Abstract
Transitions in cell states are controlled by combinatorial actions of transcription factors. For primordial germ cell (PGC) specification, BLIMP1 the key regulator apparently acts together with PRDM14 and AP2γ. To investigate their individual and combinatorial functions, we first sought an in vitro system for transcriptional readouts and ChIPseq analysis. We then integrated this data with information from single cell transcriptome analysis of normal and mutant PGCs. Here we show that BLIMP1 binds directly to repress somatic and cell proliferation genes. It also directly induces AP2γ, which together with PRDM14 initiates the PGC-specific fate. We determined the occupancy of critical genes by AP2γ, which when computed altogether with those with BLIMP1 and PRDM14, individually and cooperatively, reveals a tripartite mutually interdependent transcriptional network for PGCs. We also demonstrate that in principle, BLIMP1, AP2γ and PRDM14 are sufficient for PGC specification, and the unprecedented resetting of the epigenome towards a basal state.
Primordial germ cells (PGCs) in mice originate from the rapidly dividing post implantation epiblast cells that are primed for somatic fate, following repression of some pluripotency genes1. They also exhibit an inactive X chromosome, histone H3 lysine nine dimethylation (H3K9me2) and DNA methylation2,3. A transcriptional network for PGC specification should reverse this trend by the time 30-40 founder PGCs are established at embryonic day 7.5 (E7.5).
PGC fate is initiated by BMP4-induced expression of BLIMP1 in a few proximal epiblast cells at E6.254–8, which marks their divergence from somatic neighbours (see Fig 3b). Indeed, BLIMP1 mutant cells fail as PGCs and resemble neighbouring somatic cells7,9–11. BLIMP1 binds to a specific DNA sequence12–20 to either repress21–25 or activate26 its direct targets. Shortly after BLIMP1, there is induction of Prdm14 also by BMP427, followed by Tcfap2c encoding AP2γ28 (see Fig 3b). Genetic experiments indicate that these factors are individually critical for PGC specification. It is important however to establish if their combinatorial roles and precise targets are necessary and sufficient for PGC specification, and for the initiation of the unique epigenetic program29.
Figure 3. RNA-Seq analysis of PGCs, and BLIMP1 binding to differentially regulated genes.
(a). Cluster analysis of single-cell RNA-seq expression profiles of PGCs and somatic neighbours. The bar indicates the mean Euclidian distance between the samples. Note that E7.5 BLIMP1 mutant (KO) cells cluster next to E7.5 somatic cells, while the PGCs form distinct clusters following specification (b). Expression levels of selected gene transcripts during PGC specification (RPM=read numbers pr. million reads). The y-axis represents read numbers pr. million reads sequenced. The region shaded in blue represents BLIMP1 mutant (KO) cells. The dotted line represents the onset of PGC specification. (c). Expression of Dnmt3b, T-Brachyury, Tcfap2c and Cbx7 during PGC specification, which are all bound by BLIMP1 during PGC specification. (d). Track views of BLIMP1 binding to the genomic loci of Dnmt3b,T-Brachyury, and Tcfap2c (encoding AP2γ) and Cbx7.
In this study we combined information from different experimental models to establish how BLIMP1, PRDM14 and AP2γ contribute to PGC specification, both individually and combinatorially. We propose a tripartite transcriptional network that accounts for PGC specification and their unique properties. Indeed, co-expression of BLIMP1, AP2γ and PRDM14 in an in vitro model can substitute for cytokines in the direct induction of PGC-like cells (PGCLCs). Close scrutiny of the genetic network also provides a detailed view of how these genetic factors regulate the unique epigenetic program in germ cells, which might serve as a paradigm for wider applications in the context of tissue regeneration and experimental manipulation of cell fates.
RESULTS
We first sought an in vitro surrogate cell-culture system to examine the individual and cooperative roles of BLIMP1, PRDM14 and AP2γ, and to identify their direct targets by chromatin immunoprecipitation (ChIP) experiments, which requires large amounts of material. This is difficult with PGCs since they are relatively rare, difficult to culture, transfect and manipulate. We therefore tested BLIMP1 expression in several primary cell types, embryonic stem cells (mESCs), embryonic germ cells (EGCs) and epiblast stem cells (EpiSC), but none of them survived except for P19 embryonal carcinoma cells (P19EC)29 (Fig 1a). Indeed, P19EC cells are also appropriate for this purpose because they originate from E7.5 epiblast30, and share important properties of post implantation epiblast, the precursors of PGCs in vivo31,32.
Figure 1. BLIMP1, AP2γ and PRDM14 repress somatic regulators and induce PGC genes in P19ECs.
(a) Design and overview of the experimental approaches towards transcriptional network for PGC specification.The arrow pointing to PGCs refers to EGFP reporter labelled PGCs in mouse embryos. (b) RT-qPCR analysis of differentially regulated genes downstream of BLIMP1 in P19ECs, showing induction of the PGC genes Nanos3, Rhox9, Dppa3, Tcfap2c, Dnd1, Prdm14, Dazl and Ddx4. (c) RT-qPCR analysis showing the individual and combinatorial effect of BLIMP1, AP2γ and PRDM14 in P19ECs on the mesendodermal transcription factor genes, Eomes and T-Brachyury, the DNA methyltransferase Dnmt3b on Myc and the PGC genes Nanos3, Dnd1, Prdm1, Ddx4/Vasa and Dppa3. The error bars in panels (b) and (c) represent standard deviations for 3 independent cell cultures.
Repression of somatic program and induction of PGC genes in P19EC cells
We examined P19EC cells for transcriptional response following ectopic expression of BLIMP1-EGFP fusion protein or EGFP alone after 24h with low (24h-LO) and high expression (24h-HI), and all fluorescent cells at 48h (Fig. S1a). Whereas the 24h-HI cells showed an apoptosis response due to a strong dose dependent effect33, this was not the case with 24h-LO cells. We therefore focused mainly on 24h-LO and 48h cells (Fig S1b, Fig S2).
BLIMP1 in P19EC cells induced gene repression including mesendodermal factor Eomes, HoxA5, Evx1, Myc, and of de novo DNA methyltransferase, Dnmt3b (Fig S1b), which are amongst the key responses observed in PGCs2,34. Importantly, PGC genes, Nanos3, and Rhox9 were induced. By lowering the statistical threshold to FDR ≤ 0.05, we detected an induction of Dppa3/Stella, and Tcfap2c (encoding AP2γ ), (Fig S1b, 1b, Table S1). Furthermore, RT-qPCR revealed an induction of Dnd1 and Prdm14 at 48h, and PGC markers, Dazl and Ddx4 (Fig. 1b). While Oct4/Pou5f1 expression continued (Table S1), we noted repression of Nanog, which could explain the induction of Gata4 and FoxQ135 (Fig S1b and Table S1). Overall and in important respects, the response of P19EC to BLIMP1 approximates that seen during PGC specification in vivo.
