Abstract
Aims:
Active screening for methicillin resistant Staphylococcus aureus (MRSA) carriers remains a vital component of infection control policy in any health-care setting. The relative advantage of multiple anatomical site screening for detecting MRSA carriers is well recognized. However, this leads to increase in financial and logistical load in a developing world scenario. The objective of our study was to determine the sensitivity of MRSA screening of nose, throat, axilla, groin, perineum and the site of catheterization (central line catheter) individually among intensive care unit patients and to compare it with the sensitivity of multiple site screening.
Materials and Methods:
Active surveillance of 400 patients was done to detect MRSA colonization; 6 sites-nose, throat, axilla, perineum, groin and site of catheter were swabbed.
Result and Discussion:
The throat swab alone was able to detect maximum number of MRSA (76/90) carriers, with sensitivity of 84.4%. Next in order of sensitivity was nasal swab, which tested 77.7% of MRSA colonized patients. When multiple sites are screened, the sensitivity for MRSA detection increased to 95%.
Conclusions:
We found that though throat represent the most common site of MRSA colonization, nose or groin must also be sampled simultaneously to attain a higher sensitivity.
Keywords: Asymptomatic colonization, intensive care unit, methicillin resistant Staphylococcus aureus colonization
Introduction
The magnitude of threat caused by methicillin resistant Staphylococcus aureus (MRSA) is well recognized by infection control team of all hospitals. MRSA carriers constitute an important reservoir of infection and their effective control remains a challenge in hospital care.[1]
Active screening for MRSA carriers enables the detection of muco-cutaneous carriage among individuals without clinical infection. This is one of the vital components of MRSA control policy in any health-care facility. More than half of the reservoir of MRSA colonized patients admitted to the hospitals usually remain undetected, unless swab samples of the nose, groin, perineum, skin and wound are specifically tested for MRSA.[2] A new MRSA carrier may have tens of contacts in hospital.[2] Early detection, isolation and treatment of MRSA carrier can prevent nosocomial spread of this pathogen as well as decrease the antimicrobial burden of the hospital. Failure to detect all colonized patients may result in the underuse of infection control measures. Screening for MRSA is akin to immunization as it reduces the risk of clinical infection and prevents transmission to others. Nevertheless, performing active screening and culture for an increasing number of admitted patients, places extra demand on clinical microbiology laboratories especially in developing country like ours.
Anterior nares have classically been the only anatomic site cultured for detection of MRSA carriers. However, results from nasal swabs are not consistently helpful in diagnosing MRSA colonization. Various studies have shown that multiple anatomical site screening for MRSA is better than single nasal screening.[3] The sites suggested to screen MRSA carrier include-throat, axilla, perineum, groin and the site of catheter insertion. Nevertheless, the relative position of sampling of multiple anatomical sites for detection of MRSA colonization remains unclear; the significance of using throat swab to detect MRSA colonized patients has been contested by various authors.[4] In a developing country like ours, due to paucity of funds, there has to be a right balance stuck between the maximum MRSA detection and utilization of resources.
Therefore, the objective of this study was to determine the sensitivity of MRSA screening of nose, throat, axilla, groin, perineum and the site of catheterization individually among intensive care unit (ICU) patients and to compare it with the sensitivity of multiple site screening.
Materials and Methods
The study was conducted at a 750 bedded, tertiary care hospital, in North India over 18 months period from January 2009 to June 2010. An active surveillance of 400 adult patients was done in our multi-disciplinary ICU.
Exclusion criteria
Patient admitted for less than 48 h to ICU.
Patient admitted earlier in any other unit of our hospital or coming from other health-care settings.
Patient known to have MRSA infections.
Patients not having central line catheter.
These criteria helped us to identify only those patients who acquired MRSA carriage in our ICU, while excluding those patients who had acquired MRSA carriage from other unit or hospital.
