Abstract
In two experiments, the time course of the expression of fear in trace (hippocampus-dependent) versus delay (hippocampus-independent) conditioning was characterized with a high degree of temporal specificity using fear-potentiated startle. In experiment 1, groups of rats were given delay fear conditioning or trace fear conditioning with a 3- or 12-sec trace interval between conditioned stimulus (CS) offset and unconditioned stimulus (US) onset. During test, the delay group showed fear-potentiated startle in the presence of the CS but not after its offset, whereas the trace groups showed fear-potentiated startle both during the CS and after its offset. Experiment 2 compared the time course of fear expression after trace conditioning with the time course in two delay conditioning groups: one matched to the trace conditioning group with respect to CS duration, and the other with respect to ISI. In all groups, fear was expressed until the scheduled occurrence of the US and returned to baseline rapidly thereafter. Thus, in both trace and delay fear conditioning, ISI is a critical determinant of the time course of fear expression. These results are informative as to the possible role of neural structures, such as the hippocampus, in memory processes related to temporal information.
Although in Pavlovian delay conditioning presentations of the conditioned stimulus (CS) and the unconditioned stimulus (US) overlap, in trace conditioning a temporal gap or “trace” interval is interposed between the CS and the US. Even though eyeblink (i.e., skeletal) and fear (i.e., emotional) conditioning rely upon different neural substrates, the hippocampus has been shown to be critically involved in trace conditioning in both preparations (Moyer Jr. et al. 1990; McEchron et al. 1998; Quinn et al. 2002). Thus, the hippocampus is unlikely to be involved simply in the expression of the motoric or emotional responses associated with trace conditioning. Rather, given that trace (hippocampally dependent) and delay (hippocampally independent) conditioning differ solely with respect to the relative timing of CS and US presentation, the hippocampus would appear to be critically involved in mnemonic processes related to encoding the temporal relationship between the CS and the US. To understand hippocampal function, it is important, therefore, to characterize how the timing of conditioned responses in delay and trace conditioning relates to the timing of occurrences of the CS and US in training (see Quinn et al. 2002). In the current studies, this issue was addressed by using the acoustic startle reflex to assess the time course of the expression of conditioned fear after trace and delay conditioning.
Some evidence supporting the contention that the hippocampus is involved in the temporal aspects of memory for trace conditioning is derived from the finding that hippocampal lesions sometimes simply cause a diminution in the latency and amplitude of conditioned eyeblink responses, rather than an outright block of trace eyeblink conditioning (Port et al. 1986; Solomon et al. 1986). Similar timing effects have not been observed in trace fear conditioning (McEchron et al. 1998, 2000; Quinn et al. 2002). However, this may be related to the selection of specific behavioral measures (freezing and heart rate conditioning) as the dependent measures of fear. By using freezing, fear is observed during the CS and after its offset after delay, trace, contextual, and backward fear conditioning (Quinn et al. 2002), indicating that the time course of freezing may not be tightly coupled to the onset and offset of fear-eliciting stimuli. Although changes in heart rate show higher temporal resolution, individual differences in subjects' tendency to respond during the CS and after its offset make it difficult to detect specific shifts in timing resulting from hippocampal lesions (McEchron et al. 2000). Hence, these measures may not be ideally suited for measuring the temporal dynamics of fear conditioning.
The current study used fear-potentiated startle, or the increase in acoustic startle reflex amplitude elicited in the presence of a fearful CS, to map the time course of fear expression after trace and delay fear conditioning. The startle reflex has a nonzero baseline, is graded in magnitude, and can be elicited by the experimenter at specific time points both during and after CS presentation. This allows one to compare the strength of the subject's responses elicited at multiple time points to the subject's baseline responses, essentially providing a readout of the level of fear at any given moment. Consequently, this measure has proved useful in detecting changing levels of fear with a high degree of temporal specificity (Davis et al. 1989). The current experiments were designed specifically to determine which cues controlled the expression of conditioned fear in delay versus trace conditioning. Three possibilities were considered:
Role of CS Onset. The expression of fear in trace and delay conditioning may be related to the time of occurrence of the US in training relative to the onset of the CS, even though the CS is no longer present when the US occurs in the trace conditioning paradigm. This pattern of conditioned response magnitude has been observed in delay and trace eyeblink conditioning (Schneiderman and Gormazano 1964; Smith 1968; Smith et al. 1969; Kehoe and Napier 1991). It has also been found in delay fear conditioning, in which the magnitude of fear-potentiated startle increases gradually from CS onset until the scheduled occurrence of the US (Davis et al. 1989). Furthermore, by using a conditioned emotional response (CER) measure, Kamin (1965) found an inverse relationship between the strength of trace fear conditioning and the CS-US onset interval (i.e., interstimulus interval [ISI]), when the trace interval was held constant.
Role of CS Offset. Offset of the CS may be an essential cue for the expression of fear in trace conditioning and/or for the termination of fear in delay conditioning. Not only has CS offset been shown to serve as a cue for conditioning in other paradigms (Kamin 1965), but its close temporal proximity to the US, relative to CS onset, would tend to favor its entering into an association with the US (Schneiderman and Gormazano 1964; Ost and Lauer 1965). Interestingly, in delay fear conditioning, fear-potentiated startle decayed only slowly when the CS was extended beyond the scheduled occurrence of the US (Davis et al. 1989), suggesting that CS offset may play some role in the decay of fear. Furthermore, stimulus offset can serve as a potent inhibitor of fear in a feature-negative paradigm (Falls and Davis 1997). CS offset also appears to be influential in the timing of appetitive responses in an instrumental trace conditioning paradigm (Buhusi and Meck 2000).