We then looked at the effects of all three factors in P19EC cells following stable expression of PRDM14 and AP2γ, both individually and together. We transfected control cells and the stable lines with BLIMP1-EGFP or EGFP alone for 24 hours and examined sorted fluorescent cells. This showed repression of Eomes and T-Brachyury, while PRDM14 alone suppressed Dnmt3b (Fig 1c), its direct target36. While BLIMP1 repressed Myc, PRDM14 in combination with AP2γ modestly induced Myc expression, an effect that was overcome by BLIMP1 expression. Thus, repression of somatic regulators is complex, and may not be attributable to BLIMP1 alone.
The induction of PGC genes revealed co-operative effects of AP2γ and PRDM14, which induced Nanos3 and Prdm1 (encoding BLIMP1), with a modest induction of Ddx4. While Nanos3 induction was attenuated by BLIMP1, Dnd1 was induced by 15-fold when all three factors were present, but Dppa3 was strictly PRDM14-dependent (Fig 1c). These observations show that PRDM14 and AP2γ cooperatively induce the germ cell programme, with the additional effect of BLIMP1 on Dnd1 induction.
The analysis of P19ECs shows a response to BLIMP1, PRDM14 and AP2γ individually and collectively, with features that are pertinent to PGCs, including the repression of somatic genes and induction of PGC genes. We posit that P19EC cells are appropriate for the identification of direct targets of the three key determinants of PGC specification.
Identification of BLIMP1 targets and their relevance for PGC specification
To identify BLIMP1 targets, we transfected P19ECs with BLIMP1-EGFP, followed by ChIP sequencing (ChIPseq) using an EGFP antibody. This revealed 5046 BLIMP1 high-confidence binding peaks for 4389 protein coding and 313 non-coding genes (Table S2 and S3), including 8/11 known targets such as Myc and Id3 (Fig 2a). We observed a peak distribution with a median position of +171.5 bp relative to transcriptional start sites (TSS) (Fig. 2b,c) consistent with BLIMP1 binding on promoters and an enrichment of the previously characterized consensus binding sequence for BLIMP137 (p-value =1.1*10−388) (Fig. 2d). Notably, BLIMP1 bound to T-Brachyury, Eomes and the entire Hox gene loci (Fig. 2e and Table S3) reflecting its role in PGC specification in vivo7,9. Functional category analysis revealed a striking enrichment of BLIMP1 binding to genes encoding transcriptional regulators and of genes regulating developmental processes (Fig 2f). Moreover, BLIMP1 was bound toTcfap2c (encoding AP2γ), which is induced in PGCs (see later).
Figure 2. BLIMP1 binding to gene promoters encoding transcription factors, cell cycle and developmental regulators.
(a) Track-view of BLIMP1 ChIP-Seq density profile displayed using the UCSC genome browser centred on the Id3 and Myc genes. (b). BLIMP1 peaks distributed roughly evenly between inter- and intragenic positions, with 1248 of 2480 intergenic peaks falling within 1kb 5′ of TSS, and 527 peaks were more than 10Kb away from promoters. (TSS: transcriptional start site; TES: transcriptional end site). (c) Distribution of BLIMP1 binding relative to promoters revealing a median distance of +171.5 bp from the TSS. (d) De novo motif analysis revealed high enrichment for the BLIMP1 consensus (p-value of 1.2×10−388). (e). BLIMP1 binding profiles on Hox gene clusters are indicated in the views with their respective numbers. For example, Hoxa1 is indicated by a1, Hoxa2 is a2 and so forth. (f). Functional categories of genes bound by BLIMP1 showing the p-value for the molecular function as well as biological process GO-terms. (g). Validation of novel BLIMP1 binding regions by ChIP-qPCR in P19 EC cells. (h). A validation of novel BLIMP1 binding regions in PGCLCs by ChIP followed by whole genome amplification of precipitated and input DNA assayed by qPCR. The y-axis represents the % of signal from amplified input material at the same starting quantity of the immunoprecipitated DNA to ensure proportional amplification.
We validated Myc and several novel BLIMP1 bound regions by ChIP-qPCR in P19ECs (Fig 2g). We also validated BLIMP1 binding to Eomes, Dnmt3b, HoxB2 and Myc in PGC-like cells (PGCLCs) generated in vitro38 with a ChIP grade BLIMP1 antibody37(Fig 2h), but comprehensive analysis in PGCLCs was technically not feasible owing to limited amounts of precipitated DNA that could be generated.
To determine the significance of BLIMP1 targets, we scrutinised transcriptional changes in wild type and Prdm1 (BLIMP1) mutant PGCs from E6.25-E8.5 embryos, including E7.5 somatic neighbours, which share a common ancestry (Fig 3a). For this, we performed single-cell RNA-Seq analysis and found that all three factors; Prdm1 (encoding BLIMP1), Tcfap2c (encoding AP2γ), and Prdm14 were fully induced by E6.5 in putative PGCs, with extensive repression of somatic and cell cycle regulators, and induction of the PGC program7,2 (Fig 3b,c, S3a,b, Table S4). By contrast, we detected expression of somatic genes and a lack of expression of some germ cell genes in Prdm1 mutant cells (Fig 3b,c). Overall, the E7.5 single cell expression profiles of Prdm1 mutant cells clustered with E7.5 somatic cells and not with PGCs (Fig 3a).
We then carried out a global assessment of BLIMP1 bound genes in relation to the differentially expressed genes in PGCs (compared to the binding to the whole set of expressed genes). Indeed, BLIMP1 was bound to both repressed (Brachyury and Dnmt3b), and induced (Cbx7) genes. Notably, BLIMP1 bound to Tcfap2c (encoding AP2γ), which is induced (Fig. 3c,d and see below). We calculated the binding scores for reads both inside and outside of peak regions and their distance to promoters (see methods), as well as the defined peak regions. This revealed that the repressed genes in E7.5-E8.5 PGCs compared to either somatic cells or Prdm1 (BLIMP1) mutant cells, are enriched for BLIMP1 (Fig 4a). The repressed genes that were bound by BLIMP1 had a greater enrichment for developmental, transcriptional and Wnt-signalling function compared to the whole set of repressed genes (Fig S3a). Since misregulated gene expression in the Prdm1 mutant cells from in vivo is a direct consequence of the lack of BLIMP1 in these cells, this result further shows the functional relevance of our analysis to the processes occurring during PGC specification in vivo.
Figure 4. BLIMP1 represses the majority of its direct targets.
(a). Relative enrichment of BLIMP1 binding regions and the scores associated with genes differentially expressed between E7.5 PGCs and BLIMP1 mutant (KO) cells, and between E7.5 PGCs and E7.5 somatic cells, respectively. The x-axes indicate the log2 (fold change) and the y-axes indicate the log2 of the BLIMP1 target enrichment at each fold change-interval of differentially expressed genes over the average target frequency of the whole expression data set. (Peaks: the enrichment of peaks associated with genes in each interval at E7.5. Scores; the enrichment of binding scores calculated for genes in each interval at E7.5). (b). Binding frequency of BLIMP1 to genes associated with features on the whole microarray as well as differentially regulated genes (c). Heat map depicting the genes repressed by BLIMP1 in both P19ECs and during PGC specification. The first 4 (blue) columns refer to normalized gene expression levels in PGCs. The next 3 columns (red) reflect values of differential expression between the samples indicated. The asterisks in the final column indicate a high confidence BLIMP1 binding region associated with a gene. BLIMP1 binding was detected in 34/59 repressed genes in both data sets. (RPM: reads per million. FC: fold change).
