Abstract
Objective:
To report changes in the cardiovascular management of fluid and inotropic resistant septic shock in children based on echocardiography.
Design:
Retrospective case series.
Setting:
Tertiary care Pediatric Intensive Care Unit (PICU), Chennai.
Patients:
Twenty-two patients with unresolved septic shock after 60 ml/kg fluid plus inotropic agents in the first hour.
Interventions:
Bedside echocardiography (echo) within 6 h of admission to the PICU.
Results:
Over a 28-month period, of 37 patients with septic shock, 22 children remained in shock despite 60 ml/kg fluid and dopamine and/or dobutamine infusions as per guidelines. On clinical exam, 12 patients had warm shock and ten had cold shock, however, six exhibited an unusual pattern of cold shock with wide pulse pressures on invasive arterial monitoring. The most common echocardiographic finding was uncorrected hypovolemia in 12/22 patient while ten patients had impaired left ± right ventricular function. Echocardiography permitted an appreciation of the underlying disordered pathophysiology and a rationale for adjustment of treatment. Shock resolved in 17 (77%) and 16 patients (73%) survived to discharge.
Conclusions:
Bedside echo provided crucial information that was not apparent on clinical assessment and affords a simple noninvasive tool to determine the cause of low cardiac output in patients who remain in shock despite 60 ml/kg fluid and inotropic support. Most patients in our series had vasodilatory shock with wide pulse pressures and most common finding on echo was uncorrected hypovolemia. The echo findings allowed adjustment of therapy which was not possible based on clinical examination alone.
Keywords: Children, diagnosis, echocardiography, myocardial dysfunction, outcome, sepsis, septic shock, therapy
Introduction
Septic shock can result in complex derangements in myocardial function, vascular tone, and integrity as well as alterations in distribution of blood flow.[1,2,3,4] Based on clinical examination shock is commonly categorized as ‘warm’ shock or ‘cold’ shock, where ‘warm’ shock is associated with peripheral vasodilation, normal or increased cardiac output (CO), tachycardia, bounding pulses, warm flushed extremities, and a brisk capillary filling time (CFT).[4] ‘Cold’ shock, on the other hand is associated with low CO, cool extremities, feeble pulses, peripheral vasoconstriction, and a prolonged CFT.[4] While the physical examination is generally reliable in pinpointing the underlying hemodynamic abnormality,[1,4,5] this may not always be the case.[6]
Estimating myocardial performance and intravascular volume status from clinical examination may be difficult in patients with septic shock who have not responded to 60 ml/kg fluid and are also on inotrope and/or vasopressor combination. Under these circumstances, echocardiographic assessment may be useful in delineating the heterogeneous cardiovascular profiles. However, experience with the use of this technique is limited in pediatric septic shock.
This report summarizes our experience in 22 children who remained in shock despite 3 × 20 ml/kg fluid boluses in the first hour and were also dopamine/dobutamine refractory. Echocardiographic assessment revealed findings that complemented clinical examination and invasive monitoring and helped readjust cardiovascular management based on the disordered pathophysiology.
Materials and Methods
This is a retrospective chart review of children admitted with septic shock over a 28-month period (July 2005–September 2007) to a six bed tertiary care Pediatric Intensive Care Unit (PICU). Data extracted included: Demographic data like age, gender, presenting illness; cardiovascular and hemodynamic parameters at admission and response to volume loading; invasive central venous and arterial pressures; echocardiographic findings; change in therapy after echo; and outcome.
Diagnosis of septic shock and inclusion criteria
Patients were included if they had either a positive culture or a strong clinical suspicion of infection based on the presence of fever, leukocytosis, or leukopenia; and a focus of infection. We defined pediatric septic shock based on the American College of Critical Care Medicine and International Pediatric Sepsis Consensus Conference definitions for sepsis.[1,7]
Patients were considered to be in cold shock if their extremities were cold, pale skin, and dusky; their peripheral pulses were feeble or absent and the CFT was >3 seconds.[1,4,5] Warm shock was considered in patients with warm extremities, brisk CFT, and bounding peripheral pulses.[1,4,5]
Vasodilatory shock was diagnosed based on definitions used by Choong et al.[8] Shock in the presence of all of the following: (a) Volume resuscitation of at least 40 ml/kg; (b) at least 10 μg/kg/min of dopamine, or any dose of epinephrine, norepinephrine, or phenylephrine; and (c) clinical evidence of vasodilatory shock (invasive blood pressure monitoring (IBP) demonstrating either diastolic blood pressure (DBP) of less than half systolic blood pressure (SBP) or a pulse pressure of >40 mmHg), and two of the following: Tachycardia, warm extremities, or flash CFT. Vasoconstricted shock was considered if IBP demonstrated narrow pulse pressures ≤ 40 mmHg despite volume resuscitation of at least 40 ml/kg, absence of pressor drugs described above, and the patient had cold extremities with absent/feeble peripheral pulses and delayed CFT.