Role of Contextual Cues. In trace conditioning, but not in delay conditioning, the CS may serve as a retrieval cue for context-US associations, as suggested by Quinn et al.(2002). That is, the continuing presence of contextual cues may bridge the interval between CS offset and the eventual occurrence of the US. Under this scenario, one would expect fear to persist for an extended period after the presentation of the CS after trace but not after delay conditioning. The possibility that contextual cues play an important role in trace fear conditioning is supported by the strong level of collateral conditioning to contextual cues seen in trace fear conditioning (Rawlins and Tanner 1998), particularly when a long (i.e., 30-sec) trace interval is used (Marlin 1981).
Two experiments were conducted in order to distinguish among these possibilities. In experiment 1, rats were given either trace or delay training, which were identical with respect to CS duration but differed in terms of the CS-US onset interval (i.e., ISI). The subjects were then tested for fear-potentiated startle by using the same test sessions for all groups. The trace conditioning groups differed from the delay group in showing fear-potentiated startle that persisted after CS offset. However, because the ISI varied between groups, we could not determine whether it was the insertion of a trace interval or simply the use of longer ISIs that produced the longer lasting expression of fear in the trace conditioning groups. Thus, in experiment 2, fear-potentiated startle after trace conditioning was compared with fear-potentiated startle in two delay conditioning groups, matched to the trace group with respect to either CS duration or ISI. The results were most consistent with the interpretation that CS-US onset interval (the ISI) is a critical factor in the expression of conditioned fear after both delay and trace conditioning. These results shed light on the possible function of mnemonic structures (such as the hippocampus) that are involved specifically in the acquisition and expression of trace conditioning but not delay conditioning.
RESULTS
Experiment 1
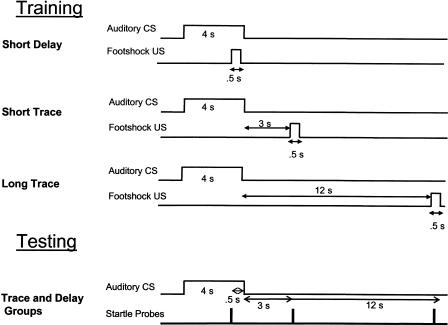
Three groups were given delay fear conditioning (SHORT DELAY, n = 10) or trace fear conditioning with either a 3-sec (SHORT TRACE, n = 12) or 12-sec (LONG TRACE n = 12) trace interval between CS offset and US onset (Fig. 1). The same CS duration (4 sec) was adopted in all groups. Fear-potentiated startle was evaluated in all three groups by using a common testing procedure. On different presentations of the CS, fear-potentiated startle was measured at the time points at which the US had occurred in training in each of the three groups (i.e., 3.5 sec into the CS, or 3 sec or 12 sec after CS offset.)
Figure 1.
Procedures for fear conditioning training and fear-potentiated startle testing in experiment 1. Animals were given delay conditioning, or trace conditioning with a 3-sec or 12-sec trace interval. A 4-sec CS was used in all conditions. All subjects underwent the same test session, in which startle was probed at three time points relative to presentation of the CS.
Findings
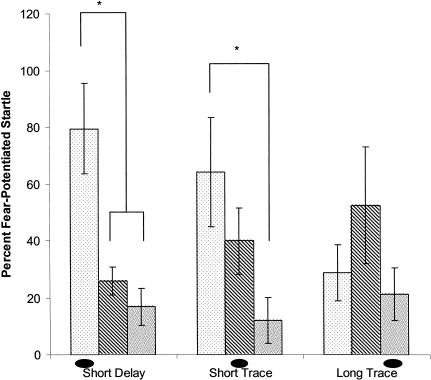
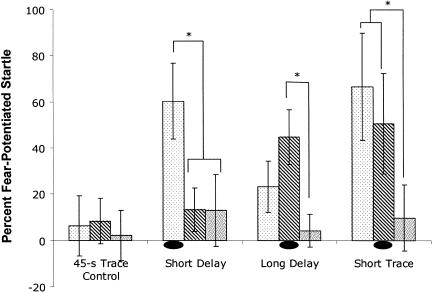
Although delay and trace conditioning produced similar peak levels of fear-potentiated startle, these peaks occurred at different times relative to the CS. This is indicated in Figure 2, which shows the magnitude of fear-potentiated startle in the three groups at each of the three probe times. After delay conditioning, fear-potentiated startle was greatest when tested during the CS. In the SHORT TRACE group, fear-potentiated startle was of similar strength during the CS and 3-sec after its offset, and in the LONG TRACE group, the highest level of fear-potentiated startle was observed 3 sec after CS offset. These observations were supported by statistical analyses. A mixed design (group × probe time) ANOVA revealed no significant main effect of group (P > 0.1), suggesting similar overall magnitude of fear-potentiated startle in delay and trace conditioning. However, fear varied as a function of the timing of the startle probe, as shown by a main effect for time of probe (F(2,62) = 14.04, P < 0.0001). Furthermore, the timing of fear expression differed across the groups, as indicated by a significant interaction between training condition and time of probe (F(4,62) = 4.727, P < 0.01). Newman-Keuls post-hoc tests indicated that fear-potentiated startle in the delay group was significantly greater during the CS than both 3 sec and 12 sec after CS offset (ts = 14.4 and 19.6, respectively, Ps < 0.01). In contrast, in rats trained with a short trace interval, fear-potentiated startle was not significantly different during the CS versus 3 sec after its offset (P > 0.1). However, fear-potentiated startle was significantly lower 12 sec after CS offset than during the CS (t = 16.2, P < 0.01). In the group trained with a long trace interval, fear-potentiated startle did not differ significantly across the three time points at which startle was measured.
Figure 2.
Fear-potentiated startle test in experiment 1. Startle was probed at the time points at which the shock had occurred in one of the groups during training: 3.5 sec into the CS (sparse shading), 3 sec after CS offset (stripes), or 12 sec after CS offset (dense shading). The oval shape denotes the time point relative to CS onset at which the US had occurred in training. The temporal pattern on fear expression was distinct in the three groups, related to differences in the time of US occurrence in training (see text for explanation). *Significantly different (P < 0.05).