Comparing the ChIPseq data with the BLIMP1-induced changes in P19ECs revealed a predominant effect on repression of direct targets. Whereas BLIMP1 bound with 24.1% of the genes in the genome (Fig. 4b, Whole Array), nearly 50% of the repressed genes were bound by BLIMP1, but binding to induced genes was close to background (Fig.4b). We further observed a striking enrichment of BLIMP1 binding on repressed genes in both P19ECs as well as in E7.5 PGCs compared to soma (Fig 4c and S3e) where 34/59 repressed genes were bound by BLIMP1. This is statistically a very high over-representation, (chi-square p-value = 1.8×10−10), which shows conclusively that the dominant effect of BLIMP1 in PGC specification is gene repression, including those required for the somatic fate.
AP2γ binds to germ cell genes and somatic regulators
Toward building a genetic network for PGC specification, we performed an unbiased scan of the BLIMP1 binding regions for transcription factor binding motifs (Fig. S4a). We found a bimodal distribution of AP2 family motifs surrounding the BLIMP1 peak (Fig S4b). Similarly, PRDM14 binding sites were also highly enriched for AP2 motifs36 (Fig. S4c,d). We therefore mapped PRDM14 and BLIMP1 binding sites to all the genes in the PGC transcriptome, and then found AP2γ motifs within the binding regions39–41. This revealed a preferential enrichment of genes regulated during PGC specification that were associated with BLIMP1 and PRDM14 binding sites that contained AP2γ motifs (Fig S4e). This strongly implies that the three factors cooperate both in gene induction and repression in PGCs. This prompted us to carry out ChIPseq analysis for AP2γ.
With P19ECs stably expressing AP2γ, we performed ChIPseq with a previously validated antibody41, and identified 3191 high confidence AP2γ binding regions that map to 1393 genes (Table S5 and S6). The peaks were enriched with the AP2 consensus motif (Fig 5a) (p-value =1.1×10−241). AP2γ binding was centred on promoters at a median position of –53bp relative to TSS, albeit the peak distribution was much broader than that of BLIMP1 (Fig 5b), perhaps implying binding to gene-distal sequence elements. Importantly, the Pou5f1 distal enhancer which is bound by PRDM14 and pluripotency transcription factors, was amongst the most strongly bound regions (Fig 5c)36,42,43. Notably, Pou5f1 expression in the post implantation epiblast and P19ECs is driven from the proximal enhancer, while the distal enhancer is utilised following PGC specification. Several somatic genes were also bound by AP2γ, including Hoxa11, HoxB13 and T-Brachyury, and regions distal to the TSS of Nanos3 and the first intron of Dppa3 (Fig 5c, d, Table S6).
Figure 5. AP2γ binds to germ cell genes and somatic regulators.
(a). De novo motif analysis revealed high enrichment for the AP2γ consensus binding site with a p-value of 1.1×10−241. (b). Distribution of AP2γ binding relative to promoters revealing a mean distance of +53 bp from the TSS. (c). Track-view of AP2γ binding on the Pou5f1 distal enhancer, to the PGC genes Dppa3 and Nanos3, and the somatic differentiation regulators HoxA10, HoxA11, Hes7 and T-Brachyury. (d). A ChIP-qPCR of AP2γ binding to the promoter of Nanos3, Dppa3/Stella as well as the distal enhancer of Oct4 (Oct4-DE). (e). Over represented gene categories sorted by cell-type specific expression at different Theiler Stages (TS) during embryonic development, TS8 corresponds roughly to E6.0, TS17 to E10.5, TS20 to E17 and TS21 to E21/P0. (f). Over represented functional categories of genes bound by AP2γ showing the p-value for the enrichment of biological process GO-terms.
Analysis of functional categories of targets revealed highly significant binding to PGC genes (e.g. Nanog, Dppa3 and Pou5f1), and E6.0 epiblast (e.g. Activin and FGF receptor genes Acvr1b, Fgfr1 and Fgfr2). This is consistent with AP2γ being involved in PGC specification from epiblast cells (Fig 5e,f Table S6). Furthermore, AP2γ was enriched for genes involved in morphogenesis and development, indicating a relevance to PGCs and somatic gene repression.
Transcriptional network for PGC specification
Next, we combined all available information for a detailed scrutiny of how the key regulators induce PGCs. With respect to gene expression in PGCs versus soma at E7.5, AP2γ was significantly enriched on both induced and repressed genes (Fig 6a), as confirmed by the hypergeometric distribution statistical test (Fig 6b). The repressed genes were also co-bound by BLIMP1-AP2γ, and by PRDM14-AP2γ, and co-binding of all three factors showed enhanced enrichment with increased degree of repression (Fig 6a, left panel), more so than with either PRDM14 or BLIMP1 alone. By contrast, the induced genes were preferentially enriched for PRDM14 and AP2γ together (Fig 6a,b right panels), where the enrichment of BLIMP1 alone or in combinations was statistically not significant (Fig 6b). While AP2γ clearly has a significant impact on gene expression genes bound by AP2γ alone were depleted of association with specific functional gene categories (Fig S5,6), unless co-bound by BLIMP1 and/or PRDM14. Hence, co-binding of BLIMP1 with the other factors mediates repression, whereas AP2γ and PRDM14 co-binding leads to gene induction, suggesting a high degree of co-regulation by these factors. Statistical testing of the overlap of binding sites for the three factors (p < 0.0001 permutation test) (Fig 6c and S8a) and the overlap of genes bound by the three factors (p < 1×10−299, chi-square test) (Fig 6d and S8b) further indicates an enrichment of co-bound genes over what would be expected by random chance, further supporting the functional relationship between the three factors.
Figure 6. A transcription factor network for PGC specification.
(a). Relative enrichment of BLIMP1, AP2γ and PRDM14 targets on differentially expressed genes between E7.5 PGCs and soma, and the combinatorial association of the peak regions to the differentially expressed genes. (b).The plots depict the hypergeometric p-values for the corresponding enrichment of BLIMP1, AP2γ and Prdm14 targets on differentially expressed genes shown in Fig 6a. (c). Venn diagrams showing the total number of genomic target sites overlapping by one base-pair or more between BLIMP1, AP2γ and PRDM14. The p-value for the enrichment of overlap between the factors of p < 0.0001 was calculated using a permutation test. See details in Fig. S8a. (d) Venn diagrams showing the total number of genes overlapping between BLIMP1, AP2γ and PRDM14. The p-value of p < 1×10−299 was calculated using a ch-square test. See details in Fig S8b (e). Track-view of BLIMP1, PRDM14 and AP2γ binding to selected repressed and induced PGC targets. (f). A transcriptional network model depicting the role of PRDM14, BLIMP1 and AP2γ during PGC specification.