Charts of children who fulfilled criteria for septic shock were reviewed if, after at least 60/kg fluid in the first hour and initiation of inotropes, hypoperfusion persisted. Patients with septic shock who were not admitted to the PICU or who did not receive invasive monitoring lines or did not receive echocardiography by cardiac technician/cardiologist were excluded. Children with underlying cardiomyopathy and or myocarditis or uncorrected congenital heart defects were also excluded.
Management of septic shock
The diagnosis and management of septic shock followed standard guidelines in all patients Specifically, after shock was recognized; stabilization of the airway, breathing, and circulation was initiated according to the American College of Critical Care Medicine/Pediatric Advanced Life Support (ACCM/PALS) Guidelines for septic shock.[1] Rapid fluid boluses were continued until shock resolved or the patient demonstrated features of fluid intolerance such as hepatomegaly, new onset or worsening respiratory signs including increased work of breathing, tachypnoea, or new rales. Intubation and ventilation was carried in all patients who were fluid intolerant and those who remained in shock despite 60 ml/kg fluid in the first hour unless they were alert. Patients also received an infusion of inotrope ± vasopressor, invasive arterial and central venous pressure (CVP) monitoring, ongoing fluid titration, stress dose hydrocortisone, and bedside trans-thoracic echocardiography.
The choice and dose of inotrope/vasopressor singly or in combination was in accordance with the ACCM/PALS guidelines, and depended on which of the two usual hemodynamic profiles described in the algorithm was applicable: “Cold shock = low CO, high/low systemic vascular resistance (SVR) state” or “warm shock = high cardiac output, low SVR state”.[1] All patients in cold shock received inotropes ± pressor depending on the blood pressure (BP); inotrope + pressor if hypotensive; and vasodilator/inotrope if normotensive.[1]
Invasive arterial BP (IBP) and CVP monitoring was initiated soon after arrival to the PICU. Following insertion of a CVP, further volume was titrated to perfusion indices and CVP targets (≥8 mmHg in nonventilated patients and ≥12 mmHg in ventilated patients).[3] Samples for central venous saturations (SCVO2) were drawn after ensuring on a check chest X-ray that the tips of the CVP were appropriately sited.
The endpoints of shock therapy included resolution of clinical signs of shock, that is, normal heart rate and blood pressure for age, urine flow >1 ml/kg/h, warm extremities with CFT <2 s and normal mental status (in nonventilated patients).[1] Additional oxygen delivery indices that corroborated shock resolution included normalization of elevated lactate or base deficit and SCVO2 > 70%.[1]
Patients who remained in shock despite 60 ml/kg fluid and dopamine/dobutamine infusions also underwent bedside echocardiography to determine the following: (a) Intravascular volume status: Phasic respiratory collapse of inferior vena cava (IVC) >50% which suggested hypovolemia in a patient with circulatory instability; (b) contractility of the cardiac chambers: Where the left ventricles (LV) and right ventricles (RV) hypercontractile, hypocontractile, or normal (c) Presence of obstructive pathology such as tamponade.
Bedside echocardiography was performed within 6 h of PICU admission by an echo technician or cardiologist. Therapy for shock preceded in accordance with the ACCM septic shock guidelines and was modified based on echocardiographic findings.
Results
Of 37 patients with septic shock that were treated over 28 months in the PICU, 22 children had shock resistant to 60 ml/kg fluid in the first hour and fulfilled inclusion criteria for analysis. Eleven had hospital-acquired sepsis including six patients following stem cell transplant [Table 1]. Twelve (54%) were culture positive. Their ages ranged from 3 months to 15 years, mean 4.9 years with four patients under 1 year of age, and 13 (59%) were female. The most common focus was pneumonia, either community or hospital acquired; including ventilator associated pneumonia (VAP) [Table 1]. None of the patients had severe malnutrition or hemoglobin less than 8 g%.