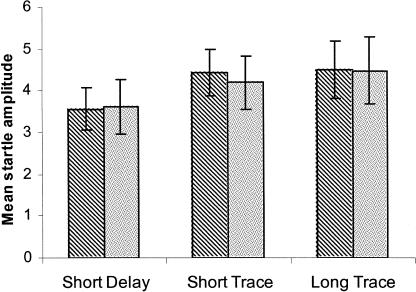
Although fear persisted after CS offset in the trace conditioning groups, startle returned to baseline levels during the interval between successive CS presentations, suggesting that presentation of the CS produced only a transient state of fear. Figure 3 shows mean startle amplitude measured on habituation trials (startle trials that occurred prior to the first presentation of the CS) and on startle-alone trials (trials during the test session in which the CS was not presented). There were no significant differences in any group between mean startle amplitude measured across these two trial types (Ps > 0.1), indicating that fear did not persist for an extended period after the point in time at which the US had occurred in training.
Figure 3.
Mean startle amplitude in each group in habituation trials (i.e., startle stimulus trials occurring before the first CS presentation, stripes) was compared with mean startle amplitude in startle-alone trials (i.e., startle stimulus test trials that were intermixed with CS-containing test trials, shading). All three groups exhibited very similar startle response levels in these two trials types, suggesting that fear dissipated completely between successive presentations of the CS.
Conclusions
In this experiment, trace and delay conditioning were compared by using different ISIs while holding CS duration constant (4 sec) across groups. After delay conditioning, robust fear-potentiated startle was observed only while the CS was present. After trace conditioning, fear-potentiated startle was observed during the CS and after its offset. In both delay and trace conditioning, the fear was transient and consistently returned to baseline during the intertrial interval. This suggests that trace conditioning did not produce a persistent fear response related to the presence of contextual cues, at least with the trace intervals (3 and 12 sec) used in this study. The temporal specificity of fear expression also suggests that the fear response was a result of the explicit association of the CS and US. However, the role of context or nonassociative factors cannot be ruled out definitively without inclusion of a control group in which the CS and US are presented in an unpaired manner.
Although the pattern of fear clearly differed in trace and delay conditioning groups, it was, however, imperfectly associated with the scheduled occurrence of the US (whether timed from CS onset or CS offset). That is, both the LONG TRACE and SHORT TRACE groups showed peak fear-potentiated startle before the exact time of US occurrence. This may suggest that a level of fear sufficient to elevate startle cannot be maintained for durations much >3 sec. It is important to note, however, that startle was not probed at intermediate time-points between 3 and 12 sec. Hence, fear in the LONG TRACE group may actually have peaked at a later time point than in the SHORT TRACE group, albeit prior to the 12-sec time point at which the shock had been delivered in training.
These data clearly serve to demonstrate that the fear response in trace conditioning, as in delay conditioning (Davis et al. 1989), shows a temporal function that is related to when the US occurred in training. A similarly timed emotional response has been suggested previously (albeit measured indirectly) in appetitive trace conditioning (Cole et al. 1995). However, the design of experiment 1 precluded further analysis of potential differences in the timing of fear expression in trace and delay conditioning. First, because CS duration was held constant across the groups, trace conditioning differed from delay conditioning not only with respect to the insertion of trace interval but also with respect to the CS-US onset interval (i.e., ISI). That is, the ISI was longer in both trace groups than in the delay group. To evaluate the relative importance of ISI in timing fear responses, it is important also to equate ISI across trace and delay groups.
Second, these data were not informative as to whether the expression of fear in delay and trace conditioning shows equivalent increase and decay functions around the scheduled occurrence of the US. If CS offset serves as a signal for the inhibition of fear in delay conditioning (see Role of CS Offset), one would predict a more rapid decay of fear in delay conditioning than in trace conditioning, in which there is no cue that similarly signals the termination of footshock. In experiment 1, startle was measured at the three time points at which the shock had been delivered to the different groups in training: before CS offset, 3 sec after CS offset, and 12 sec after CS offset. Thus, even though startle was measured at the point of scheduled occurrence of the US, the groups differed with regard to the timing of startle measurement before and after the scheduled occurrence of the US. For example, in the SHORT DELAY group, startle was measured 3 sec after the scheduled termination of the US, whereas in the SHORT TRACE group, startle was only measured 8.5 sec after the scheduled occurrence of the US (i.e., 12 sec after CS offset). Thus, even though fear-potentiated startle had decayed in both groups at these respective time points, it is not clear whether fear decayed at the same rate. Similarly, although startle was measured 3.5 sec prior to the scheduled occurrence of the US in the SHORT TRACE group, there was no measure of startle prior to the scheduled occurrence of the US in the delay group. Hence, the rate of both the increase and decay of fear in delay and trace conditioning could not be clearly ascertained in experiment 1.
Experiment 2
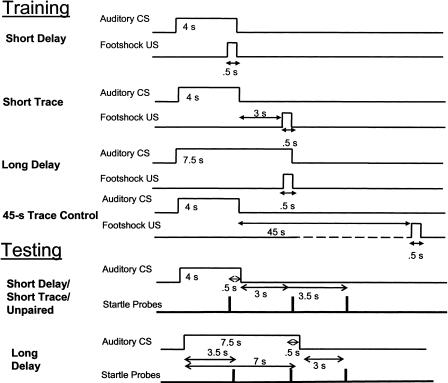
Experiment 2 was designed to examine whether the expression of fear after delay and trace conditioning is controlled by the same cues and follows the same time course based upon the ISI, or whether the expression of fear in trace and delay conditioning differs because of the presence of a temporal gap between the CS and US in the trace conditioning paradigm. To address these issues, we compared the time course of fear after trace conditioning (SHORT TRACE) with the time course of fear in two delay groups: one matched to the trace conditioning group with respect to the CS duration (SHORT DELAY) and one matched with respect to the ISI (LONG DELAY). So that the increase and decay functions of fear expression could be compared, the startle response was measured at similar time points shortly before, after, and at the time of the scheduled occurrence of the US in delay and trace conditioning. In addition, to test for any nonassociative effects of CS and US presentation, a control group (45-S TRACE CONTROL) was also included in which the US was presented 45 sec after CS offset. To ensure that any differences seen in the time course of fear-potentiated startle in the delay- and trace conditioned-groups were not the result of weaker conditioning in the trace group, one additional group (TRACE OVERTRAIN) was given twice as many training sessions as were the other groups. The procedures are illustrated in Figure 4.