BLIMP1, AP2γ and PRDM14 co-binding on repressed genes included developmental and signalling genes, particularly those of Fgf and Wnt signalling. BLIMP1 was preferentially bound to proliferation genes, and PRDM14 and AP2γ to cell-motility and cytoskeleton organization genes (Fig S5a,S6a), indicating initiation of PGC migration. All three factors are over-represented on induced genes involved in actin cytoskeleton organization and intracellular signalling cascades (Fig S5b,S6b), although PRDM14, either alone or with AP2γ, is predominant over BLIMP1. Critically, PRDM14 binds to PGC specific genes, including Tcfap2c (encoding AP2γ) , Sox2 and Kit as well as Dnd1, Nanos3, Dppa3, Prdm1 (encoding BLIMP1) and Prdm14 (Fig 6e, S6b). Furthermore, AP2γ binds to Dppa3 as well as Nanos3, and acts cooperatively with PRDM14 in the induction of Nanos3 (Fig 1c, 6e and S6b). All three factors are involved in the induction of Dnd1 (Fig 1c). Thus, after the induction of AP2γ by BLIMP1, it cooperates with PRDM14 to induce PGC genes.
In the context of a compendium of transcription factors in mESCs44, BLIMP1 clustered predominantly with self-renewal and polycomb factors, consistent with somatic gene repression (Fig 7 and S7). PRDM14 clustered with pluripotency transcription factors, but AP2γ associated weakly with factors in the compendium (Fig S7). This underlines the context-dependent combinatorial action of these three factors, which as expected is not fully captured in comparison to mESCs factors.
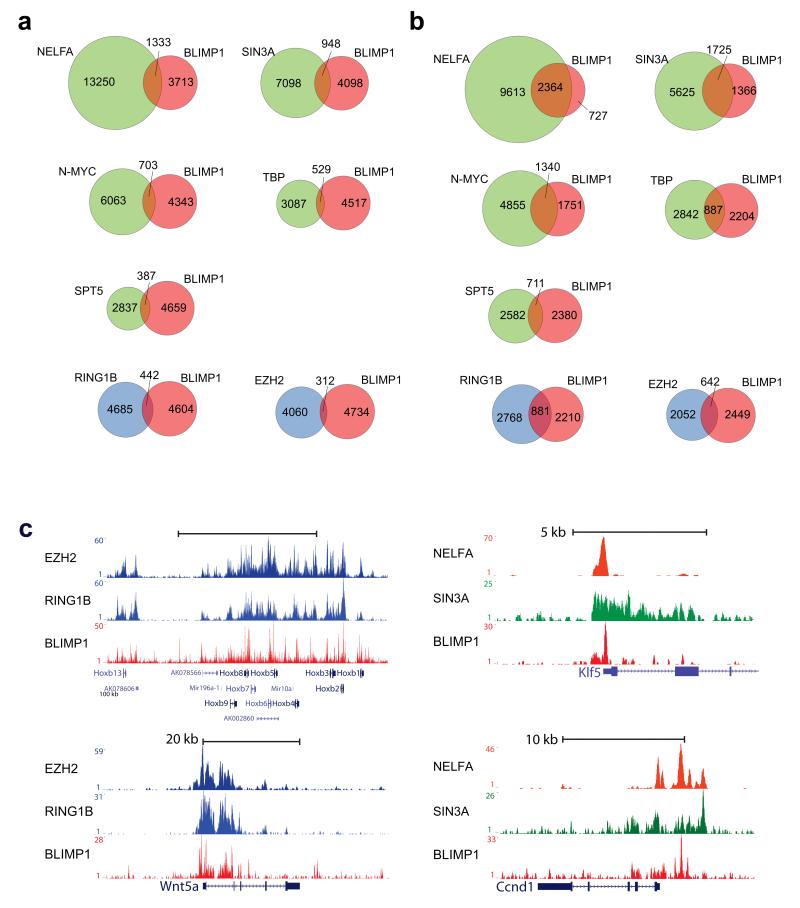
Figure 7. BLIMP1 binds to targets of mESC self-renewal regulators and Polycomb proteins.
(a). Venn diagrams showing the total number of target sites overlapping by one base-pair or more between BLIMP1 on one hand and the indicated transcription factors on the other. (b) Venn diagrams showing the total number of genes overlapping between BLIMP1 on one hand and indicated transcription factors on the other hand. In (a) and (b), the circles for the “self-renewal” cluster genes are indicated in green and the “polycomb” cluster genes in blue (c) Track-view of BLIMP1 binding on example genomic loci including the views for EZH2, RING1B, NELFA as well as SIN3A where appropriate.
A key role of the transcriptional network is in initiating epigenetic reprogramming in PGCs. Consistently, BLIMP1 and PRDM14 repress Kdm6b encoding a H3K27 demethylase, while PRDM14 mediates the induction of the H3K9 demethylase Kdm4b, and together with BLIMP1, induces Kdm3a (Fig S5a,b). This ensures the erasure of H3K9me2 that is prerequisite for global DNA demethylation45. The two factors also repress de novo DNA methyltransferase, Dnmt3b as well as Uhrf1, encoding an accessory protein for the maintenance DNA methyltransferase, DNMT1; this facilitates global DNA demethylation in PGCs (Fig S6a). BLIMP1 and PRDM14 each bind to one of the two promoters to repress Uhrf1 in PGCs (Fig 6e), while PRDM14 alone in mESC is insufficient to do so46. The regulation of histone- and DNA methylases links PGC specification to the dynamic and genome wide epigenetic reprogramming47. BLIMP1 and PRDM14 bind extensively to differentially expressed genes during PGC specification, while AP2γ binds to a subset of them and perhaps acts as a facilitator of key events, except perhaps for chromatin modifications
The proposed transcriptional network is interdependent, since Tcfap2c (encoding AP2γ) expression is dependent on BLIMP1. Expression of Prdm14 and Prdm1 (encoding BLIMP1) expression is mutually interdependent as revealed by genetic experiments7,27. PRDM14 also binds Tcfap2c and could enhance Tcfap2c expression after it’s induction by BLIMP1. This supports an obligatory functional relationship between BLIMP1, PRDM14 and AP2γ as critical regulators of PGC specification.
Direct induction of PGC-like fate by BLIMP1, AP2γ and PRDM14 in vitro
Next we asked whether BLIMP1, AP2γ and PRDM14 are sufficient to directly induce PGC fate and used the in vitro induction of PGCLCs to test this premise29. PGCLCs can be induced in response to cytokines (Fig 8a, left panel), which we observed in 47.4-52.6% of the cells after 4 days. Using the same reporter cells harbouring doxycycline inducible constructs for the three transcription factors, we observed induction of PGCLCs in the absence of cytokines in ~45-60% of the cells (Fig 8a, right panel). Both the overall response and transcriptional analysis by qPCR using the sorted fluorescent PGCLCs were remarkably similar to those induced by cytokines with respect to the key PGC, epigenetic and somatic cell regulators. The response to the transcription factors was slightly enhanced, (Fig 8b and S8c) as reflected in the higher induction of Dppa3 and Nanos3, and a more pronounced repression of HoxB1 and T-Brachyury. This observation establishes the principle that the proposed transcriptional network delineating specific and combined functions of BLIMP1, AP2γ and PRDM14 accounts for the necessary gene expression changes for PGC specification. Further work in the future will advance our knowledge of how these factors, both individually and in combination, induce PGC fate.