For each patient included in our study, swab sample were taken from following 6 sites-nose, throat, axilla, perineum, groin, and site of catheter (central line catheter). Each patient was sampled only once; each swab was streaked onto Blood agar and Mac Conkey agar (Hi-Media, India) and incubated at 37°C for 24 h. Colonies suggestive of S. aureus were further identified by Gram stain, catalase and slide and tube coagulase. The isolates were confirmed as MRSA by disc diffusion test using 30 mg cefoxitin disc on Mueller Hinton agar, as per Clinical Laboratory Standards Institute CLSI recommendations.[5]
Results
During the study multiple-site screening for carriage of MRSA was performed on 400 patients, out of which 90 patients had carriage of MRSA at one or more site, giving an overall positivity of 22.5%.
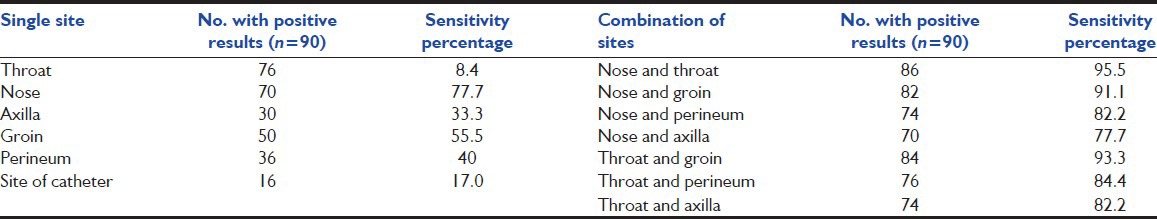
Table 1 shows the sensitivity of different sites for detecting an MRSA colonized patient. Being positive at any screening site, at any time was used as the denominator. The throat swab alone was able to detect maximum number of MRSA (76/90) carriers, with sensitivity of 84.4%. Next, in order of sensitivity was nasal swab which detected 77.7% of MRSA colonized patients. Swabs taken from groin and perineum could only detect 50 and 36 MRSA carriers, thus, having sensitivity of 55.5% and 40.0% respectively. The swabs from axilla and catheter site had sensitivity of 33.0% and lower. The sensitivity of detection of MRSA carriage improved immensely when the combination of two sites was done. A total of 86/90 (95.5%) patients were positive in the nose and throat swab, 84 (93.3%) in the throat and groin and 82 (91.1%) in the nasal and groin swab. Similarly, combination of perineum swabs with either nasal or throat increased the rate of MRSA detection to 82.2% respectively.
Table 1.
Positivity rates of sampling sites and their sensitivity
Discussion
It is a well-known fact that hand washing by the health-care workers before and after touching any patient, is the single most important tool for preventing spread of MRSA amongst different patients.[1,4] Since MRSA is always a cross transmitted infections, decreasing MRSA pressure in ICUs and other high-risk areas. Nevertheless, accurate and efficient detection as well as management of MRSA carriers in any health-care setting would reduce the antibiotic burden of the hospital and improve the over-all outcome of the patients.
The prevalence of MRSA is 35% in our institute.[6] The overall MRSA carriage rate among the ICU patients was found to be 22.5%. This rate is in concordance with the rate of MRSA colonized patients in ICU setting in a study done in Australia by Marshall.[7]
The anatomical site, which most often yielded positive result was throat swab (84.4%) followed by nasal swab (77.7%). Marshall and Nilsson et al., in their respective studies, found throat swab to be better than nasal swab for MRSA detection.[7,8] The sensitivity of nasal swab (77.7%) observed in this study was consistent with other studies which showed sensitivity between 78.0% and 93.0%.[3,9] In addition the sensitivity of single swab from axilla, groin, and perineum for surveillance of MRSA carriers in this study was similar to that done by Lautenbach et al. and Meurman et al.[10,11]
However, our results clearly show that culturing nose or any single anatomical site is insufficient for efficient detection of MRSA carriers. When multiple sites are screened the sensitivity for MRSA detection increases to 95%, as shown by other authors as well.[10,11,12] In our study, combination of two site sampling, namely throat with nose or throat with groin or nose with groin were most appropriate and least number of MRSA colonized patients were missed with these two swabs combination. We found significant concordance between the results of nose and throat swabs. This association makes intuitive sense because of the close anatomical connection between the two. In addition, among the ICU patients, the majority of patients had endotracheal tube and nasogastric tubes, which may have impaired the normal anatomy of the area and facilitated spread of MRSA between the two sites.