Table 1.
Spectrum and type of infections and features of shock
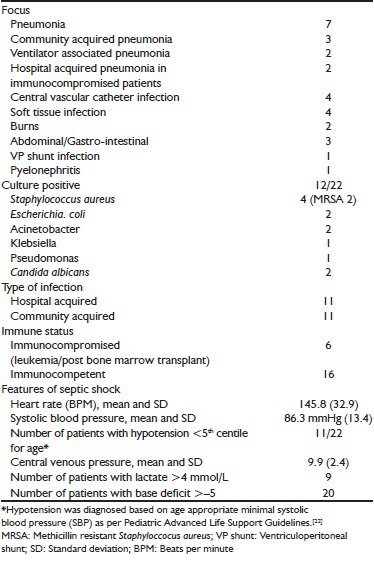
On arrival to the PICU, all 22 patients remained tachycardic and hypoperfused despite having received at least 60 ml/kg fluid and dopamine ± dobutamine at 5-10 μg/kg/min. Based on clinical examination, 12 had warm septic shock [Figure 1, Group A] and ten had cold septic shock. Eleven patients were hypotensive [Table 1]. Hypotension was diagnosed based on age appropriate minimal systolic blood pressure (SBP) as per Pediatric Advanced Life Support Guidelines.[22] Overall, eight patients developed hepatomegaly ± new onset/worsening respiratory insufficiency during volume resuscitation; and as per guidelines,[1] further fluids were temporarily discontinued or infused at a slower rate. Seventeen children were intubated and mechanically ventilated.
Figure 1.
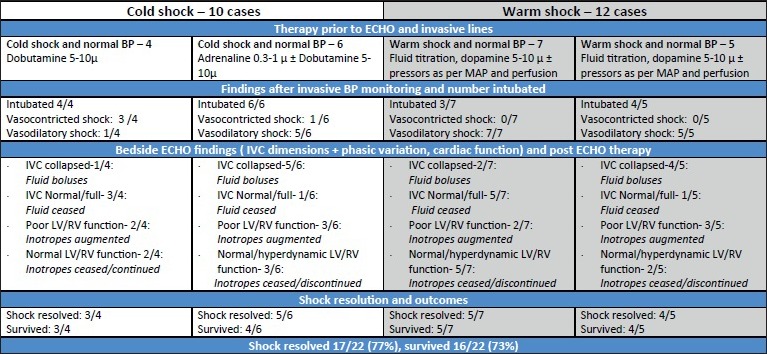
Twenty-two patients with fluid refractory and dopamine/dobutamine resistant shock as per ACCM/PALS Guidelines admitted to PICU over study period. All cases received at least 60 ml/kg isotonic fluid, correction of glucose and ionized calcium, invasive arterial and CVP monitoring and bedside ECHO screening. BP: Blood pressure; SBP: Systolic blood pressure; LV: Left ventricle; RV: Right ventricle; IVC: Inferior vena cava; MAP: Mean arterial blood pressure
Cardiovascular support prior to echo examination and IBP
The ten patients with cold shock were considered on physical examination to have fluid refractory cold shock due to possible septic myocardial dysfunction with high SVR and were started on inotropes (±pressors if hypotensive) as per guidelines [Figure 1].
The 12 patients with warm shock received further fluid boluses and pressors titrated to age appropriate mean arterial blood pressures (MAP) and DBP.
IBP and echo findings
Following IBP placement, patients were further categorized as having vasodilatory or vasoconstricted shock [Figure 1]; notably, 6 of 10 patients with cold shock demonstrated vasodilatory shock on IBP.
The commonest echocardiography finding was uncorrected hypovolemia as evidenced by phasic respiratory collapse of the IVC in 12/22 patients with both cold and warm shock.
Myocardial contractility was impaired in ten patients (45%), predominantly involving diminished LV function. Most had mild or moderate LV dysfunction; however, three patients had severe hypokinesia with gross dilatation of all cardiac chambers. Two children with pneumonia as a primary focus had right ventricular (RV) dilatation. Twelve (54.5%) patients had normal or hyperdynamic cardiac function. None had diastolic dysfunction or pericardial tamponade.
Changes in therapy after echo and invasive arterial monitoring
Cardiovascular therapy was modified depending on (a) volume status, (b) vasoconstricted vs vasodilatory shock, and (c) myocardial function.