Figure 4.
Procedures for fear conditioning training and fear-potentiated startle testing in experiment 2. Subjects were given either trace or delay conditioning, or presentations of the CS and US separated by a 45-sec trace interval (45-sec TRACE CONTROL). Delay conditioning was conducted in two groups, using either a 4-sec or 7.5-sec CS. In the fear-potentiated startle test, startle was probed at the same three time points relative to CS onset in all four groups.
Findings
As shown in Figure 5, in the SHORT TRACE group and in both delay groups fear-potentiated startle was observed until the scheduled occurrence of the US, at which point it declined rapidly. The 45-sec TRACE CONTROL group showed no fear-potentiated startle at any of the test intervals. These observations were supported by statistical analyses. A two-way mixed-design (group × probe time) ANOVA found a significant main effect for probe time (F(2,128) = 12.5, P < 0.0001) and a significant group by probe time interaction (F(6,128) = 3.7, P < 0.01), suggesting that different patterns of fear were produced by the several training conditions. Fear-potentiated startle in the SHORT TRACE group was significantly higher at the 3.5- and 7.0-sec (i.e., scheduled occurrence of the US) intervals than at the 10.5-sec interval, as indicated by Newman-Keuls post-hoc tests (ts = 19.9 and 10.2, Ps < 0.01 and 0.05, respectively). The SHORT DELAY group showed significantly higher fear-potentiated startle at the 3.5-sec interval than at the other intervals (ts = 14.9 and 15.1, Ps < 0.01). The LONG DELAY group showed peak fear-potentiated startle at the 7.0-sec interval (i.e., the scheduled occurrence of the US in this group). However, fear-potentiated startle was not statistically different at this interval compared with the 3.5-sec interval (P > 0.1). Fear-potentiated startle was significantly greater at the 7.0-sec interval than at the 10.5-sec interval (t = 11.0, P < 0.05).
Figure 5.
Fear-potentiated startle test in experiment 2. Startle was probed 3.5 sec after CS onset (sparse shading), 7.0 sec after CS onset (stripes), and 10.5 sec after CS onset (dense shading). The oval shape denotes the time point relative to CS onset at which the US had occurred in training. Fear-potentiated startle was observed both before and coincident with, but not after, the scheduled occurrence of the US (3.5 sec for the SHORT DELAY, 7.0 sec for the SHORT TRACE and LONG DELAY groups). *Significantly different (P < 0.05).
Comparisons between the groups revealed similar levels of fear-potentiated startle in the SHORT DELAY group and in the SHORT TRACE group at the 3.5-sec interval (P > 0.1). Also, the LONG DELAY group produced similar levels of fear-potentiated startle compared with that of the SHORT TRACE group at the 7.0-sec interval (P > 0.1), even though the CS was present at this time in the delay group but not in the trace group.
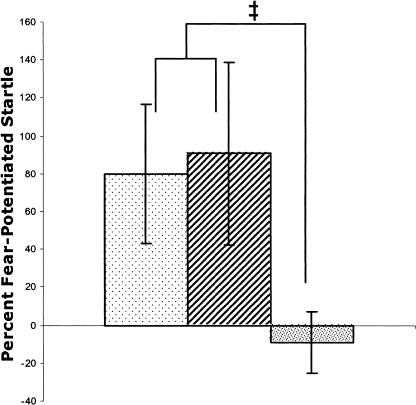
The TRACE OVERTRAIN group showed elevated fear-potentiated startle at the 3.5-sec interval and the 7.0-sec interval (Fig.6). A repeated-measures ANOVA revealed a significant effect for probe time (F(2,7) = 3.74, P < 0.05). Subsequent preplanned repeated-measures two-tailed t-tests revealed no significant differences between the 3.5-sec and the 7.0-sec interval (P > 0.10). There were trends toward higher fear-potentiated startle at the 3.5- and 7.0-sec intervals than at the 10.5-sec interval (ts = 2.2 and 1.9; Ps < 0.10).
Figure 6.
Fear-potentiated startle test after overtraining of trace conditioning. Startle was probed 3.5 sec after CS onset (sparse shading), 7.0 sec after CS onset (stripes), and 10.5 sec after CS onset (dense shading). Fear-potentiated startle was observed before and during the anticipated occurrence of the US, but not 3-sec after its offset. There were no significant differences between groups. ‡P < 0.10.
Conclusions
Experiment 2 supported the outcome of experiment 1 by further demonstrating a temporally specific pattern of fear in both trace and delay conditioning. Such was the precision of the timing of fear in delay and trace conditioning groups that there was no significant fear-potentiated startle as soon as 3 sec after the point in time of scheduled occurrence of the US. Because CS offset does not signal US occurrence in trace conditioning, and the decay of fear is similarly rapid in trace and delay conditioning, it is therefore unnecessary to posit a role for CS offset in the inhibition of fear in delay conditioning. Furthermore, there was no significant level of fear-potentiated startle at any of the startle probe intervals in the group trained with a 45-sec trace interval (45-S TRACE CONTROL). Hence, not only was fear a discretely timed response but it appeared to be related specifically to the relative timing of CS and US presentations in training.
