Abstract
Background:
Pilon fractures are challenging to manage because of the complexity of the injury pattern and the risk of significant complications. The soft tissue injury and handling of the soft tissue envelope are crucial in pilon fracture outcomes. The purpose of this study was to evaluate the early rate of complications using the strategy of “soft tissue control” for operative treatment of complex pilon fractures.
Materials and Methods:
36 complex pilon fractures were treated with the “soft tissue control” strategy. Patients followed the standard staged protocol, anterolateral approach to the distal tibia, the “no-touch” technique and incisional negative pressure wound therapy for pilon fractures. Patients were examined clinically at 2-3 weeks and then 8 weeks for complications associated with the surgical technique.
Results:
All fractures were AO/OTA (Orthopaedic Trauma Association) type C fractures (61% C3, 22% C2 and 16% C1). Only one patient developed superficial infection and resolved with antibiotics and local wound care. None developed deep infection.
Conclusions:
The strategy of soft tissue control for treatment of pilon fractures resulted in relatively low incidence of early wound complications in patients with complex pilon fractures.
Keywords: Negative pressure wound therapy, pilon fractures, soft tissue control, staged protocol
INTRODUCTION
Pilon fractures are among the most serious fractures that involve the ankle joint and continue to present a challenge to the orthopedic surgeon. They are often the result of violent trauma and are associated with significant soft tissue damage, osseous comminution and articular surface disruption. Management principles were originally outlined by Rüedi and Allgöwer1 and included reconstruction of the fibula as well as the articular surface of the tibia. The subsequent literature initially revealed high soft tissue complication rates when using these techniques and principles in high-energy injuries.2,3,4,5,6 Current opinion is that the high rates of infection and wound healing problems may be secondary to operating through swollen, compromised soft tissues. This has led to a staged approach to management for high-energy pilon fractures with initial restoration of fibular length and tibial external fixation followed by delayed open reduction internal fixation of the tibia when soft tissue swelling subsides.7,8,9,10,11 In addition to using a staged protocol for high-energy injuries, meticulous handling of soft tissues is mandatory to minimize soft tissue complications.6,11 Careful attention must be directed to soft tissue retraction and wound management. We recommend the staged protocol, anterolateral approach to the distal tibia, the “no-touch” technique where there is no retractor placed in the soft tissues yet adequate visualization is present to permit reduction of the fracture and applying incisional negative pressure wound therapy (NPWT), which we called “soft tissue control,” for treatment the complex pilon fractures.
The purpose of this study was to evaluate the early rate of complications with using the strategy of “soft tissue control” for operative treatment of complex pilon fractures.
MATERIALS AND METHODS
36 close pilon fractures were treated with the two stage protocol at our institution between February 2008 and February 2011. There were 22 men and 14 women. The interval between injury and presentation ranged from 1 h to 24 h (average, 6.7 h). Patients eligible for inclusion were between ages 18 and 65 years with closed pilon fractures OTA (Orthopaedic Trauma Association) 43C. Patients were excluded if they had a chronic disorder of their soft tissues, since this study evaluated soft tissue outcomes. The mechanisms of injury were high-energy falls (n=21), motor vehicle accidents (n=11), crushing injury (n=4). The fractures were classified according to the AO/ASIF (Association for the Study of Internal Fixation) system: 6 were classified as type C1, 8 type C2 and 22 type C3.
