Abstract
Tranexamic acid (TA) act as anti-fibrinolytic agent and is widely used to limit bleeding in clinical practice. Tranexemic acid bind with plasminogen and prevent its conversion to plasmin, which limits the fibrinolytic pathway, so there is a theoretical risk of increasing thrombosis with high or prolonged therapy with TA. We encountered a case of acute arterial thrombosis following inadvertent administration of high dose of TA. A 27-years-old male with no other co-morbidity was ordered intravenous 1 gm TA to control excessive bleeding from previous bladder injury, but by mistake, he received 10 gm of TA. The patient developed signs and symptoms of acute ischemia in the right lower limb, which was diagnosed as acute iliac arterial thrombosis by computed tomography (CT) angiography. The patient was managed with systemic heparinization, fasciotomy for impending gangrene and other supportive care following which he recovered fully within a few days. Caution should be exercised for all prophylactic use, especially with high dosage or prolonged therapy with TA.
Keywords: Arterial thrombosis, hemorrhage control, thrombo-embolism, tranexamic acid
Introduction
Early administration of tranexamic acid has been recommended to reduce hemorrhage and blood transfusion in traumatic bleeding.[1,2] Tranexamic acid is an anti-fibrinolytic agent that binds with the plasminogen and prevent its conversion to plasmin.[3] Within recommended dose intravenous, Tranexamic acid (TA) is effective in reducing hemorrhage and blood transfusion requirement in various clinical conditions with minimal or negligible side effects.[1] Because of the mechanism of action of TA is stabilization of clot, there is a theoretical risk of excessive clot formation with vessel occlusion in patients with thrombophilic tendencies or use of very high dose and or prolonged therapy with TA. Several reports of – thromboembolic phenomena including cerebral arterial thrombosis have been published in the literature.[4,5,6,7] We encountered a case of acute arterial thrombosis of a lower limb with acute ischemic changes following an inadvertent very high dose of TA in a patient with no known hematological disorder.
Case Report
A 27-year-old male with no known co-morbidity sustained penetrating injuries to the perineum, rectum, bladder neck, and bowel following a fall on a sharp object. After initial resuscitation, he underwent surgical repair of his injury with ileostomy and primary repair of bladder neck injury. The perineum was contaminated with rectal content. After debridement and hemostasia, a delayed closure was planned. Patient had a stormy course in the intensive care unit (ICU), with the delayed abdominal closure being followed by wound dehiscence, ventilator-associated pneumonia and adult respiratory distress syndrome (ARDS). He was managed with ventilator support, antibiotics, parenteral nutritional, and thromboprophylasix with low molecular weight heparin (LMWH). Despite his stormy course, he was recovering from ARDS and healing perineal wound; In the 3rd week of his ICU admission, he had sudden bleeding from the bladder neck manifesting as hematuria through Foleys catheter and pericatheter bleeding from the urethra. The bleeding was managed with surgical exploration of perineal wound and direct compression. He was transfused with two unit of packed red blood cell (PRBC), and 1 gm of TA was ordered. But, by mistake, the patient received 10 gm of TA as bolus, followed by 1 gm infusion for 8 hrs. Patient was stabilized and was doing well on a ventilator until the attending nursing staff noticed pallor and coldness of the right lower limb after 6-8 hours of TA administration. Suspecting acute arterial occlusion, an urgent contrast angiography was done, which revealed arterial thrombosis involving right common iliac artery extending from the bifurcation of aorta up to the proximal femoral artery [Figure 1].
Figure 1.
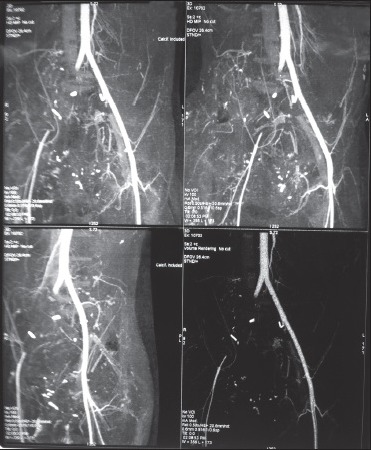
CT angiography showing thrombosis of right common iliac artery and proximal femoral artery
Consultation of a vascular surgeon was sought; the patient was systemically heparinized as per the hospital protocol to keep activated partial throboplastin time (aPTT) 2-2.5 times the control value. A few hours later, there was increasing pallor of the limb with loss of arterial pulsations in the entire lower limb with associated swelling. Immediate decompressive fasciotomy was done to prevent further ischemic injury. In view of worsening ischemic changes, the patient was planned for aorto-femoral bypass grafting. But, before he was prepared for the operating room, there was return of blood flow, skin color and arterial pulsations to the lower limb. Surgical intervention was postpone. Eventually, the patient recovered well from the arterial thrombosis and also from ARDS and was discharged from the ICU after about 10 days from the arterial thrombosis on LMWH prophylaxis.