Figure 8. Co-expression of BLIMP1, AP2γ and PRDM14 induces PGC-like cell fate in vitro.
(a). Bright-field and fluorescent microscopy images of the Oct4 reporter ESC line (GOF) in culture. The images show ESCs, EpiLCs (stage preceding PGCLC), and PGCLCs as indicated. The left panels show the induction of PGCLCs using cytokines, and the right panels show the induction of PGCLCs without cytokines using doxycycline dependent induction of BLIMP1, AP2γ and PRDM14 from GOF cells harbouring stable doxycycline-responsive constructs. The numbers on the figure panels indicate the ratio of fluorescent cells induced at each time-point. (b). RT-qPCR analysis of sorted fluorescent PGCLCs on Day2 and 4 of either cytokine or doxycycline induction, as well as EpiLCs. Panels (a and b) show a representative of two identical experiments.
DISCUSSION
We present a comprehensive examination of the initiation of PGC specification by the combinatorial roles of BLIMP1, AP2γ and PRDM14, leading to a unique epigenetic program culminating in the epigenetic basal state48,49. Co-expression of the three factors is by itself apparently sufficient to induce PGC-like fate in the absence of cytokines, and supports the proposed tripartite genetic network for PGC specification.
BLIMP1, PRDM14 and AP2γ contribute to the repression of mesodermal genes in PGCs to set them apart from their somatic neighbours; until then, these cells are indistinguishable from each other. BLIMP1 has a dominant function in this respect, which is reinforced by PRDM14 and AP2γ. By contrast, PRDM14 and AP2γ together are associated with gene induction. Notably, Tcfapc2 (encoding AP2γ) is a direct target of BLIMP1 and induced by it in P19EC cells in vitro, and probably maintained thereafter by PRDM14 in PGCs. Tcfap2c fails to be induced in BLIMP1 mutant PGC-like cells in vivo and its induction by BLIMP1 is perhaps the vital link for the initiation of the PGC-specific genes.
The high overlap of AP2γ targets with that of BLIMP1 and PRDM14 implies that it cooperates, augments or otherwise modulates the response of a subset of the targets. Furthermore, the distinct and predominant binding of BLIMP1 to promoter regions as opposed to gene-distal regulatory regions binding noted for PRDM1450, suggests parallel mechanisms in regulating transcription. The collaborative role of AP2γ is also reflected in its broad distribution that is centred on promoters, but potentially, encompasses distal elements.
This comprehensive insight on PGC specification in mice, may facilitate investigations on germ cells in other mammals, including humans, and enhance an understanding of context dependent functions of transcription factors. For example, AP2γ has a role in trophectoderm differentiation in conjunction with CDX2 and EOMES, whereas it participates in the repression of Eomes in PGCs. BLIMP1 also drives cell fate commitment in several different lineages, while PRDM14 is crucial for pluripotency in ES cells50–52. These differences are presumably linked to the molecular control of competence, which precedes and ‘anticipates’ specific cell fate decisions.
A fundamental property of early germ cells are the unique epigenetic changes that ensue following PGC specification, leading to global erasure of DNA methylation and acquisition of a basal epigenetic state. The mechanism that regulates this unique resetting of the epigenome in germ cells could in principle be extended towards approaches for modifying epigenetic states of normal and diseased tissues. This study may help towards achieving wider objects of general interest in the field of regenerative medicine.
Supplementary Material
Table S1. Microarray Expression Data. This table shows the genome wide differential gene expression in P19EC cells upon transient transfection of BLIMP1. The first four columns show the Entrez Gene ID, the gene symbol and the Illumina Probe ID as well as the gene definition. The next 3 columns give the false discovery rate (FDR) for each comparison and the last 3 columns show the log2 fold change (LFC) for each comparison.
Table S2. BLIMP1 Peaks. A list of the high-confidence binding regions of BLIMP1.
Table S5. AP2γ Peaks. A list of the high-confidence binding regions for AP2γ.
Table S6. AP2γ Peaks With Gene Annotation. Gene annotations for the high-confidence binding regions of AP2γ.
Table S7. Primer Sequences. The table shows the primer sequences of primers used for RT-qPCR as well as ChIP-qPCR experiments in 3′ to 5′ direction.
Table S3. BLIMP1 Peaks with Gene Annotation. Gene annotations for the high-confidence binding regions of BLIMP1.
Table S4. Single Cell PGC RNAseq Expression Data. This table shows the genome wide differential gene expression in nascent PGCs (E6.5, E7.5 and E8.5) and somatic neighbours (E7.5 soma) as well as Blimp1 null PGC-like cells (E7.5 Blimp1KO), assayed by single cell RNAseq. The first column shows the RefSeqID, the second column the gene symbol, the third column shows the gene localization and strand. The values indicate the expression levels in log2(reads pr. million).
Figure S1. Expression profiling of P19ECs with ectopic expression of BLIMP1. (a). Flow cytometric plot showing the fluorescence intensity of EGFP and BLIMP1-EGFP transfected cells. Gates employed for cell sorting are indicated. (b) Heat map showing differentially regulated genes in P19ECs upon BLIMP1 expression. Each column represents a time-point assayed in triplicate. The colours indicate the z-score for differential expression. (c). Gene ontology analysis of the top 10% of genes repressed and induced upon BLIMP1 expression in P19ECs.
Figure S2.Functional categories of differentially expressed genes upon BLIMP1 expression in P19ECs. (a-f). Gene ontology analysis of all genes with significant expression changes (FDR < 0.005) in P19ECs upon BLIMP1 expression for both induced and repressed genes for each of the 3 comparisons performed.
Figure S3. RNAseq analysis during PGC specification; integrative analysis of PGC transcriptome, BLIMP1 induced changes in P19EC profiles, and BLIMP1targets. (a and b). Gene ontology analysis showing functional categories of genes repressed and induced respectively, between E7.5 PGCs and somatic cells and the genes from the comparison that are bound by BLIMP1. (c). Relative enrichment of BLIMP1 binding regions and the scores associated with genes differentially expressed between E8.5 PGCs and E7.5 Prdm1 (encoding BLIMP1)-KO PGC–like cells. (d). Correlation analysis of differentially expressed genes during PGC specification and upon BLIMP1 expression in P19ECs. The Pearson correlation coefficients are indicated in the bottom half for each pair-wise comparison and each point on the plot indicates the differential expression of a gene in the comparisons indicated on the x- and y-axes respectively by the juxtaposition to the squares along the diagonal. (e). Relative enrichment of BLIMP1 binding regions associated with genes that are differentially expressed both upon BLIMP1 expression in P19ECs as well as during PGC specification. The x-axis indicates the log2 (fold change) and the y-axis indicates the log2 of the BLIMP1 target enrichment at each fold change-interval of differentially expressed genes over the average target frequency of the whole expression data set. Peaks: the enrichment of peaks associated with genes in each interval differential expression expression level interval Scores; the enrichment of binding scores calculated for genes in each interval. Intersect: The enrichment of peaks associated with genes differentially expressed in both comparisons, in each interval of differential expression. (f). ChIP sequencing tracks from the UCSC browser of genes showing example genomic loci of genes bound by BLIMP1 and repressed in both PGCs and P19ECs upon BLIMP1 expression.