The commonest change was more confident fluid augmentation in patients with echo features of hypovolemia.
The seven patients with vasodilatory shock and hyperdynamic/normal LV function received agents with predominant vasopressor activity (noradrenaline ± vasopressin) targeted to clinical indices of perfusion, age appropriate mean arterial pressure (MAP), and SCVO2 > 70%. Of the five patients with warm shock and LV dysfunction, inotropy (dobutamine or adrenaline in inotropic dose) was also initiated in addition to fluid titration and vasopressors. Shock resolved in 9/12 children with warm septic shock.
Six patients with the unusual presentation of cold shock and a vasodilatory state on IBP [Figure 1] had volume deficits in 5/6, while three of them also had depressed LV function.
Therapy in these patients was three-pronged and included filling, further inotropy (dobutamine/adrenaline) with simultaneous titration of the lowest dose of vasopressor (noradrenaline) that resulted in an acceptable MAP for age [Table 1]. Post echo therapy, an improvement was observed in the stroke volume (SV) permitting “unmasking” of the underlying vasodilatory shock in five of six patients and a change in clinical picture from a cold to a warm well-perfused state.
Among the four patients with vasoconstricted shock on arterial monitoring, echo revealed underfilled IVC in one and decreased cardiac function in two. This group also received further fluids and more inotropy (dobutamine/adrenaline) depending on the extent of LV dysfunction, and perfusion improved in three of four patients in this group.
Outcomes
Following echocardiographic and IBP directed changes in fluid and cardiovascular therapy, shock resolved in 17 of 22 children (77%). Repeat echo after 48 h showed normalization of cardiac function in 7/10 patients who had impaired heart function on initial echo.
Five children failed to improve and died of refractory shock and multiorgan failure; one died of progressive neurological events, with an overall mortality of 27%.
Discussion
We present these cases to illustrate three findings. First, in patients who remain in shock despite three fluid boluses and first line inotropes/vasopressor, focused bedside echocardiographic screening is a useful modality that can be used as a serial, noninvasive monitoring tool to better judge intravascular volume, and myocardial function. Second, the most common reason for unresolved shock despite 60 ml/kg fluid and dopamine/dobutamine infusions in the first hour was a persistent intravascular volume deficit which was not apparent on clinical examination. Third, majority of the children with septic shock in our study had vasodilatory shock.
Persistent fluid refractory shock in children can be secondary to an inappropriate cardiovascular support regimen rather than an inexorable or refractory shock process.[2] This contention is supported by Ceneviva et al. who reported that 44 of 50 of children with persistent fluid refractory septic shock improved following a change in cardiac support directed by pulmonary artery catheterization.[2] However, in resource limited environments such as ours, expensive technology is limited. An echocardiographic machine or at least a black and white ultrasound machine is usually available in most centers. Bedside echocardiography can directly image the great veins; ventricular size and contractility; and has emerged as an important, noninvasive, portable, and rapid diagnostic tool in the ER and ICU to facilitate early detection of potentially reversible and time-dependent conditions.[9,10,11,12,13,14]
A patient may have fluid refractory shock despite initial resuscitation with 60 ml/kg fluid plus dopamine/dobutamine because hypovolemia may not be fully corrected, cardiac output remains poor and/or because of abnormal vascular tone (vasodilatation/vasoconstriction). Basic screening techniques can complement standard monitoring and provide an answer to important questions at the bedside, that is, does the patient with unresolved shock despite 60 ml/kg fluid plus dopamine/dobutamine need more volume, inotropes or vasopressors, or a combination of these?
In these 22 cases who remained in shock despite reasonable filling and vasoactive therapy as per standard guidelines, a combination of uncorrected fluid deficits (in 12/22 patients) and decreased cardiac function (10/22 patients) contributed to the hypoperfusion. However, this information could not be deduced just from physical examination. Following echo, the treatment could be customized to better address the specific deranged pathophysiology.
Regarding fluid resuscitation, although all 22 patients had received at least 60 ml/kg in 1 hour, and further CVP targeted filling after central line placement over half the patients had evidence of uncorrected hypovolemia on echo. While it is well-known that large fluid deficits can exist in septic shock and adequate volume therapy is one of the keystones of management,[1] there may be some reluctance to continue aggressive filling as the downside of over-resuscitation includes longer ICU stays and worse outcomes.[1,14,15] Moreover, getting the balance quite right in terms of volume resuscitation may be challenging, especially in ventilated patients where the CVP readings may be unreliable.[16] In these situations, bedside echo documentation of inspiratory collapse of the IVC permits more confident fluid administration.
