Abstract
The turnip yellow mosaic virus genomic RNA terminates at its 3' end in a tRNA-like structure that is capable of specific valylation. By directed mutation, the aminoacylation specificity has been switched from valine to methionine, a novel specificity for viral tRNA-like structures. The switch to methionine specificity, assayed in vitro under physiological buffer conditions with wheat germ methionyl-tRNA synthetase, required mutation of the anticodon loop and the acceptor stem pseudoknot. The resultant methionylatable genomes are infectious and stable in plants, but genomes that lack strong methionine acceptance (as previously shown with regard to valine acceptance) replicate poorly. The results indicate that amplification of turnip yellow mosaic virus RNA requires aminoacylation, but that neither the natural (valine) specificity nor interaction specifically with valyl-tRNA synthetase is crucial.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barciszewska M., Dirheimer G., Keith G. The nucleotide sequence of methionine elongator tRNA from wheat germ. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1161–1168. doi: 10.1016/0006-291x(83)90684-8. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Chazal P., Thomes J. C., Julien R. Méthionyl-tRNA synthétase des embryons de blé: dissociation en sous-unités. Eur J Biochem. 1977 Mar 1;73(2):607–615. doi: 10.1111/j.1432-1033.1977.tb11356.x. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Bransom K. L. Genomic RNA sequence of turnip yellow mosaic virus isolate TYMC, a cDNA-based clone with verified infectivity. Plant Mol Biol. 1992 Jan;18(2):403–406. doi: 10.1007/BF00034967. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Florentz C., Giege R. Valylation of tRNA-like transcripts from cloned cDNA of turnip yellow mosaic virus RNA demonstrate that the L-shaped region at the 3' end of the viral RNA is not sufficient for optimal aminoacylation. Biochimie. 1988 Dec;70(12):1719–1727. doi: 10.1016/0300-9084(88)90030-2. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Hall T. C. Mutational analysis of the sequence and structural requirements in brome mosaic virus RNA for minus strand promoter activity. J Mol Biol. 1988 May 5;201(1):31–40. doi: 10.1016/0022-2836(88)90436-6. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Tsai C. H., Florentz C., Giegé R. Specific valylation of turnip yellow mosaic virus RNA by wheat germ valyl-tRNA synthetase determined by three anticodon loop nucleotides. Biochemistry. 1992 Sep 29;31(38):9183–9189. doi: 10.1021/bi00153a010. [DOI] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Gargouri-Bouzid R., David C., Haenni A. L. The 3' promoter region involved in RNA synthesis directed by the turnip yellow mosaic virus genome in vitro. FEBS Lett. 1991 Dec 2;294(1-2):56–58. doi: 10.1016/0014-5793(91)81342-6. [DOI] [PubMed] [Google Scholar]
- Hall T. C. Transfer RNA-like structures in viral genomes. Int Rev Cytol. 1979;60:1–26. doi: 10.1016/s0074-7696(08)61257-7. [DOI] [PubMed] [Google Scholar]
- Joshi R. L., Ravel J. M., Haenni A. L. Interaction of turnip yellow mosaic virus Val-RNA with eukaryotic elongation factor EF-1 [alpha]. Search for a function. EMBO J. 1986 Jun;5(6):1143–1148. doi: 10.1002/j.1460-2075.1986.tb04339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Chapeville F., Haenni A. L. Turnip yellow mosaic virus RNA is aminoacylated in vivo in Chinese cabbage leaves. EMBO J. 1982;1(8):935–938. doi: 10.1002/j.1460-2075.1982.tb01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukubowski H., Pawelkiewicz J. The plant aminoacyl-tRNA synthetases. Purification and characterization of valyl-tRNA, tryptophanyl-tRNA and seryl-tRNA synthetases from yellow-lupin seeds. Eur J Biochem. 1975 Mar 17;52(2):301–310. doi: 10.1111/j.1432-1033.1975.tb03998.x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol Cell Biol. 1989 Nov;9(11):5073–5080. doi: 10.1128/mcb.9.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahser F. C., Marsh L. E., Hall T. C. Contributions of the brome mosaic virus RNA-3 3'-nontranslated region to replication and translation. J Virol. 1993 Jun;67(6):3295–3303. doi: 10.1128/jvi.67.6.3295-3303.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