Figure S4. AP2γ motif analysis on BLIMP1 and PRDM14 binding regions. (a). TRANSFAC motif scanning of the BLIMP1 binding regions. The enrichment scores and p-values for the enrichment of each motif are indicated. (b). The distribution of the BLIMP1, AP2 alpha- and the AP2γ motifs on the BLIMP1 binding regions. (c). TRANSFAC motif scanning of the PRDM14 binding regions. d). The distribution of the PRDM14 motif, AP2 alpha and AP2γ motifs around the centre of the binding regions. (e). Relative enrichment of BLIMP1, and PRDM14 targets on differentially expressed genes between E7.5 soma and PGCs filtered by the association of an AP2γ motif in the peak region and the combinatorial association of the peak regions to the differentially expressed genes.
Figure S5. GO-Term clustering of genes differentially associated with BLIMP1, AP2γ and Prdm14 during PGC specification. Clustering analysis of the GO-terms associated with genes that are repressed (a). or induced (b). in PGCs compared to neighbouring somatic cells at E7.5 and bound by different combinations of either BLIMP1, AP2γ or Prdm14. The dark blue colour indicates that fewer than 4 genes were mapped to the indicated GO-term.
Figure S6. A transcription factor network for PGC specification. BLIMP1, AP2γ and Prdm14 binding to differentially expressed genes at E7.5 between PGCs and soma. (a). Repressed genes. (b). Induced genes. The yellow nodes indicate the BLIMP1, PRDM14 and AP2γ peak and associations. The smaller nodes indicate genes bound by the factors, with the binding associations indicated by lines connected to the yellow nodes. The colours of the genenodes indicate functional categories as shown.
Figure S7. A compendium of mESC transcription factor integrated profiles. (a). A full hierarchical clustering analysis of the genome-wide BLIMP1 and AP2γ binding patterns together with binding patterns of transcriptional regulators from mESCs. The red lines above the heat-maps indicate the main clusters, showing the pluripotency cluster (Oct4 etc), polycomb-cluster (Ring1b etc), self-renewal/proliferation cluster (N-Myc etc), and a genome-architectural cluster (CTCF etc). The combinatorial binding pattern analysis was performed by generating a unified data matrix based on 165,607 unique peak regions, indicating for each factor whether it was bound or not. Subsequently the hierarchical clustering and Pearson’s correlation coefficients shown in the heat map were used to investigate global relationships. The colours indicate level of correlation for all pairwise comparisons as indicated on the figure. Note that BLIMP1 associates most strongly with the self-renewal cluster and has high correlation with polycomb factors. PRDM14 associates with the pluripotency cluster whereas AP2γ binding does not correlate highly with mESC transcriptional regulators.
Figure S8. Statistical testing for binding overlap between BLIMP1, Ap2γ and PRDM14 and co-expression of BLIMP1, AP2γ and PRDM14 induces PGC-like cell fate in vitro. (a). Number of observed and expected overlap in genomic binding sites of BLIMP1, AP2γ and PRDM14, and a scatterplot showing the observed against expected overlap in genomic binding sites of: 1. AP2γ and BLIMP1, 2. BLIMP1 and PRDM14, and 3. AP2γ and PRDM14. The p-value for the enrichment of overlap in binding sites is p < 0.0001 for all comparisons. (b). A contingency table for the calculation of a chi-square p-value for the overlap of genes bound by different combinations of BLIMP1, AP2g and PRDM14. The calculated p-value based on this table was p < 1×10−299. The total number of genes (34274) represents the number of unique gene identifiers in Ensembl that were a basis for the gene annotation of the respective transcription factor binding sites. (c). RT-qPCR analysis of sorted fluorescent PGCLCs on Day2 and 4 of either cytokine or doxycycline induction, as well as EpiLCs. The experiment is the second of two experiments performed. The first experiment is shown in Figure 8.
ACKNOWLEDGEMENTS
We thank Nigel Miller for flow-cytometry, and Matthew Trotter, Charles Bradshaw and George Allen for bioinformatics analysis. We thank Roopsha Sengupta and Jamie Hackett for critically reading the manuscript.This work was supported by grants from the Wellcome Trust and HFSP to M.A.S and the ERC to E.M.
REFERENCES
- 1.Fuhrmann G, et al. Mouse germline restriction of Oct4 expression by germ cell nuclear factor. Dev Cell. 2001;1:377–387. doi: 10.1016/s1534-5807(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 2.Seki Y, et al. Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Developmental Biology. 2005;278:440–458. doi: 10.1016/j.ydbio.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Borgel J, et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nature genetics. 2010;42:1093–100. doi: 10.1038/ng.708. [DOI] [PubMed] [Google Scholar]
- 4.McLaren A, Lawson KA. How is the mouse germ-cell lineage established? Differentiation; research in biological diversity. 2005;73:435–7. doi: 10.1111/j.1432-0436.2005.00049.x. [DOI] [PubMed] [Google Scholar]
- 5.Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418:293–300. doi: 10.1038/nature00927. [DOI] [PubMed] [Google Scholar]
- 6.Yamaji M, et al. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat Genet. 2008;40:1016–1022. doi: 10.1038/ng.186. [DOI] [PubMed] [Google Scholar]
- 7.Kurimoto K, et al. Complex genome-wide transcription dynamics orchestrated by Blimp1 for the specification of the germ cell lineage in mice. Genes & development. 2008;22:1617–35. doi: 10.1101/gad.1649908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson EJ, et al. Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, heart and sensory vibrissae in mice. Development (Cambridge, England) 2007;134:4335–45. doi: 10.1242/dev.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohinata Y, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- 10.Vincent SD, et al. The zinc finger transcriptional repressor Blimp1/Prdm1 is dispensable for early axis formation but is required for specification of primordial germ cells in the mouse. Development (Cambridge, England) 2005;132:1315–25. doi: 10.1242/dev.01711. [DOI] [PubMed] [Google Scholar]
- 11.Saitou M, Payer B, O’Carroll D, Ohinata Y, Surani MA. Blimp1 and the emergence of the germ line during development in the mouse. Cell Cycle. 2005;4:1736–1740. doi: 10.4161/cc.4.12.2209. [DOI] [PubMed] [Google Scholar]
- 12.Turner CA, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 13.Keller AD, Maniatis T. Identification and characterization of a novel repressor of beta-interferon gene expression. Genes & Development. 1991;5:868–879. doi: 10.1101/gad.5.5.868. [DOI] [PubMed] [Google Scholar]