As in experiment 1, it was also notable that the continued presence of the CS was not required for robust fear-potentiated startle to be observed. Thus, in the SHORT TRACE group, fear was approximately as strong 3 sec after CS offset as it was during the CS in the delay groups (Fig. 5). Moreover, in both groups conditioned with a 3-sec trace interval (SHORT TRACE and TRACE OVERTRAIN), fear-potentiated startle was robust during the CS, as well as 3 sec after its offset (Figs. 5, 6), suggesting that CS offset is not required in trace conditioning for expression of fear (see Role of CS Offset). Hence, the results of experiment 2 are most consistent with the possibility that the ISI is the critical temporal component encoded in both delay and trace conditioning.
DISCUSSION
By using the fear-potentiated startle reflex, this study compared the time course of fear expression after trace and delay Pavlovian conditioning. In experiment 1, trace and delay conditioning produced different patterns of fear when the CS duration was held constant. Fear-potentiated startle was greatest during CS presentation in both delay conditioning and in trace conditioning when the trace interval was short (3 sec), but fear-potentiated startle was greatest after the offset of the CS when the trace interval was long (12 sec). Experiment 2 compared trace conditioning with delay conditioning in two groups, equated with the trace group with respect to either CS duration or ISI. This experiment also included startle probes in close temporal proximity to CS offset and scheduled US occurrence in order to compare the characteristics of the increase and decay of fear in delay and trace conditioning. Once again, this experiment indicated that given sufficient training, fear was maximally expressed in both delay and trace conditioning before or around the time of scheduled occurrence of the US. Furthermore, in both delay and trace paradigms, fear was observed to have dissipated completely as soon as 3 sec after the time point of scheduled US presentation.
The most parsimonious interpretation of the current data set is that both delay and trace fear conditioning are underpinned by a common learning mechanism, related to encoding the time that elapses between CS onset and US onset. However, one cannot entirely rule out the possibility that CS offset additionally serves as a “safety signal” in delay conditioning and/or as an excitatory cue in trace conditioning. Such a determination would require extending the duration of the CS during test sessions beyond the duration used in training. Unfortunately, a substantial diminution in fear-potentiated startle resulted in an experiment in which CS duration was varied in test (data not shown), a finding, also shown in other preparations, that may be attributable to an enhancement of extinction (Kehoe and Napier 1991; Haselgrove and Pearce 2003). Nonetheless, any role for CS offset in either delay or trace fear conditioning is likely to be relatively minor. In trace conditioning, fear-potentiated startle was observed to be robust prior to CS offset, even after extensive training, making it unlikely that CS offset plays a central role in triggering a state of fear. Similarly, the decay of fear in trace conditioning was very rapid (within 3 sec) after the time of scheduled US occurrence, meaning that one does not have to invoke any additional mechanisms to explain the rapid decay of fear seen after CS offset in delay conditioning.
It has long been known that the conditioned eyeblink response comes to coincide with the time of US presentation in trace and in delay conditioning, if sufficient training is provided (Schneiderman and Gormazano 1964; Smith 1968; Smith et al. 1969; Kehoe and Napier 1991). Delay fear conditioning, measured by using fear-potentiated startle, is also expressed maximally at a time point that is coincident with ISI (Davis et al. 1989). In contrast, by using heart-rate or freezing as a measure of conditioning, fear has been observed to persist, and even on occasion to peak, after CS offset in delay and in trace conditioning (Quinn et al. 2002; McEchron et al. 2003; Weitemier and Ryabinin 2003), even though the hippocampus appears to encode the CS-US interval (McEchron et al. 2003). This difference between freezing and fear-potentiated startle could be at least in part attributable to differences in sensitivity of the two behavioral measures to contextual fear. Fear-potentiated startle is insensitive to contextual conditioning after the first habituation trials (McNish et al. 1997), which are routinely excluded from the analysis of baseline startle when assessing fear-potentiated startle to an explicit CS. In the current study, we demonstrated that startle returned to baseline levels between successive CS presentations. In addition, there was no significant fear-potentiated startle in a group trained with a 45-sec trace interval (45-S TRACE CONTROL). This strongly suggests that fear-potentiated startle measured by using the current procedure was also insensitive to contextual fear. It is possible, therefore, that freezing and fear-potentiated startle measured after CS offset reflect somewhat different associative phenomena. That is, perhaps freezing is sensitive to fear resulting from a CS-context configural association, even when the CS is presented in a novel context (Quinn et al. 2002), whereas fear-potentiated startle is sensitive to fear resulting from the CS alone.
This study also differed from the freezing studies in using shorter trace intervals. In a CER conditioning procedure, Marlin (1981) found that a 30-sec trace interval—the interval most typically used in freezing studies—did not produce measurable fear to the CS but did produce robust contextual conditioning. This is consistent with the weaker fear-potentiated startle (in numerical, but not statistical, terms) observed in the current study when a long (12-sec) trace interval was used, and the absence of fear-potentiated startle when a 45-sec interval was used. Hence, an association between the CS and contextual cues may be encouraged by the use of longer trace intervals. Indeed, if, under these circumstances, acquisition of conditioned fear were only possible through the recruitment of contextual cues, it would be difficult to distinguish the effects of hippocampal lesions on trace conditioning per se from the known effects of hippocampal lesions on contextual fear conditioning (Phillips and LeDoux 1994; Maren et al. 1997).
The use of short trace intervals might also have been responsible for the considerable strength of trace conditioning obtained in this study, which was not significantly weaker than was delay conditioning. Previous research has indicated that the strength of trace conditioning is related to both the trace interval and overall ISI (Smith 1968; Smith et al. 1969; Marlin 1981; Blazis and Moore 1991). Several studies have found differences between trace and delay conditioning when compared in terms of rate of acquisition (Schneiderman and Gormazano 1964; Smith et al. 1969; Beylin et al. 2001). Even though trace conditioning is acquired more slowly than is delay conditioning, it appears to reach a similar asymptote after sufficient training (Ellison 1964). In pilot studies, we found trace conditioning to be less reliably produced than is delay fear conditioning after 1 d of training, using our standard acquisition and testing procedures (data not shown). Thus, in studies designed to test the effects of specific manipulations or interventions (e.g., involving the hippocampus) in which it is important to equate the strength of trace conditioning with the strength of delay conditioning, the use of a short trace interval and/or a large amount of training is probably recommended.