With respect to vascular tone and cardiac output (CO), adult patients with septic shock typically have a hyperdynamic state with a high CO, and a low SVR state resulting in a warm vasodilatory state.[3] Even when the myocardial function is depressed, the CO can be elevated.[17]
Although hemodynamic studies in pediatric septic shock are limited, Ceneviva et al., in 1998, have suggested that a greater proportion of children have cold shock with low CO and high SVR.[2] However, vasodilatory shock may not be uncommon in children with septic shock. Brierley et al. in 2008 have described distinct hemodynamic patterns of fluid resistant pediatric septic shock depending on the cause. The authors reported that a greater proportion of central vascular catheter (CVC) infections manifested the adult-pattern hemodynamics of warm vasodilatory shock with high cardiac index while patients with community-acquired sepsis had shock with low cardiac index.[18] In 2009, a Canadian multicenter trial have reported that the most common cause of vasodilatory shock in children was sepsis in 78% of 69 children studied.[8]
The ACCM-PALS guidelines categorize pediatric septic shock as having warm or cold shock on the basis of clinical signs,[1] and recommend therapy accordingly, although the clinician's ability to judge hemodynamic parameters is known to be poor.[6]
In our series, 18 of 22 patients manifested with vasodilatory shock. However, the initial clinical examination in six patients did not fit the typical pictures of a hyperdynamic vasodilatory shock. These six patients were unusual as they were cold on physical exam but had a vasodilatory state on IBP. Echo demonstrated persistent hypovolemia in the majority (5/6) and decreased cardiac function in 3/6. While the physical examination in most patients is a good indicator of the underlying hemodynamic state to permit categorization into warm (= low SVR) or cold (= high SVR) shock; this may not always be the case.
A low SV can lead to a clinical picture of cold shock despite a wide pulse pressure.[19] Important causes of a greatly decreased SV are uncorrected fluid deficits alone or fluid deficits coexisting with septic myocardial dysfunction.[19,20,21] Kumar et al.,[20] Hunter et al.,[19] and Ceneviva et al.,[2] have described a volume resuscitation-dependent hyperdynamic circulatory state in adults and children. Following volume repletion and targeted inotropy, stroke volume improved, and the underlying warm vasodilatory state became clinically more apparent.
This suggests that, in a patient with cold shock but wide pulse pressures, an important initial strategy is to improve stroke volume with more fluids, followed by inotropy ± pressors guided by invasive pressures and echo findings. Furthermore, categorization on the basis of arterial pressure monitoring into vasodilatory versus vasoconstricted shock rather than cold versus warm shock may permit more appropriate targeted hemodynamic therapy at the bedside.
Limitations of our study include the inherent problems of a retrospective analysis. Further, we did not utilize dynamic measures of preload responsiveness to assess and guide volume therapy as, during the study period, there was only limited emerging data regarding utility of these in children. Finally, validation of the echo findings by performing pulmonary artery catheterization may have further strengthened our findings; however, this was unavailable at our hospital.
Conclusion
Patients may exhibit fluid and dopamine/dobutamine refractory septic shock due to an inappropriate cardiovascular support regimen rather than an inexorable or refractory shock process. However, this may not be obvious on clinical examination. Bedside echocardiography can complement information obtained by clinical exam and invasive monitoring in patients with septic shock.
In our study, most patients in our study had warm shock. However, some patients with vasodilatory shock had atypical clinical findings on initial physical examination. Information obtained by a combination of clinical exam, invasive pressures, and echo permitted optimization of the stroke volume by change or intensification of existing therapy resulted in an improved cardiac output and resolution of shock in most patients in this group. Adequate training and quality control is necessary if echocardiography is to be useful at the bedside in the hands of the intensivist.