Discussion
TA is generally safe and effective in most of the studies with negligible side effects. Even though there is published data to show TA reduced blood loss, the studies may be underpowered to identify thrombotic complications. Tranexamic acid is a competitive inhibitor of plasminogen activation, and at much higher concentrations, a non-competitive inhibitor of plasmin and prolonged the thrombin time. Tranexamic acid has the potential to cause thrombotic disorders and is contraindicated in patients with active thrombotic or embolic disorders. It should not be prescribed for patients with risk factors for thrombo-embolic disease unless the potential benefits clearly outweigh the potential for harm.[8] Tranexamic acid (TA) has been used since years with a 10-fold difference in dosing. Very high doses of TA up to 10 gm has been safely used, especially in cardiac surgeries.[9] There are few reports of thrombo-embolic events associated with TA use, mainly deep venous thrombosis and pulmonary embolism.[4,5,6] Two of these patient had bleeding disorders-idiopathic thrombocytopenic purpura and acquired hemophilia. Another patient had subarachnoid hemorrhage. Two reports of arterial thrombosis has been reported in literature, both of whom were on oral TA for menorrhagia and developed cerebral arterial thrombosis.[7] There are 56 reports of deep vein thrombosis, pulmonary embolism or both and these include reports of cerebral and retinal vein thrombosis in the World Health Organization's international drug monitoring database. Additionally, there are 22 reports of cerebral embolism and 9 of arterial thrombosis.[8]
We could not find any other precipitating cause of acute arterial thrombosis in this patient. Our patient had no history of any hematological or thrombophilic disorder. The arterial thrombosis occurred after 6-8 hrs of TA administration, providing circumstantial evidence for TA as cause of vascular occlusion. The only prothrombotic state in our patient was prolonged ICU stay, which generally causes venous thrombosis. Our patient recovered after stopping the TA infusion, institution of systemic heparinization, and fasciotomy, which indicate an acute ischemic event. However, there are reports of use of very high-dose TA, especially in cardiac surgery where the actual effect of TA may be masked by systemic heparinization, which is invariably instituted after cardiac surgery.
Sundstrom A et al., conducted a study using database from general practitioners using TA for menorrhagia from 1992-1998. They found 134 cases of venous thrombo-embolism (VTE), but association of TA did not reach statistical significance.[10]
Though the literature relating thrombo-embolic events with TA is inconsistent, there might be an association with very high or prolonged doses of TA in susceptible patients. Therefore caution should be exercised for all TA use, especially with high doses and prolonged usage.
References
- 1.CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage: A randomised, placebo controlled trial. The Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 2.Dzik WH, Blajchman MA, Fergusson D, Hameed M, Henry B, Kirkpatrick AW, et al. Clinical review: Canadian National Advisory Committee on Blood and Blood Products: Massive Transfusion Consensus Conference 2011: Report of the panel. Critical Care. 2011;15:242. doi: 10.1186/cc10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramstrom G, Blomback M, Egberg N, Johnsson H, Ljungberg B, Schulman S. Oral surgery in patients with hereditary bleeding disorders. A survey of treatment in the Stockholm area (1974-1985) Int J Oral Maxillofac Surg. 1989;18:320–2. doi: 10.1016/s0901-5027(89)80026-8. [DOI] [PubMed] [Google Scholar]
- 4.Endo Y, Nishimura S, Miura A. Deep-vein thrombosis induced by tranexamic acid in idiopathic thrombocytopenic purpura [letter] JAMA. 1988;259:3561–2. doi: 10.1001/jama.1988.03720240023026. [DOI] [PubMed] [Google Scholar]
- 5.Woo KS, Tse LK, Woo JL. Massive pulmonary thromboembolism after tranexamic acid antifibrinolytic therapy. Br J Clin Pract. 1989;43:465–6. [PubMed] [Google Scholar]
- 6.Taparia M, Cordingley FT, Leahy MF. Pulmonary embolism associated with tranexamic acid in severe acquired haemophilia. Eur J Haematol. 2002;68:307–9. doi: 10.1034/j.1600-0609.2002.01607.x. [DOI] [PubMed] [Google Scholar]
- 7.Rydin E, Lundberg PO. Tranexamic acid and intracranial thrombosis. Lancet. 1976;2:49. doi: 10.1016/s0140-6736(76)93013-0. [DOI] [PubMed] [Google Scholar]
- 8.Ruth Savage. Thrombosis with Tranexamic Acid for Menorrhagia. Prescriber Update. 2003;24:26–7. [Google Scholar]
- 9.Hardy JF, Belisle S, Dupont C, Harel F, Robitaille D, Roy M. Prophylactic tranexamic acid and epsilon-aminocaproic acid for primary myocardial revascularization. Ann Thorac Surg. 1998;65:371–6. doi: 10.1016/s0003-4975(97)01016-3. [DOI] [PubMed] [Google Scholar]
- 10.Sundström A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: A case-control study using the General Practice Research Database. BJOG. 2009;116:91–7. doi: 10.1111/j.1471-0528.2008.01926.x. [DOI] [PubMed] [Google Scholar]


