Abstract
Background and Aims:
Given choice, bacteria prefer a community-based, surface-bound colony to an individual existence. The inclination for bacteria to become surface bound is so ubiquitous in diverse ecosystems that it suggests a strong survival strategy and selective advantage for surface dwellers over their free-ranging counterparts. Virtually any surface, biotic or abiotic (animal, mineral, or vegetable) is suitable for bacterial colonization and biofilm formation. Thus, a biofilm is “a functional consortium of microorganisms organized within an extensive exopolymeric matrix.”
Materials and Methods:
The present study was undertaken to detect biofilm production from the repertoire stocks of Acinetobacter baumannii (A. baumannii) and Pseudomonas aeruginosa (P. aeruginosa) obtained from clinical specimens. The tube method was performed to qualitatively detect biofilm production.
Results:
A total of 109 isolates of both organisms were included in the study, out of which 42% (46/109) isolates showed biofilm detection. Among the biofilm producers, 57% of P. aeruginosa and 73% of A. baumannii showed multidrug resistance (MDR) pattern which was statistically significant in comparison to nonbiofilm producers (P < 0.001).
Conclusion:
To the best of our knowledge, this is the only study to have tested the biofilm production in both P. aeruginosa and A. baumannii in a single study. Biofilm production and MDR pattern were found to be significantly higher in A. baumannii than P. aeruginosa. Antibiotic resistance was significantly higher among biofilm producing P. aeruginosa than non producers. Similarly, antibiotic resistance was significantly higher among biofilm producing A. baumannii than non producers.
Keywords: Acinetobacter baumannii, biofilm, multidrug resistant, Pseudomonas aeruginosa
Introduction
Biofilm is defined as “a structured community of bacterial cells enclosed in a self-produced polymeric matrix adherent to an inert or living surface.” Biofilm-producing organisms are far more resistant to antimicrobial agents than organisms which do not. In some extreme cases, the concentrations of antimicrobials required to achieve bactericidal activity against adherent organisms can be three- to four-fold higher than for those bacteria which do not produce biofilm, depending on the species and drug combination.[1]
Biofilms have great importance for public health because of their role in certain infectious diseases and a variety of device related infections. A greater understanding of biofilm processes should lead to novel and effective strategies for biofilm control resulting in improved patient management.[2] Several aspects make biofilm formation a relevant process such as being a mechanism for antibiotic resistance, transfer of resistance plasmids, and a medium for intercellular communication.[3]
Mechanisms responsible for antimicrobial resistance in organisms producing biofilms may be delayed penetration of the antimicrobial agents through the biofilm matrix, altered growth rate of biofilm organisms, and other physiological changes due to the biofilm mode of growth.[4] Thus, the ability to form biofilm could be an effective strategy to enhance the survival and persistence under stressed conditions like host invasion or following antibiotic treatment.[5]
Infection with multidrug resistant (MDR) strains of Pseudomonas aeruginosa (P. aeruginosa) and Acinetobacter baumannii (A. baumannii) are of great concern for hospitalized patients. A. baumannii thought to be a nonpathogenic organism 2 decades back has emerged as an important and problematic human pathogen causing several types of infection including pneumonia, meningitis, septicemia, and urinary tract infections. It ranks second only to P. aeruginosa among the nosocomial, aerobic, nonfermentative, gram negative bacilli pathogen.[5] Both of these organisms have high potential for biofilm production which might explain their outstanding antibiotic resistance, survival properties, and increased virulence.
Keeping these facts in mind, the present study was undertaken with the aims and objectives to detect biofilm production and its association with MDR among the clinical isolates of A. baumannii and P. aeruginosa in our hospital.
Materials and Methods
Samples
A total number of 109 isolates were included in the study, out of which 49 were P. aeruginosa and 60 were A. baumannii. All isolates were obtained from clinical samples received from intensive care unit, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences (NEIGRIHMS), between July and October 2010. The isolates taken for the study as per the site of isolation were shown in Table 1. All isolates were stored at 4°C till testing for biofilm production.
Table 1.