The timing of trace fear-potentiated startle established here can be used in future studies to understand the role of the hippocampus in this form of learning. It has been important, for example, to ascertain whether a hippocampal lesion or inactivation completely blocks trace conditioning, or whether it disrupts timing of the expression of fear. It may also be informative to compare the effects of pretraining and posttraining disruptions of hippocampal function by using the present set of behavioral procedures. The facts that (1) trace conditioning only differs procedurally from delay conditioning by virtue of the insertion of a trace interval after CS offset, and (2) trace but not delay conditioning is disrupted by hippocampal manipulations, might suggest a role for the hippocampus in retaining the CS representation in working memory until the US occurs. This would be consistent with the involvement of the hippocampus in a number of working memory tasks (see Lee and Kesner 2003). On the other hand, the facts that both posttraining hippocampal lesions (Kim et al. 1995) and interference with hippocampal neurogenesis (Shors et al. 2001) disrupt trace eyeblink conditioning suggest a role for the hippocampus in long-term plasticity for trace memories.
Reconciling these two apparently contrasting notions of hippocampal function in trace conditioning may be facilitated by studying the effects of hippocampal manipulations on the long-term memory for the timing of conditioned fear responses, an approach that is currently under way (Shi et al. 2000; Mathews et al. 2001). Moreover, in light of recent accounts of spatial learning in terms of associations between successive events separated along the dimension of time (Wallenstein et al. 1998; Foster et al. 2000; Fortin et al. 2002), these studies may be important in interpreting the role of the hippocampus not only in trace conditioning per se but also in hippocampally dependent forms of learning more generally.
MATERIALS AND METHODS
Subjects
Male albino Sprague-Dawley rats (Charles River, Wilmington, MA) weighing between 300 and 400 g at the start of the experiment were used in the present studies. Animals were housed in groups of eight to a cage and were maintained on a 12-h light/dark cycle with food and water continuously available. Animals were allowed to acclimate to the experimental housing for 2 weeks after arrival in the colony. All experimental procedures conformed to the Guidelines for the Humane Care and Use of Laboratory Animals of the Institutional Animal Care and Use Committee from the University of Minnesota.
Apparatus
Animals were tested in four identical 8 × 15 × 15-cm stabilimeter devices. Each stabilimeter consisted of a Plexiglas cage, which rested on four compression springs and was located within a ventilated sound-attenuating chamber. Cage movement resulted in displacement of a type 338B35 accelerometer (PCB Piezotronics) attached to the top of each cage. The resultant voltage of the accelerometer was proportional to the velocity of the cage displacement. This signal was amplified by a signal processing unit (482820 PCB Piezotronics). An InstruNet 100b board (GW Instruments) interfaced to a Macintosh G3 microcomputer digitized the analog output of the accelerometer on a scale of 0 to 10 units. Startle amplitude was defined as the peak accelerometer voltage that occurred during the first 200 msec after onset of the startle stimulus. High-frequency speakers (Radio Shack Super-tweeters, range 5 to 40 kHz, 40-1310b) located 5 cm behind each cage delivered the startle stimuli. The startle stimuli were 50-msec (increase-decay, 5 msec) bursts of white noise (low pass, 22 kHz) at various intensities. The ventilation fans of the sound-attenuating chamber elevated background noise to 65 dB. The foot shock was a 0.5-sec, 0.6-mA constant current scrambled shock, delivered by a shock generator (SGS-004, by BRS-LVE) through the four bars that made up the bottom of the stabilimeter. Shock intensity was measured with a 1-kΩ resistor across a differential channel of an oscilloscope in series with a 100-kΩ resistor connected between two floor bars in each cage. Current was defined as the root mean square voltage across the kΩ resistor where mA = 0.707 × 0.5 × peak-to-peak voltage. The CS was a 4-sec or a 7.5-sec, 75-dB band pass-filtered noise, with high and low cut-offs set at 4 kHz and 24 dB per octave attenuation. The noise was generated by the computer and delivered through a low-frequency speaker (Radio Shack woofer, model 40-1024A) situated 15 cm from the cage.
Baseline Startle Sessions
To acclimate the rats to the apparatus and startle stimuli, and to measure levels of baseline startle, the naive rats underwent 2 days of baseline startle testing prior to training. After a 5-min acclimation period, they were presented with 28 startle stimuli, seven at each of four intensities (90, 95, 100, 105 dB). The various stimulus intensities were presented in a semirandom order with a 45-sec ISI. Startle amplitudes for the second day alone were averaged across all 28 startle stimuli and were used to match the animals into groups with similar overall mean startle amplitude (see Davis et al. 1989; Falls et al. 1992).
Fear-Conditioning Procedure
After baseline startle sessions, acquisition was conducted over three successive days for both experiments 1 and 2, except for one group in experiment 2 that was given 6 d of training (see below). On each day, the rats were presented with 16 tone-shock pairings after a 5-min acclimation period. The interval between shocks was variable, with a mean of 2.75 min (range, 2.0 to 3.5 min). In experiment 1, for delay conditioning, the 0.5-sec shock overlapped and coterminated with a 4-sec CS (SHORT DELAY; n = 10). For trace conditioning, the onset of the shock occurred 3 sec (SHORT TRACE; n = 12) or 12 sec (LONG TRACE; n = 12) after offset of the 4-sec CS. In experiment 2, for the delay-conditioned animals, the 0.5-sec shock overlapped and coterminated with either a 4-sec (SHORT DELAY; n = 12) or a 7.5-sec CS (LONG DELAY; n = 12). In the trace-conditioned group, the onset of the shock occurred 3 sec after offset of the 4-sec CS (SHORT TRACE; n = 11). In the control group, the shock occurred 45 sec after CS offset (45-sec TRACE CONTROL; n = 7). To ensure that any differences seen between trace and delay conditioning were not a function of strength of learning, an additional group was given more extensive trace conditioning training (OVERTRAINED TRACE; n = 8). Training was conducted by using the same arrangement of stimuli as in the SHORT TRACE group, except that 6 days of training were given. In addition, to monitor the strength of fear-potentiated startle acquired through training, animals in the OVERTRAINED TRACE GROUP were given several fear-potentiated startle test trials prior to training trials on days 2, 4, and 6 of training (see below).