This assumes importance in a developing world health-care setting where a balance has to be struck between excessive workload and limited resources. Thus, two site screening appears to be an efficient screening method for MRSA carriers in an attempt to decrease the laboratory workload and cost associated with multiple site sampling.
Conclusion
We found that though throat represent the most common site of MRSA colonization, nose or groin must also be sampled simultaneously to attain a higher sensitivity. This partly off-sets the higher cost and increased laboratory workload associated with multisite screening while achieving similar sensitivity.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Girou E, Pujade G, Legrand P, Cizeau F, Brun-Buisson C. Selective screening of carriers for control of methicillin-resistant Staphylococcus aureus (MRSA) in high-risk hospital areas with a high level of endemic MRSA. Clin Infect Dis. 1998;27:543–50. doi: 10.1086/514695. [DOI] [PubMed] [Google Scholar]
- 2.Girou E, Azar J, Wolkenstein P, Cizeau F, Brun-Buisson C, Roujeau JC. Comparison of systematic versus selective screening for methicillin-resistant Staphylococcus aureus carriage in a high-risk dermatology ward. Infect Control Hosp Epidemiol. 2000;21:583–7. doi: 10.1086/501807. [DOI] [PubMed] [Google Scholar]
- 3.Lucet JC, Chevret S, Durand-Zaleski I, Chastang C, Régnier B Multicenter Study Group. Prevalence and risk factors for carriage of methicillin-resistant Staphylococcus aureus at admission to the intensive care unit: Results of a multicenter study. Arch Intern Med. 2003;163:181–8. doi: 10.1001/archinte.163.2.181. [DOI] [PubMed] [Google Scholar]
- 4.Manian FA, Senkel D, Zack J, Meyer L. Routine screening for methicillin-resistant Staphylococcus aureus among patients newly admitted to an acute rehabilitation unit. Infect Control Hosp Epidemiol. 2002;23:516–9. doi: 10.1086/502099. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. Wayne, PA, USA: CLSI; 2011. Performance Standards for Antimicrobial Susceptibility Testing: Twenty First Informational Supplement M100-S21. [Google Scholar]
- 6.Datta P, Gulati N, Singla N, Rani Vasdeva H, Bala K, Chander J, et al. Evaluation of various methods for the detection of meticillin-resistant Staphylococcus aureus strains and susceptibility patterns. J Med Microbiol. 2011;60:1613–6. doi: 10.1099/jmm.0.032219-0. [DOI] [PubMed] [Google Scholar]
- 7.Marshall C, Spelman D. Re: Is throat screening necessary to detect methicillin-resistant Staphylococcus aureus colonization in patients upon admission to an intensive care unit? J Clin Microbiol. 2007;45:3855. doi: 10.1128/JCM.01176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsson P, Ripa T. Staphylococcus aureus throat colonization is more frequent than colonization in the anterior nares. J Clin Microbiol. 2006;44:3334–9. doi: 10.1128/JCM.00880-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanford MD, Widmer AF, Bale MJ, Jones RN, Wenzel RP. Efficient detection and long-term persistence of the carriage of methicillin-resistant staphylococcus aureus. Clin Infect Dis. 1994;19:1123–8. doi: 10.1093/clinids/19.6.1123. [DOI] [PubMed] [Google Scholar]
- 10.Lautenbach E, Nachamkin I, Hu B, Fishman NO, Tolomeo P, Prasad P, et al. Surveillance cultures for detection of methicillin-resistant Staphylococcus aureus: Diagnostic yield of anatomic sites and comparison of provider- and patient-collected samples. Infect Control Hosp Epidemiol. 2009;30:380–2. doi: 10.1086/596045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meurman O, Routamaa M, Peltonen R. Screening for methicillin-resistant Staphylococcus aureus: Which anatomical sites to culture? J Hosp Infect. 2005;61:351–3. doi: 10.1016/j.jhin.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Eveillard M, de Lassence A, Lancien E, Barnaud G, Ricard JD, Joly-Guillou ML. Evaluation of a strategy of screening multiple anatomical sites for methicillin-resistant Staphylococcus aureus at admission to a teaching hospital. Infect Control Hosp Epidemiol. 2006;27:181–4. doi: 10.1086/500627. [DOI] [PubMed] [Google Scholar]