Number of isolates taken for study as per the site of isolation

The isolates were identified to species level by gram stain, cultural characteristics, biochemical reactions, and antibiotic sensitivity test performed by “Kirby Bauer Disk Diffusion Method” and interpreted as per standard protocols.[6]
Antibiotics tested were: Amikacin (Ak), Ciprofloxacin (Cf), Ceftazidime (Ca), Cefoperazone (Cs), Ceftriaxone (Ci), Gentamicin (G), Norfloxacin (Nx), Ofloxacin (Of), Piperacillin (P), and Imipenem (I).
Biofilm production
Biofilm production was qualitatively tested for all isolates by the “Test tube method” as described by Christensen et al.,[7] after revival of the strains. Methicillin-sensitive Staphylococcus aureus (MSSA) ATCC-25923 and P. aeruginosa ATCC-27853 were used as negative and positive controls, respectively.
Revival of the strains
The stock cultures were inoculated in Eppendorf tubes each containing 300 μL of peptone water and incubated overnight at 37°C. The strains were then subcultured onto Mueller Hilton Agar (MHA) and incubated overnight at 37°C. Subsequently, the isolates from MHA were reinoculated into Eppendorf tubes containing peptone water and incubated at 37°C for 4 hrs.
Test tube method
Glass test tubes (13 × 10 cm) filled with 2.6 mL of Brain Heart Infusion Broth (BHIB) with 1% glucose were inoculated with a loopful of culture from the Eppendorf tubes and incubated overnight at 37°C.[7] BHIB with 1% glucose inoculated separately with MSSA ATCC-25923 and P. aeruginosa ATCC-27853 were included as negative and positive controls, respectively.
After overnight incubation at 37°C, the test tubes were checked for turbidity. The contents of the tubes were carefully pipetted out and 2 mL of 0.25% safranin solution was added to each tube. After 1 min, the tubes were emptied of their contents with a pipette and placed upside down on a blotting paper. The results of the test were read after overnight standing at room temperature.
All samples were tested in triplicates.
Statistical analysis
Statistical evaluation was done using “Student's t-test.”
Results
A total of 46 of the 109 isolates (42.2%) showed biofilm production. Biofilm was detected in 16/49 (33%) of P. aeruginosa and 30/60 (50%) of A. baumannii. The test was considered positive when there was an adherent layer of stained material on the inner surface of the tubes. Isolates which showed stained material only at the liquid-air interface were considered negative. The test was repeated in triplicates.
Biofilm production was graded as described by Stepanovic et al.,[8] [Figure 1].
Figure 1.
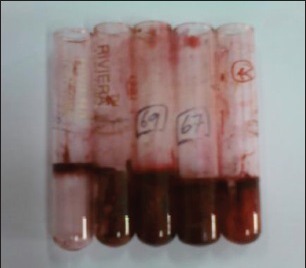
(L-R) 1st tube Methicillin-sensitive Staphylococcus aureus ATCC-25923 (negative control), 2nd Pseudomonas aeruginosa ATCC-27853 (positive control), Tubes 3, 4, 5 test isolates
Biofilm positive isolates with respect to the site of isolation is shown below [Table 2]. Both A. baumannii and P. aeruginosa from sterile fluid showed 100% biofilm production. The least propensity of biofilm production was shown by A. baumannii (25%) isolated from urine samples and P. aeruginosa (27.58%) isolated from respiratory samples.
Table 2.
Biofilm positivity with respect to the specimens

In our study, biofilm producing P. aeruginosa showed 81%, 75%, 69%, 69%, 63%, and 63% resistance to ceftazidime, cefoperazone, ofloxacin, amikacin, ciprofloxacin, and ceftriaxone, respectively. Biofilm producing A. baumannii showed 93%, 90%, 80%, 77%, and 73% resistance to cefoperazone, ceftazidime, ceftriaxone, ofloxacin, and ciprofloxacin, respectively.
