Pathway (i) produces waste water from the separation process, and (ii) suffers from coke generation. Regeneration of catalyst by burning coke not only consumes organic carbon resources but also contributes to more CO2 emission. A very recent report describes one integrated catalytic process combining hydropressing with zeolite catalysis for bio-oil upgrading.33 Producing polyols and alcohols from pyrolysis oil at the pre-hydropressing step decreased the total hydrogen consumption and reduced the coke generation following zeolite catalysis. Therefore, the bimetallic Pt-Pd/ZrO2 presented here could be a promising hydrotreating catalyst in integrated catalytic processes for bio-oil upgrading.
Unlike in HDO of cyclopentanone, Pd/ZrO2 showed higher activity than Pt/ZrO2 in the HDO of the aromatic ketone acetophenone. As shown in Fig. 4, the conversion of acetophenone and TOF (at 140°C) was 80% and 0.42 s−1 for Pd/ZrO2, and 32% and 0.15 s−1 for Pt/ZrO2, respectively. Increasing the Pd content from 25 to 75 at% in the bimetallic catalysts was accompanied by an increase of acetophenone conversion from 53% to 96%. However, the TOF still reached a maximum of 0.5 s−1 for Pt50Pd50/ZrO2 at a conversion of 87%. Again the activity is enhanced by applying the bimetallic catalysts. Although a direct comparison of the activity of the bimetallic catalysts to others reported in the literature18-20, 34-38 is difficult due to different conditions applied (temperature, solvent etc.), we can safely conclude that the flame-derived bimetallic catalysts are among those possessing the highest activity.
Fig. 4.
Influence of the Pd content of Pt-Pd/ZrO2 catalysts on the conversion (open circles) and TOF (full circles) of acetophenone. Conditions: 140°C, 60 bar hydrogen pressure, 3 h reaction time.
Pt-Pd/ZrO2 catalysts were able to totally remove the oxygen from acetophenone and converted this activated ketone to ethylbenzene (EB) as shown in Fig. 5. EB is one desired compound in biofuel, since it only consumes limited hydrogen during HDO and has a high RON (Research Octane Number) of 124. Due to the competitive hydrogenation of the carbonyl group and the phenyl group, different possible products can be formed during the HDO of acetophenone, as shown in Scheme 1. Beside its higher activity, Pd/ZrO2 also showed higher selectivity to EB (SEB=64%) than Pt/ZrO2 (SEB=14) (Fig. 5). For bimetallic Pt-Pd/ZrO2, the selectivity to EB increased from 8%, to 50% and 83%, when the Pd content was increased from 25% to 50% and 75%. Reversely, when the Pt content was increased from 0% to 100%, the selectivity of Pt-Pd/ZrO2 to CE+EC+CMK increased from 0% to 62%. Pt sites seem to favour hydrogenation of the phenyl group and Pd sites that of the carbonyl group. For bimetallic catalysts, the surface Pd and Pt sites still kept their specific catalytic properties though their electronic properties have been changed and activity was enhanced.
Fig. 5.
Influence of Pd content of Pt-Pd/ZrO2 catalysts on the selectivity to products at an acetophenone conversion of ca. 60%: Selectivity to C=O bond HDO affording EB (open squares); Selectivity to phenyl ring hydrogenation products affording CE+EC+CMK (full squares). Beside these products 1-phenylethanol (PhE) was the only by-product. Conditions: 160°C, 60 bar hydrogen pressure, 3 h reaction time.
Scheme 1.
Hydrodeoxygenation of acetophenone.
In the bimetallic Pt-Pd particles prepared by wetness impregnation, Pd segregation to the surface was experimentally evidenced.39 Pt-Pd/ZrO2 prepared by wetness impregnation did not promote the activity in tetraline hydrogenation, and showed poor activity in decahydroquinoline hydrodenitrogenation as compared to the Pt/ZrO2 monometallic system.28 Probing the surface by combined DRIFTS and CO adsorption as well as the catalytic results of the flame-made bimetallic Pt-Pd/ZrO2 did not indicate substantial surface segregation of Pd and led to highly dispersed nanoparticles. These may be the properties responsible for the high activity of these catalysts in the solvent-free hydrodeoxygenation of cyclopentane and acetophenone.