- 14.Morgan M. a J., et al. Blimp-1/Prdm1 alternative promoter usage during mouse development and plasma cell differentiation. Molecular and cellular biology. 2009;29:5813–27. doi: 10.1128/MCB.00670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martins GA, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nature immunology. 2006;7:457–65. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 16.Kallies A, et al. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nature immunology. 2006;7:466–74. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- 17.Chan Y-H, et al. Absence of the transcriptional repressor Blimp-1 in hematopoietic lineages reveals its role in dendritic cell homeostatic development and function. Journal of immunology (Baltimore, Md. : 1950) 2009;183:7039–46. doi: 10.4049/jimmunol.0901543. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa K, et al. Blimp1-mediated repression of negative regulators is required for osteoclast differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3117–22. doi: 10.1073/pnas.0912779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang DH, Angelin-Duclos C, Calame K. BLIMP-1: trigger for differentiation of myeloid lineage. Nature immunology. 2000;1:169–76. doi: 10.1038/77861. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B. Tolerogenic function of Blimp-1 in dendritic cells. The Journal of experimental medicine. 2011;208:2193–2199. doi: 10.1084/jem.20110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Angelin-Duclos C, Greenwood J, Liao J, Calame K. Transcriptional Repression by Blimp-1 (PRDI-BF1) Involves Recruitment of Histone Deacetylase. Molecular and Cellular Biology. 2000;20:2592–2603. doi: 10.1128/mcb.20.7.2592-2603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren B, Chee KJ, Kim TH, Maniatis T. PRDI-BF1/Blimp-1 repression is mediated by corepressors of the Groucho family of proteins. Genes & Development. 1999;13:125–137. doi: 10.1101/gad.13.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gyory I, Wu J, Fejér G, Seto E, Wright KL. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nature immunology. 2004;5:299–308. doi: 10.1038/ni1046. [DOI] [PubMed] [Google Scholar]
- 24.Ancelin K, et al. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol. 2006;8:623–630. doi: 10.1038/ncb1413. [DOI] [PubMed] [Google Scholar]
- 25.Su S-T, et al. Involvement of histone demethylase LSD1 in Blimp-1-mediated gene repression during plasma cell differentiation. Molecular and cellular biology. 2009;29:1421–31. doi: 10.1128/MCB.01158-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cretney E, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 27.Yamaji M, et al. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nature Genetics. 2008;40:1016–1022. doi: 10.1038/ng.186. [DOI] [PubMed] [Google Scholar]
- 28.Weber S, et al. Critical function of AP-2 gamma/TCFAP2C in mouse embryonic germ cell maintenance. Biology of reproduction. 2010;82:214–23. doi: 10.1095/biolreprod.109.078717. [DOI] [PubMed] [Google Scholar]
- 29.Hackett J. a, Zylicz JJ, Surani MA. Parallel mechanisms of epigenetic reprogramming in the germline. Trends in genetics : TIG 1–11. 2012 doi: 10.1016/j.tig.2012.01.005. doi:10.1016/j.tig.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 30.MCBURNEY M. Isolation of male embryonal carcinoma cells and their chromosome replication patterns*1. Developmental Biology. 1982;89:503–508. doi: 10.1016/0012-1606(82)90338-4. [DOI] [PubMed] [Google Scholar]
- 31.Yeom YI, et al. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development (Cambridge, England) 1996;122:881–94. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- 32.Chambers I, et al. Functional Expression Cloning of Nanog, a Pluripotency Sustaining Factor in Embryonic Stem Cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 33.Ancelin K, et al. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol. 2006;8:623–630. doi: 10.1038/ncb1413. [DOI] [PubMed] [Google Scholar]
- 34.Yabuta Y, Kurimoto K, Ohinata Y, Seki Y, Saitou M. Gene Expression Dynamics During Germline Specification in Mice Identified by Quantitative Single-Cell Gene Expression Profiling. Biol Reprod. 2006;75:705–716. doi: 10.1095/biolreprod.106.053686. [DOI] [PubMed] [Google Scholar]
- 35.Mitsui K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–42. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 36.Ma Z, Swigut T, Valouev A, Rada-Iglesias A, Wysocka J. Sequence-specific regulator Prdm14 safeguards mouse ESCs from entering extraembryonic endoderm fates. Nature structural & molecular biology. 2011;18:120–7. doi: 10.1038/nsmb.2000. [DOI] [PubMed] [Google Scholar]
- 37.Kuo TC, Calame KL. B lymphocyte-induced maturation protein (Blimp)-1, IFN regulatory factor (IRF)-1, and IRF-2 can bind to the same regulatory sites. J Immunol. 2004;173:5556–5563. doi: 10.4049/jimmunol.173.9.5556. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146:519–32. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 39.Woodfield GW, Chen Y, Bair TB, Domann FE, Weigel RJ. Identification of Primary Gene Targets of TFAP2C in Hormone Responsive Breast Carcinoma Cells. 2010;962:948–962. doi: 10.1002/gcc.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan SK, et al. AP-2γ regulates oestrogen receptor-mediated long-range chromatin interaction and gene transcription. The EMBO journal. 2011;30:2569–81. doi: 10.1038/emboj.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kidder BL, Palmer S. Examination of transcriptional networks reveals an important role for TCFAP2C, SMARCA4, and EOMES in trophoblast stem cell maintenance. Genome research. 2010;20:458–72. doi: 10.1101/gr.101469.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loh YH, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–17. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 44.Sugimoto Toshimi, Diamanti Evangelia, Joshi Anagha, Hannah Rebecca, Ohtsuka Satoshi, Göttgens Berthold, Niwa Hitoshi, Smith Austin. G. M. ESRRB is a pivotal target of the GSK3 /Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell. 2012 doi: 10.1016/j.stem.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura T, et al. PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature. 2012:2–7. doi: 10.1038/nature11093. doi:10.1038/nature11093. [DOI] [PubMed] [Google Scholar]
- 46.Grabole Nils, Tischler Julia, Leitch Harry, Hackett Jamie, Kim Shinseog, Tang Fuchou, Magnúsdóttir Erna. A. S. Prdm14 promotes germline fate and naïve pluripotency by modulating signalling and the epigenome. EMBO Reports. 2013 doi: 10.1038/embor.2013.67. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hajkova P, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 48.Hajkova P, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 49.Hajkova P, et al. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature. 2008;452:877–881. doi: 10.1038/nature06714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Z, Swigut T, Valouev A, Rada-Iglesias A, Wysocka J. Sequence-specific regulator Prdm14 safeguards mouse ESCs from entering extraembryonic endoderm fates. Nature structural & molecular biology. 2011;18:120–7. doi: 10.1038/nsmb.2000. [DOI] [PubMed] [Google Scholar]
- 51.Kuckenberg P, et al. The transcription factor TCFAP2C/AP-2gamma cooperates with CDX2 to maintain trophectoderm formation. Molecular and cellular biology. 2010;30:3310–20. doi: 10.1128/MCB.01215-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chia N-Y, et al. A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature. 2010;468:316–20. doi: 10.1038/nature09531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Microarray Expression Data. This table shows the genome wide differential gene expression in P19EC cells upon transient transfection of BLIMP1. The first four columns show the Entrez Gene ID, the gene symbol and the Illumina Probe ID as well as the gene definition. The next 3 columns give the false discovery rate (FDR) for each comparison and the last 3 columns show the log2 fold change (LFC) for each comparison.
Table S2. BLIMP1 Peaks. A list of the high-confidence binding regions of BLIMP1.
Table S5. AP2γ Peaks. A list of the high-confidence binding regions for AP2γ.