Testing Procedure
All subjects were given a test session 24 to 48 h after completion of acquisition. After a 5-min acclimation period, 30 startle stimuli (15 at each of 95 and 105 dB) were delivered in an intermixed sequence to habituate the rodents to the startle stimulus. Only two dB levels were used during the test session to minimize the overall number of trials and, thus, extinction. Only the last 20 stimuli were retained for analysis (habituation trials). These were followed immediately by presentations of the test trials. The number and type of test trial was a function of condition (see below). For experiment 1, startle stimuli (95 and 105 dB) were presented either alone (startle-alone trials), 0.5 sec prior to offset of the CS, 3 sec after offset of the CS, or 12 sec after offset of the 4-sec CS (i.e., the time of US onset during acquisition for each one of the groups; Fig. 1). There were eight of each of the three CS-containing test trials and 16 of the startle-alone test trials (trials in which the startle stimulus was presented without the CS), presented in a pseudorandom order. The CS duration was fixed at 4 sec. The interval between successive startle stimuli was 45 sec throughout the session. The CS-containing test trials were compared with the startle-alone trials to measure fear-potentiated startle. For experiment 2, startle stimuli (95 and 105 dB) were presented either alone (startle-alone trials), 3.5 sec after CS onset, 7.0 sec after CS onset, or 10.5 sec after CS onset (Fig. 4). There were six of each of the three CS-containing test trials and six of the startle alone test trials (three at each dB level), presented in a pseudorandom order. The CS duration varied as a function of the acquisition group. Thus, although the SHORT TRACE, the SHORT DELAY group, and the 45-S TRACE CONTROL group received identical test sessions, the LONG DELAY group was tested with a longer CS duration. The interval between successive startle stimuli was 45 sec. In the OVERTRAINING TRACE group, short tests occurred on days 2, 4, and 6 of the 6 days of training in order to monitor strength of conditioning (Kim and Davis 1993). These tests consisted of 10 startle stimuli presented alone, followed by six CS-startle trials intermixed with two startle-alone trials that occurred before training began. On each of two CS-startle trials, the startle stimulus was presented 3.5, 7.0, and 10.5 sec after CS onset. The startle stimuli (105 dB) were presented at 45-sec intervals. On days 1, 3, and 5 of training, the animals were given the same total number (i.e., 18) of 105-dB startle stimuli before training, but without presentation of the CS. On day 7, the testing procedure was identical to that of the SHORT TRACE group.
Grouping and Statistics
Percentage of fear-potentiated startle was calculated by comparing mean startle obtained on each CS trial type to mean startle obtained on startle-alone trials (i.e., [CS-startle - startle alone]/startle alone × 100%). These data were analyzed by using ANOVA, as in other studies (Davis et al. 1989; Falls et al. 1992). Two-way mixed-design (group × startle probe time) ANOVAs were used in both experiments 1 and 2, followed by Neuman-Keul's post-hoc tests where appropriate. One animal in the SHORT TRACE group in experiment 2 was excluded as a statistical outlier for having fear-potentiated startle >2.6 SD above the mean (i.e., P < 0.01).
Acknowledgments
Special thanks are extended to Kirsten Westergard, Nathaniel Kreager, and Mark Liszewski for their help in gathering data.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.66004.
References
- Beylin, A.V., Gandhi, C.C., Wood, G.E., Talk, A.C., Matzel, L.D., and Shors, T.J. 2001. The role of the hippocampus in trace conditioning: Temporal discontinuity or task difficulty? Neurobiol. Learn. Mem. 76: 447-461. [DOI] [PubMed] [Google Scholar]
- Blazis, D.E. and Moore, J.W. 1991. Conditioned stimulus duration in classical trace conditioning: Test of a real-time neural network model. Behav. Brain Res. 43: 73-78. [DOI] [PubMed] [Google Scholar]
- Buhusi, C.V. and Meck, W.H. 2000. Timing for the absence of a stimulus: The gap paradigm reversed. J. Exp. Psychol. Anim. Behav. Process 26: 305-322. [DOI] [PubMed] [Google Scholar]
- Cole, R.P., Barnet, R.C., and Miller, R.R. 1995. Effect of relative stimulus validity: Learning or performance deficit? J. Exp. Psychol. Anim. Behav. Process 21: 293-303. [DOI] [PubMed] [Google Scholar]
- Davis, M., Schlesinger, L.S., and Sorenson, C.A. 1989. Temporal specificity of fear conditioning: Effects of different conditioned stimulus-unconditioned stimulus intervals on the fear-potentiated startle effect. J. Exp. Psychol. Anim. Behav. Process 15: 295-310. [PubMed] [Google Scholar]
- Ellison, G.D. 1964. Differential salivary conditioning to traces. J. Comp. Physiol. Psychol. 57: 373-380. [DOI] [PubMed] [Google Scholar]
- Falls, W.A. and Davis, M. 1997. Inhibition of fear-potentiated startle can be detected after the offset of a feature trained in a serial feature-negative discrimination. J. Exp. Psychol. Anim. Behav. Process 23: 3-14. [DOI] [PubMed] [Google Scholar]
- Falls, W.A., Miserendino, M.J., and Davis, M. 1992. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J. Neurosci. 12: 854-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin, N.J., Agster, K.L., and Eichenbaum, H.B. 2002. Critical role of the hippocampus in memory for sequences of events. Nat. Neurosci. 5: 458-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, D.J., Morris, R.G., and Dayan, P. 2000. A model of hippocampally dependent navigation, using the temporal difference learning rule. Hippocampus 10: 1-16. [DOI] [PubMed] [Google Scholar]
- Haselgrove, M. and Pearce, J.M. 2003. Facilitation of extinction by an increase or a decrease in trial duration. J. Exp. Psychol. Anim. Behav. Process 29: 153-166. [DOI] [PubMed] [Google Scholar]
- Kamin, L.J. 1965. Temporal and intensity characteristics of the conditioned stimulus. In Classical conditioning: A symposium (ed. W. F. Prosaky), pp 118-147. Appleton-Century-Crofts, New York.