Finally, the most active Pt50Pd50/ZrO2 catalyst has been subjected to repetitive catalytic tests and showed the same activity and selectivity for HDO of cyclopentanone and acetophenone after washing (n-hexane), filtering, and drying (vacuum overnight at 30°C).
Conclusions
Flame-derived bimetallic Pt-Pd/ZrO2 catalysts show excellent catalytic performance in the solvent-free hydrodeoxygenation of important components of bio-oil, such as cyclopentane and acetophenone. Synthesis of bimetallic Pt-Pd catalysts by flame-spray pyrolysis results in homogeneous dispersion of Pt and Pd and allows easy control of the metal loading on the ZrO2 surface. The as-prepared bimetallic Pt-Pd/ZrO2 afforded high activity in the HDO conversion of both cyclopentanone and acetophenone. Among the Pt-Pd/ZrO2 catalysts with different atomic ratio Pt:Pd, the Pt50Pd50/ZrO2 showed the highest activity (TOF). Although the electronic properties of bimetallic catalysts changed compared to their monometallic counterparts, the Pd and Pt active sites still kept their specific properties concerning product selectivity. The catalysts could be reused without significant loss in activity and selectivity after appropriate treatment. The substrate/metal molar ratio under solvent-free condition was in the range between 1814 and 4406, which is much higher than that in the hydrogenation with solvent. This efficient solvent-free hydrogenation of bio-oil constituents may present a significant step towards the development of green chemistry.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures and methods. See DOI: 10.1039/b000000x/
references
- 1.Dresselhaus MS, Thomas IL. Nature. 2001;414:332–337. doi: 10.1038/35104599. [DOI] [PubMed] [Google Scholar]
- 2.Potocnik J. Science. 2007;315:810–811. doi: 10.1126/science.1139086. [DOI] [PubMed] [Google Scholar]
- 3.Huber GW, Iborra S, Corma A. Chem. Rev. 2006;106:4044–4098. doi: 10.1021/cr068360d. [DOI] [PubMed] [Google Scholar]
- 4.Zhao C, Kou Y, Lemonidou AA, Li XB, Lercher JA. Angew. Chem. Int. Ed. 2009;48:3987–3990. doi: 10.1002/anie.200900404. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Yao J, Li HR, Su DS, Antonietti M. J. Am. Chem. Soc. 2011;133:2362–2365. doi: 10.1021/ja109856y. [DOI] [PubMed] [Google Scholar]
- 6.Zhao C, Kou Y, Lemonidou AA, Li XB, Lercher JA. Chem. Commun. 2010;46:412–414. doi: 10.1039/b916822b. [DOI] [PubMed] [Google Scholar]
- 7.Yan N, Yuan YA, Dykeman R, Kou YA, Dyson PJ. Angew. Chem. Int. Ed. 2010;49:5549–5553. doi: 10.1002/anie.201001531. [DOI] [PubMed] [Google Scholar]
- 8.Hong D-Y, Miller SJ, Agrawal PK, Jones CW. Chem. Commun. 2010;46:1038–1040. doi: 10.1039/b918209h. [DOI] [PubMed] [Google Scholar]
- 9.Peng J, Chen P, Lou H, Zheng XM. Bioresource Technol. 2009;100:3415–3418. doi: 10.1016/j.biortech.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Gayubo AG, Aguayo AT, Atutxa A, Valle B, Bilbao J. J. Chem. Tech. Biot. 2005;80:1244–1251. [Google Scholar]
- 11.Gayubo AG, Aguayo AT, Atutxa A, Prieto R, Bilbao J. Energy Fuel. 2004;18:1640–1647. [Google Scholar]