Resistance to amikacin (69% vs. 21%), ceftazidime (81% vs. 27%), ciprofloxacin (63% vs. 24%), ceftriaxone (63% vs. 24%), cefoperazone (75% vs. 21%), ofloxacin (69% vs. 15%) were comparatively higher among biofilm producing P. aeruginosa than nonproducer (Statistically significant; P < 0.001). Resistance to amikacin (73% vs. 37%), ceftazidime (90% vs. 40%), ciprofloxacin (73% vs. 23%), ceftriaxone (80% vs. 30%), cefoperazone (93% vs. 27%), ofloxacin (77% vs. 27%) were comparatively higher among biofilm producing A. baumannii than nonproducer (statistically significant; P < 0.001). Resistance pattern of biofilm producing and nonproducing P. aeruginosa and A. baumannii are shown in Figures 2 and 3.
Figure 2.
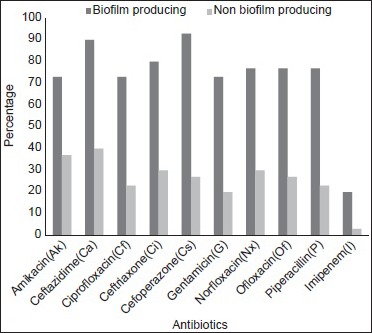
Resistance pattern of biofilm producing and nonproducing Acinetobacter baumannii
Figure 3.
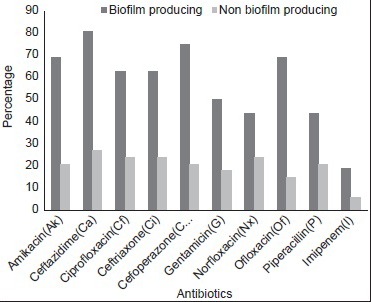
Resistance pattern of biofilm producing and nonproducing Pseudomonas aeruginosa
Discussion
Bacterial cells have grown in the biofilm phenotype for billions of years, as a part of their successful strategy to colonize most of this planet and most of its life forms. We have only recognized this distinct phenotype as the predominant mode of bacterial growth for the last 2 decades. A. baumannii and P. aeruginosa infections present a global medical challenge as opportunistic pathogens which are successful at colonizing and persisting in the hospital environment. A considerable percentage of patients are at risk of being infected with isolates capable of producing biofilm. This will unnecessarily increase the hospital load and amount of time and money spent by the patients. They are able to resist desiccation and survive on inanimate surfaces for years.[9,10]
Characteristically, gradients of nutrients and oxygen exist from the top to the bottom of biofilms and these gradients are associated with decreased bacterial metabolic activity and increased doubling times of the bacterial cells. It is these more or less dormant cells that are responsible for some of the tolerance to antibiotics. Biofilm growth is associated with an increased level of mutations as well as with quorum-sensing-regulated mechanisms. Conventional resistance mechanisms such as chromosomal beta-lactamase, upregulated efflux pumps, and mutations in antibiotic target molecules in bacteria also contribute to the survival of biofilms. Biofilms can be prevented by early aggressive antibiotic prophylaxis or therapy and they can be treated by chronic suppressive therapy.[11] A promising strategy may be the use of enzymes that can dissolve the biofilm matrix (e.g., DNase, F-actin, and alginate lyase) as well as quorum-sensing inhibitors that increase biofilm susceptibility to antibiotics.[11]
Biofilm production in A. baumannii and P. aeruginosa promote increased colonization and persistence leading to higher rates of device-related infections. The MDR pattern can be transferred to the other organisms that initially do not show such resistance. The clinicians who are informed about the relevance of biofilms in infection can use this knowledge to make sound decision that affect patient's health and safety. Interest in these organisms has been growing rapidly because of the emergence of MDR strains, some of which are pan-resistant to antimicrobial agents.[9,10]
The tube method is simple to perform and allows us the benefit to survey the extent of this current invasion of our health-care facilities but reading of the results may be difficult.[8] However, as adherence alone may not complete the cycle of process of biofilm formation, there might be many other mechanisms that could explain adherence.[12,13] There may be difference in the interpretation between the observers particularly for weak reactions.[8]
The present study showed a propensity among the clinical isolates of P. aeruginosa and A. baumannii to form biofilm as 33% and 50%, respectively. Similar studies on biofilm detection showed (62-63%) biofilm production among isolates of A. baumannii.[5,14,15]
Biofilm producing P. aeruginosa showed >55% resistance to aminoglycoside, fluoroquinolones, and b-lactam group of antibiotics, respectively. Nonbiofilm producing P. aeruginosa showed 20% resistance to aminoglycoside, fluoroquinolones, and b-lactam group of antibiotics, respectively (Statistically significant; P < 0.001). Biofilm producing A. baumannii showed >70% resistance to aminoglycoside, fluoroquinolone, and b-lactam group of antibiotics, respectively. Nonbiofilm producing A. baumannii showed 25% resistance to aminoglycoside, fluoroquinolone, and b-lactam group of antibiotics, respectively (Statistically significant; P < 0.001).