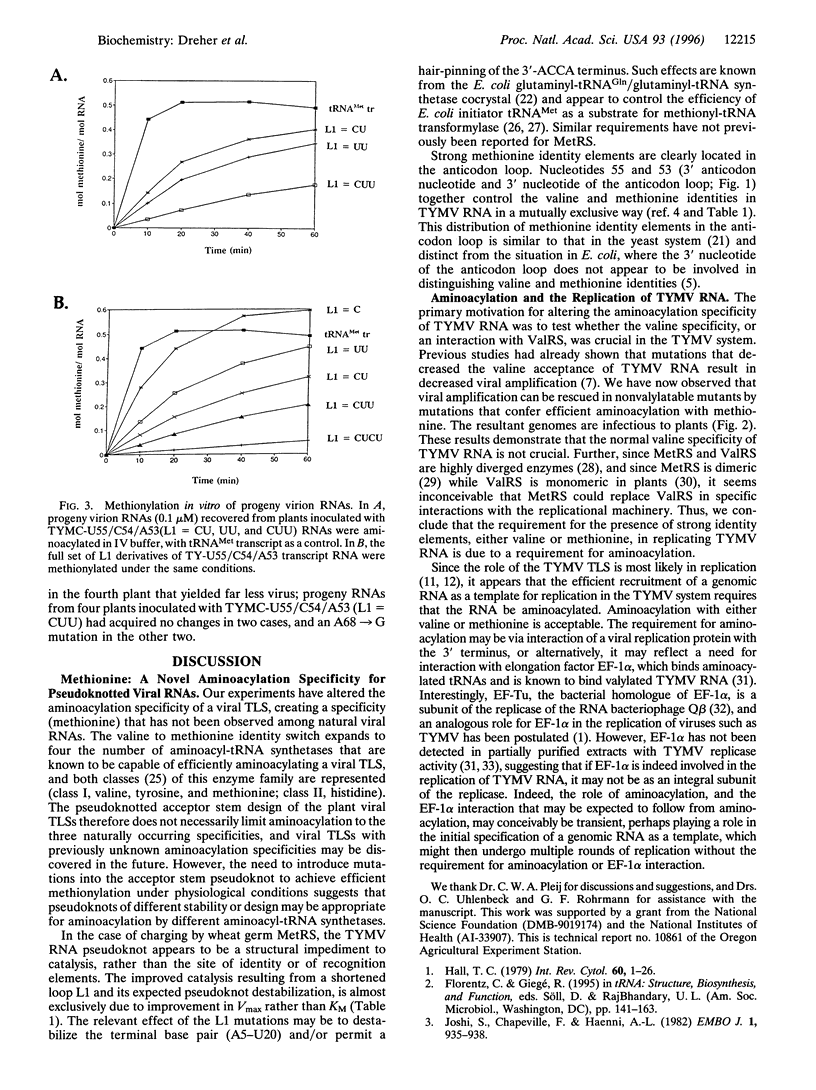
- Mans R. M., Van Steeg M. H., Verlaan P. W., Pleij C. W., Bosch L. Mutational analysis of the pseudoknot in the tRNA-like structure of turnip yellow mosaic virus RNA. Aminoacylation efficiency and RNA pseudoknot stability. J Mol Biol. 1992 Jan 5;223(1):221–232. doi: 10.1016/0022-2836(92)90727-2. [DOI] [PubMed] [Google Scholar]
- Nagel G. M., Doolittle R. F. Evolution and relatedness in two aminoacyl-tRNA synthetase families. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8121–8125. doi: 10.1073/pnas.88.18.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulikowska J., Wojtaszek P., Korcz A., Michalski Z., Candresse T., Twardowski T. Immunochemical properties of elongation factors 1 of plant origin. Eur J Biochem. 1988 Jan 15;171(1-2):131–136. doi: 10.1111/j.1432-1033.1988.tb13768.x. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., Uhlenbeck O. C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Anticodon switching changes the identity of methionine and valine transfer RNAs. Science. 1988 Nov 4;242(4879):765–768. doi: 10.1126/science.3055296. [DOI] [PubMed] [Google Scholar]
- Senger B., Despons L., Walter P., Fasiolo F. The anticodon triplet is not sufficient to confer methionine acceptance to a transfer RNA. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10768–10771. doi: 10.1073/pnas.89.22.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuzeski J. M., Bozarth C. S., Dreher T. W. The turnip yellow mosaic virus tRNA-like structure cannot be replaced by generic tRNA-like elements or by heterologous 3' untranslated regions known to enhance mRNA expression and stability. J Virol. 1996 Apr;70(4):2107–2115. doi: 10.1128/jvi.70.4.2107-2115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Himeno H., Asahara H., Hasegawa T., Shimizu M. Identity determinants of E. coli tRNA(Val). Biochem Biophys Res Commun. 1991 Jun 14;177(2):619–623. doi: 10.1016/0006-291x(91)91833-x. [DOI] [PubMed] [Google Scholar]
- Tsai C. H., Dreher T. W. In vitro transcription of RNAs with defined 3' termini from PCR-generated templates. Biotechniques. 1993 Jan;14(1):58–61. [PubMed] [Google Scholar]
- Tsai C. H., Dreher T. W. Second-site suppressor mutations assist in studying the function of the 3' noncoding region of turnip yellow mosaic virus RNA. J Virol. 1992 Sep;66(9):5190–5199. doi: 10.1128/jvi.66.9.5190-5199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. H., Dreher T. W. Turnip yellow mosaic virus RNAs with anticodon loop substitutions that result in decreased valylation fail to replicate efficiently. J Virol. 1991 Jun;65(6):3060–3067. doi: 10.1128/jvi.65.6.3060-3067.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland J. J., Dreher T. W. Cis-preferential replication of the turnip yellow mosaic virus RNA genome. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6095–6099. doi: 10.1073/pnas.90.13.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland J. J., Dreher T. W. Infectious TYMV RNA from cloned cDNA: effects in vitro and in vivo of point substitutions in the initiation codons of two extensively overlapping ORFs. Nucleic Acids Res. 1989 Jun 26;17(12):4675–4687. doi: 10.1093/nar/17.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]