- 12.Gayubo AG, Aguayo AT, Atutxa A, Aguado R, Olazar M, Bilbao J. Ind. Eng. Chem. Res. 2004;43:2619–2626. [Google Scholar]
- 13.Gayubo AG, Aguayo AT, Atutxa A, Aguado R, Bilbao J. Ind. Eng. Chem. Res. 2004;43:2610–2618. [Google Scholar]
- 14.Vitolo S, Bresci B, Seggiani M, Gallo MG. Fuel. 2001;80:17–26. [Google Scholar]
- 15.Vitolo S, Seggiani M, Frediani P, Ambrosini G, Politi L. Fuel. 1999;78:1147–1159. [Google Scholar]
- 16.Huang J, Long W, Agrawal PK, Jones CW. J. Phys. Chem. C. 2009;113:16702–16710. [Google Scholar]
- 17.Corma A, Iborra S, Velty A. Chem. Rev. 2007;107:2411–2502. doi: 10.1021/cr050989d. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Jiang Y, van Vegten N, Hunger M, Baiker A. J. Catal. 2011;281:352–360. [Google Scholar]
- 19.Schimmoeller B, Hoxha F, Mallat T, Krumeich F, Pratsinis SE, Baiker A. Appl. Catal.A. 2010;374:48–57. [Google Scholar]
- 20.Jutz F, Andanson JM, Baiker A. J. Catal. 2009;268:356–366. [Google Scholar]
- 21.Rousset JL, Bertolini JC, Miegge P. Phys. Rev. B. 1996;53:4947–4957. doi: 10.1103/physrevb.53.4947. [DOI] [PubMed] [Google Scholar]
- 22.Ertl G, Knözinger H, Schüth F, Weitkamp J. Handbook of Heterogeneous Catalysis. 2nd Edition Wiley-VCH; Weinheim, Germany: 2008. [Google Scholar]
- 23.Huang J, van Vegten N, Jiang Y, Hunger M, Baiker A. Angew. Chem. Int. Ed. 2010;49:7776–7781. doi: 10.1002/anie.201003391. [DOI] [PubMed] [Google Scholar]
- 24.van Vegten N, Maciejewski M, Krumeich F, Baiker A. Appl. Catal.B. 2009;93:38–49. [Google Scholar]
- 25.Buchel R, Strobel R, Krumeich F, Baiker A, Pratsinis SE. J. Catal. 2009;261:201–207. [Google Scholar]
- 26.Strobel R, Baiker A, Pratsinis SE. Adv. Powder Technol. 2006;17:457–480. [Google Scholar]
- 27.Schimmoeller B, Pratsinis SE, Baiker A. Chemcatchem. 2011;3:1234–1256. [Google Scholar]
- 28.Devers E, Geantet C, Afanasiev P, Vrinat M, Aouine M, Zotin JL. Appl. Catal.A. 2007;322:172–177. [Google Scholar]
- 29.Hoxha F, Schimmoeller B, Cakl Z, Urakawa A, Mallat T, Pratsinis SE, Baiker A. J. Catal. 2010;271:115–124. [Google Scholar]
- 30.van Santen RA. J. Chem. Soc., Faraday Trans. 1987;83:1915–1934. [Google Scholar]
- 31.Tessier D, Rakai A, Bozonverduraz F. J. Chem. Soc., Faraday Trans. 1992;88:741–749. [Google Scholar]
- 32.Wolter K, Seiferth O, Libuda J, Kuhlenbeck H, Baumer M, Freund HJ. Surf. Sci. 1998;404:428–432. [Google Scholar]
- 33.Vispute TP, Zhang H, Sanna A, Xiao R, Huber GW. Science. 2010;330:1222–1227. doi: 10.1126/science.1194218. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XB. React. Kinet. Mech. and Cat. 2011;102:417–424. [Google Scholar]
- 35.Hiyoshi N, Sato O, Yamaguchi A, Shirai M. Chem. Commun. 2011;47:11546–11548. doi: 10.1039/c1cc13130c. [DOI] [PubMed] [Google Scholar]
- 36.Bertero NM, Trasarti AF, Apesteguia CR, Marchi AJ. Appl. Catal.A. 2011;394:228–238. [Google Scholar]
- 37.Reddy BM, Rao KN, Reddy GK. Catal. Lett. 2009;131:328–336. [Google Scholar]
- 38.Liu H, Lu G, Guo Y, Wang Y, Guo Y. Catal. Commun. 2009;10:1324–1329. [Google Scholar]
- 39.Fiermans L, De Gryse R, De Doncker G, Jacobs PA, Martens JA. J. Catal. 2000;193:108–114. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.