In our study, 57% of P. aeruginosa and 73% of A. baumannii which were biofilm producers showed MDR pattern, while a similar study reported only 32% of P. aeruginosa with MDR pattern.[10] This study could be more statistically significant if we could perform the test with larger number of samples and if possible use interdepartmental and interhospital approaches.
Conclusion
A greater understanding of the nature and intercellular communications within the biofilm and their role in serious infections of A. baumannii and P. aeruginosa will help in development of new and more effective treatment resulting in improved patient management. In the present study, biofilm production and MDR pattern were found to be significantly higher in A. baumannii than P. aeruginosa. Antibiotic resistance was significantly higher among biofilm producing P. aeruginosa than nonproducer. Similarly, antibiotic resistance was significantly higher among biofilm producing A. baumannii than nonproducer.
The percentage of biofilm production seen in individual hospital along with their antibiotic susceptibility pattern could inform antibiotic choice and could help us formulate hospital antibiotic policy. Though it may not be possible to stop biofilm production, following antibiotic policy such as early aggressive antibiotic prophylaxis or therapy and chronic suppressive therapy reduces biofilm production in the context of device-related infections.[11] Novel treatment strategies such as phage therapy, quorum-sensing inhibition, and induced biofilm-dispersion have been documented in the literature.[11] Further research should be done in this field to provide us with the vital knowledge to combat this real threat.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Dunne WM., Jr Bacterial adhesion: Seen any good biofilms lately? Clin Microbiol Rev. 2002;15:155–66. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donlan RM. Biofilms: Microbial life on surfaces. Emerg Infect Dis. 2002;8:881–90. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donlan RM. Biofilm formation: A clinically relevant microbiological process. Clin Infect Dis. 2001;33:1387–92. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 4.Donlan RM, Costerton JW. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao RS, Karthika RU, Singh SP, Shashikala P, Kanungo R, Jayachandran S, et al. Correlation between biofilm production and multiple drug resistance in Imipenem resistant clinical isolates of Acinetobacter baumanii. Indian J Med Microbiol. 2008;26:333–7. doi: 10.4103/0255-0857.43566. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. Vol. 29. Wayne: CLSI; 2009. Performance standards for antimicrobial susceptibility testing, 19th informational supplement, M100-S19; pp. 44–7. [Google Scholar]
- 7.Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime producing strains of staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–26. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stepanovic S, Vukovic D, Dakic I, Savic B, Vlahovic MS. A modified microtiter-plate test for quantification of Staphylococcus biofilm formation. J Microbiol Methods. 2000;40:175–9. doi: 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 9.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: Multidrug resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–51. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 10.Navon-Venezia S, Ben-Ami R, Carmeli Y. Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the health care setting. Curr Opin Infect Dis. 2005;18:306–13. doi: 10.1097/01.qco.0000171920.44809.f0. [DOI] [PubMed] [Google Scholar]
- 11.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–32. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Costeron JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 13.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Bano J, Marti S, Soto S, Fernandez-Cuenca F, Cisneros JM, Pachon J, et al. Spanish Group for the Study of Nosocomial Infections (GEIH). Biofilm formation in Acinetobacter baumannii: Associated features snd clinical implications. Clin Microbiol Infect. 2008;14:276–8. doi: 10.1111/j.1469-0691.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 15.Marti S, Rodriguez-Bano J, Catel-Ferreira M, Jouenne T, Vila J, Seifert H, et al. Biofilm formation at the solid-liquid and air-liquid interfaces by Acinetobacter species. BMC Res Notes. 2011;4:5. doi: 10.1186/1756-0500-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