Acknowledgment
We are grateful to Dr. Ramesh Venkataraman, MD, Consultant, Critical Care Unit, Apollo Hospitals, Chennai for his helpful comments and valuable input; and to all of my colleagues at Apollo and Mehta Children's hospital for their commitment to high quality patient care and enthusiastic support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–88. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceneviva G, Paschall JA, Maffei F, Carcillo JA. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics. 1998;102:e19. doi: 10.1542/peds.102.2.e19. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. International Surviving Sepsis Campaign Guidelines Committee, American Association of Critical-Care Nurses, American College of Chest Physicians, American College of Emergency Physicians, Canadian Critical Care Society, European Society of Clinical Microbiology and Infectious Diseases. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. [Google Scholar]
- 4.Melendez E, Bachur R. Advances in the emergency management of pediatric sepsis. Curr Opin Pediatr. 2006;18:245–53. doi: 10.1097/01.mop.0000193305.55635.ff. [DOI] [PubMed] [Google Scholar]
- 5.Khilnani P, Singhi S, Lodha R, Santhanam I, Sachdev A, Chugh K, et al. Pediatric Sepsis Guidelines: Summary for resource-limited countries. Indian J Crit Care Med. 2010;14:41–52. doi: 10.4103/0972-5229.63029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tibby SM, Hatherill M, Marsh MJ, Murdoch IA. Clinicians’ abilities to estimate cardiac index in ventilated children and infants. Arch Dis Child. 1997;77:516–8. doi: 10.1136/adc.77.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 8.Choong K, Bohn D, Fraser DD, Gaboury I, Hutchison JS, Joffe AR, et al. Vasopressin in pediatric vasodilatory shock: A multicenter randomized controlled trial. Am J Respir Crit Care Med. 2009;180:632–9. doi: 10.1164/rccm.200902-0221OC. [DOI] [PubMed] [Google Scholar]
- 9.Brown JM. Use of echocardiography for hemodynamic monitoring. Crit Care Med. 2002;30:1361–4. doi: 10.1097/00003246-200206000-00039. [DOI] [PubMed] [Google Scholar]
- 10.Vignon P, Chastagner C, François B, Martaillé JF, Normand S, Bonnivard M, et al. Diagnostic ability of hand-held echocardiography in ventilated critically ill patients. Crit Care. 2003;7:R84–91. doi: 10.1186/cc2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pershad J, Myers S, Plouman C, Rosson C, Elam K, Wan J, et al. Bedside limited echocardiography by the emergency physician is accurate during evaluation of the critically ill patient. Pediatrics. 2004;114:e667–71. doi: 10.1542/peds.2004-0881. [DOI] [PubMed] [Google Scholar]
- 12.Randazzo MR, Snoey ER, Levitt MA, Binder K. Accuracy of emergency physician assessment of left ventricular ejection fraction and central venous pressure using echocardiography. Acad Emerg Med. 2003;10:973–7. doi: 10.1111/j.1553-2712.2003.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 13.Beaulieu Y. Specific skill set and goals of focused echocardiography for critical care clinicians. Crit Care Med. 2007;35:S144–9. doi: 10.1097/01.CCM.0000260682.62472.67. [DOI] [PubMed] [Google Scholar]
- 14.Ranjit S, Kissoon N, Jayakumar I. Aggressive management of dengue shock syndrome may decrease mortality rate: A suggested protocol. Pediatr Crit Care Med. 2005;6:412–9. doi: 10.1097/01.PCC.0000163676.75693.BF. [DOI] [PubMed] [Google Scholar]
- 15.Foland FA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: A retrospective analysis. Crit Care Med. 2004;32:1771–6. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 16.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–8. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- 17.Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–42. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 18.Brierley J, Peters MJ. Distinct hemodynamic patterns of septic shock at presentation to pediatric intensive care. Pediatrics. 2008;122:752–9. doi: 10.1542/peds.2007-1979. [DOI] [PubMed] [Google Scholar]
- 19.Hunter JD, Doddi M. Sepsis and the heart. Br J Anaesth. 2010;104:3–11. doi: 10.1093/bja/aep339. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock: Part I. Clinical manifestation of cardiovascular dysfunction. J Cardiothorac Vasc Anesth. 2001;15:364–76. doi: 10.1053/jcan.2001.22317. [DOI] [PubMed] [Google Scholar]
- 21.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: Myocardial depression in sepsis and septic shock. Crit Care. 2002;6:500–8. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinman ME, Chameides L, Schexnayder SM, Samson RA, Hazinski MF, Atkins DL, et al. Pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2010;126:e1361–99. doi: 10.1542/peds.2010-2972D. [DOI] [PubMed] [Google Scholar]