- Kehoe, E.J. and Napier, R.M. 1991. In the blink of an eye: Real-time stimulus factors in delay and trace conditioning of the rabbit's nictitating membrane response. Q. J. Exp. Psychol. B 43: 257-277. [PubMed] [Google Scholar]
- Kim, J.J., Clark, R.E., and Thompson, R.F. 1995. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav. Neurosci. 109: 195-203. [DOI] [PubMed] [Google Scholar]
- Kim, M. and Davis, M. 1993. Electrolytic lesions of the amygdala block acquisition and expression of fear-potentiated startle even with extensive training but do not prevent reacquisition. Behav. Neurosci. 107: 580-595. [DOI] [PubMed] [Google Scholar]
- Lee, I. and Kesner, R.P. 2003. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J. Neurosci. 23: 1517-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren, S., Aharonov, G., and Fanselow, M.S. 1997. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav. Brain Res. 88: 261-274. [DOI] [PubMed] [Google Scholar]
- Marlin, N. 1981. Contextual associations in trace conditioning. Anim. Learn. Behav. 9: 519-523. [Google Scholar]
- Mathews, E., Shi, C.-J., and Davis, M. 2001. Muscimol administration into dorsal hippocampus blocks the acquisition of trace and contextual fear conditioning measured with freezing and fear-potentiated startle. Soc. Neurosci. Abstracts, 27: Program 531.15.1403.
- McEchron, M.D., Bouwmeester, H., Tseng, W., Weiss, C., and Disterhoft, J.F. 1998. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus 8: 638-646. [DOI] [PubMed] [Google Scholar]
- McEchron, M.D., Tseng, W., and Disterhoft, J.F. 2000. Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus 10: 739-751. [DOI] [PubMed] [Google Scholar]
- ____. 2003. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J. Neurosci. 23: 1535-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNish, K.A., Gewirtz, J.C., and Davis, M. 1997. Evidence of contextual fear after lesions of the hippocampus: A disruption of freezing but not fear-potentiated startle. J. Neurosci. 17: 9353-9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer Jr., J.R., Deyo, R.A., and Disterhoft, J.F. 1990. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav. Neurosci. 104: 243-252. [DOI] [PubMed] [Google Scholar]
- Ost, J.W.P. and Lauer, D.W. 1965. Some investigations of classical salivary conditioning in the dog. In Classical conditioning: A symposium (ed. W.F. Prosaky), pp 192-207. Appleton-Century-Crofts, New York.
- Phillips, R.G. and LeDoux, J.E. 1994. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn. Mem. 1: 34-44. [PubMed] [Google Scholar]
- Port, R.L., Romano, A.G., Steinmetz, J.E., Mikhail, A.A., and Patterson, M.M. 1986. Retention and acquisition of classical trace conditioned responses by rabbits with hippocampal lesions. Behav. Neurosci. 100: 745-752. [DOI] [PubMed] [Google Scholar]
- Quinn, J.J., Oommen, S.S., Morrison, G.E., and Fanselow, M.S. 2002. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus 12: 495-504. [DOI] [PubMed] [Google Scholar]
- Rawlins, J.N. and Tanner, J. 1998. The effects of hippocampal aspiration lesions on conditioning to the CS and to a background stimulus in trace conditioned suppression. Behav. Brain Res. 91: 61-72. [DOI] [PubMed] [Google Scholar]
- Schneiderman, N. and Gormazano, I. 1964. Conditioning of the nictitating membrane of the rabbit as a function of the CS-US interval. J. Comp. Physiol. Psychol. 57: 188-195. [DOI] [PubMed] [Google Scholar]
- Shi, C.-J., Mathews, E., and Davis, M. 2000. Muscimol administration into dorsal hippocampus blocks the expression of trace fear conditioning, but not context fear, measured with fear-potentiated startle. Soc. Neurosci. Abstracts, 26: Program 171.11.
- Shors, T.J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T., and Gould, E. 2001. Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372-376. [DOI] [PubMed] [Google Scholar]
- Smith, M.C. 1968. CS-US interval and US intensity in classical conditioning of the rabbits nictitating membrane response. J. Comp. Physiol. Psychol. 66: 679-687. [DOI] [PubMed] [Google Scholar]
- Smith, M.C., Coleman, S.R., and Gormazano, I. 1969. Classical Conditioning of the rabbit's nictitating membrane response at backward, simultaneous, and forward CS-US intervals. J. Comp. Physiol. Psychol. 69: 226-231. [DOI] [PubMed] [Google Scholar]
- Solomon, P.R., Vander Schaaf, E.R., Thompson, R.F., and Weisz, D.J. 1986. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav. Neurosci. 100: 729-744. [DOI] [PubMed] [Google Scholar]
- Wallenstein, G.V., Eichenbaum, H., and Hasselmo, M.E. 1998. The hippocampus as an associator of discontiguous events. Trends Neurosci. 21: 317-323. [DOI] [PubMed] [Google Scholar]
- Weitemier, A.Z. and Ryabinin, A.E. 2003. Alcohol-induced memory impairment in trace fear conditioning: A hippocampus-specific effect. Hippocampus 13: 305-315. [DOI] [PubMed] [Google Scholar]