Table S6. AP2γ Peaks With Gene Annotation. Gene annotations for the high-confidence binding regions of AP2γ.
Table S7. Primer Sequences. The table shows the primer sequences of primers used for RT-qPCR as well as ChIP-qPCR experiments in 3′ to 5′ direction.
Table S3. BLIMP1 Peaks with Gene Annotation. Gene annotations for the high-confidence binding regions of BLIMP1.
Table S4. Single Cell PGC RNAseq Expression Data. This table shows the genome wide differential gene expression in nascent PGCs (E6.5, E7.5 and E8.5) and somatic neighbours (E7.5 soma) as well as Blimp1 null PGC-like cells (E7.5 Blimp1KO), assayed by single cell RNAseq. The first column shows the RefSeqID, the second column the gene symbol, the third column shows the gene localization and strand. The values indicate the expression levels in log2(reads pr. million).
Figure S1. Expression profiling of P19ECs with ectopic expression of BLIMP1. (a). Flow cytometric plot showing the fluorescence intensity of EGFP and BLIMP1-EGFP transfected cells. Gates employed for cell sorting are indicated. (b) Heat map showing differentially regulated genes in P19ECs upon BLIMP1 expression. Each column represents a time-point assayed in triplicate. The colours indicate the z-score for differential expression. (c). Gene ontology analysis of the top 10% of genes repressed and induced upon BLIMP1 expression in P19ECs.
Figure S2.Functional categories of differentially expressed genes upon BLIMP1 expression in P19ECs. (a-f). Gene ontology analysis of all genes with significant expression changes (FDR < 0.005) in P19ECs upon BLIMP1 expression for both induced and repressed genes for each of the 3 comparisons performed.
Figure S3. RNAseq analysis during PGC specification; integrative analysis of PGC transcriptome, BLIMP1 induced changes in P19EC profiles, and BLIMP1targets. (a and b). Gene ontology analysis showing functional categories of genes repressed and induced respectively, between E7.5 PGCs and somatic cells and the genes from the comparison that are bound by BLIMP1. (c). Relative enrichment of BLIMP1 binding regions and the scores associated with genes differentially expressed between E8.5 PGCs and E7.5 Prdm1 (encoding BLIMP1)-KO PGC–like cells. (d). Correlation analysis of differentially expressed genes during PGC specification and upon BLIMP1 expression in P19ECs. The Pearson correlation coefficients are indicated in the bottom half for each pair-wise comparison and each point on the plot indicates the differential expression of a gene in the comparisons indicated on the x- and y-axes respectively by the juxtaposition to the squares along the diagonal. (e). Relative enrichment of BLIMP1 binding regions associated with genes that are differentially expressed both upon BLIMP1 expression in P19ECs as well as during PGC specification. The x-axis indicates the log2 (fold change) and the y-axis indicates the log2 of the BLIMP1 target enrichment at each fold change-interval of differentially expressed genes over the average target frequency of the whole expression data set. Peaks: the enrichment of peaks associated with genes in each interval differential expression expression level interval Scores; the enrichment of binding scores calculated for genes in each interval. Intersect: The enrichment of peaks associated with genes differentially expressed in both comparisons, in each interval of differential expression. (f). ChIP sequencing tracks from the UCSC browser of genes showing example genomic loci of genes bound by BLIMP1 and repressed in both PGCs and P19ECs upon BLIMP1 expression.
Figure S4. AP2γ motif analysis on BLIMP1 and PRDM14 binding regions. (a). TRANSFAC motif scanning of the BLIMP1 binding regions. The enrichment scores and p-values for the enrichment of each motif are indicated. (b). The distribution of the BLIMP1, AP2 alpha- and the AP2γ motifs on the BLIMP1 binding regions. (c). TRANSFAC motif scanning of the PRDM14 binding regions. d). The distribution of the PRDM14 motif, AP2 alpha and AP2γ motifs around the centre of the binding regions. (e). Relative enrichment of BLIMP1, and PRDM14 targets on differentially expressed genes between E7.5 soma and PGCs filtered by the association of an AP2γ motif in the peak region and the combinatorial association of the peak regions to the differentially expressed genes.
Figure S5. GO-Term clustering of genes differentially associated with BLIMP1, AP2γ and Prdm14 during PGC specification. Clustering analysis of the GO-terms associated with genes that are repressed (a). or induced (b). in PGCs compared to neighbouring somatic cells at E7.5 and bound by different combinations of either BLIMP1, AP2γ or Prdm14. The dark blue colour indicates that fewer than 4 genes were mapped to the indicated GO-term.
Figure S6. A transcription factor network for PGC specification. BLIMP1, AP2γ and Prdm14 binding to differentially expressed genes at E7.5 between PGCs and soma. (a). Repressed genes. (b). Induced genes. The yellow nodes indicate the BLIMP1, PRDM14 and AP2γ peak and associations. The smaller nodes indicate genes bound by the factors, with the binding associations indicated by lines connected to the yellow nodes. The colours of the genenodes indicate functional categories as shown.
Figure S7. A compendium of mESC transcription factor integrated profiles. (a). A full hierarchical clustering analysis of the genome-wide BLIMP1 and AP2γ binding patterns together with binding patterns of transcriptional regulators from mESCs. The red lines above the heat-maps indicate the main clusters, showing the pluripotency cluster (Oct4 etc), polycomb-cluster (Ring1b etc), self-renewal/proliferation cluster (N-Myc etc), and a genome-architectural cluster (CTCF etc). The combinatorial binding pattern analysis was performed by generating a unified data matrix based on 165,607 unique peak regions, indicating for each factor whether it was bound or not. Subsequently the hierarchical clustering and Pearson’s correlation coefficients shown in the heat map were used to investigate global relationships. The colours indicate level of correlation for all pairwise comparisons as indicated on the figure. Note that BLIMP1 associates most strongly with the self-renewal cluster and has high correlation with polycomb factors. PRDM14 associates with the pluripotency cluster whereas AP2γ binding does not correlate highly with mESC transcriptional regulators.
Figure S8. Statistical testing for binding overlap between BLIMP1, Ap2γ and PRDM14 and co-expression of BLIMP1, AP2γ and PRDM14 induces PGC-like cell fate in vitro. (a). Number of observed and expected overlap in genomic binding sites of BLIMP1, AP2γ and PRDM14, and a scatterplot showing the observed against expected overlap in genomic binding sites of: 1. AP2γ and BLIMP1, 2. BLIMP1 and PRDM14, and 3. AP2γ and PRDM14. The p-value for the enrichment of overlap in binding sites is p < 0.0001 for all comparisons. (b). A contingency table for the calculation of a chi-square p-value for the overlap of genes bound by different combinations of BLIMP1, AP2g and PRDM14. The calculated p-value based on this table was p < 1×10−299. The total number of genes (34274) represents the number of unique gene identifiers in Ensembl that were a basis for the gene annotation of the respective transcription factor binding sites. (c). RT-qPCR analysis of sorted fluorescent PGCLCs on Day2 and 4 of either cytokine or doxycycline induction, as well as EpiLCs. The experiment is the second of two experiments performed. The first experiment is shown in Figure 8.