The protocol for the treatment consisted of several specific steps. After the initial evaluation in the emergency department, the limb was pulled out to length and placed in a well padded splint. As soon as the patient was medically cleared, he or she was brought to the operating room. The limb was then elevated and the tourniquet was used to decrease bleeding. All cases were associated with fibular fracture. Immediate open reduction and internal fixation of fibular fracture with wound closure was performed. The fibula posterolateral approach at least 6 cm away from the planned anterolateral pilon incision, was chosen. If radiographs revealed a pilon fracture with an associated fracture of the posterior malleolus that was displaced, a posterolateral incision was made midway between the posterior border of the fibula and lateral aspect of the Achilles tendon. The posterolateral approach gave excellent visualization of the posterior aspect of the tibia and posterior malleolar fragment as well as the fibula without creating extensive surgical flaps.12 Through the same incision, open reduction and internal fixation (ORIF) of the posterolateral fragment of the tibia and fibula fractures were achieved. Once completed, the tourniquet was deflated and a medially placed external fixator that spans the ankle joint was applied. The external fixation pins were placed well away from the subsequent surgical incision both proximally and distally. After reduction, the external fixator was assembled [Figure 1]. The purpose of so doing was to maintain tibial length and make the distal tibial fractures relative stable; thus, allowing soft tissue healing. Once edema had resolved, generally within 7-14 days postinjury, surgery could be performed. The fixator was removed, but the pins were left in place. The limb was cleaned and draped in the sterile field from the level of the tourniquet to the toes. The frame was initially removed and flashed for sterility so that could be reused intraoperatively as a distractor. The limb was then exanguinated by elevation and the tourniquet inflated. The anterolateral approach was useful for many complex and complete articular and partial articular fracture patterns. The incision was centered at the ankle parallel to the fourth metatarsal distally and between the tibia and fibula proximally as described by Herscovici et al.13 Full-thickness skin flaps were maintained while dissecting through the subcutaneous tissues. The superficial peroneal nerve would likely cross proximal to the ankle in the surgical field and was identified, mobilized and protected throughout the exposure. The fascia over the anterior compartment of the distal tibia and the extensor compartment was incised sharply and the anterior compartment tendons were all retracted medially. During the procedure, the full-thickness skin flap was gently lifted with a small skin retractor and K-wire was placed distally on the anterior medial portion of the ankle joint proximal to the articular fracture fragments. An additional K-wire was used proximally for retraction. When placed, the K-wires were bent at a right angle to protect any assistants during this procedure. The K-wires used for retraction permitted excellent visualization of the metaphyseal-diaphyseal region and exposure to the medial aspect of the distal tibia. Reduction should proceed to reconstruct the joint surface. During this time, with the retracting K-wires in place, there was no excess traction on the skin and soft tissues. Once adequate reduction of the fracture was obtained, fixation was done. The retraction K-wires were then removed once the anterolateral plate was applied [Figure 1c]. If needed, a second, medially based incision was used for reduction of separate medial malleolar fractures and for percutaneous medial plate or screw application [Figure 2]. Wound closure then beginning with deep Vicryl sutures (Ethicon, Somerville, New Jersey). The extensor retinaculum was closed with interrupted 2.0 Vicryl sutures. The subcutaneous tissue was closed with interrupted 2.0 Vicryl sutures and the skin was closed with interrupted 2.0 nylon vertical mattress sutures. The vacuum assisted closure system (Kinetic Concepts Inc., San Antonio, TX) was used to achieve NPWT over the surgical incision including anterolateral and medial incision if existed. NPWT were applied in the operating room and then changed on postoperative day 2 and every 1-2 days thereafter until wound drainage was minimal. Early limb elevation was done to minimize tension on the skin closure. The limb was placed in a splint for the first 1-2 weeks to maximize soft tissue recovery and then ankle mobilization was started.
Figure 1.
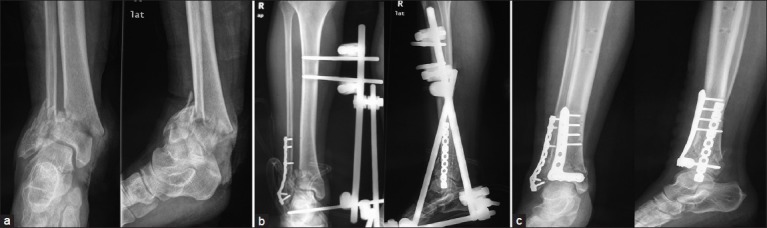
Anteroposterior and lateral X-rays of anke joint showing (a) C2 pilon fracture (b) Internal fixation of the fibula and application of a medial external fixator (c) Anterolateral plate in situ through the anterolateral incision of pilon fracture
Figure 2.
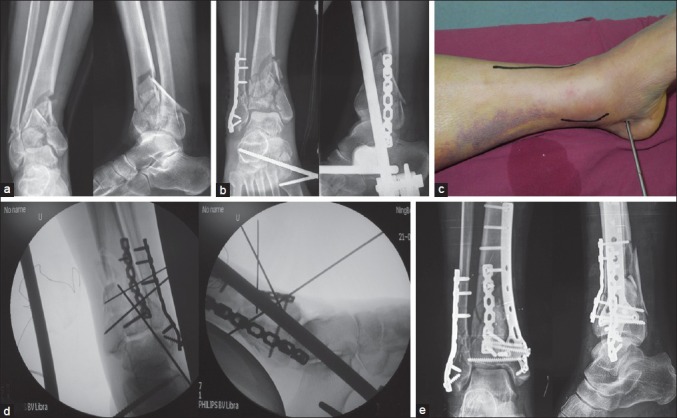
(a) Anteroposterior and lateral X-rays of ankle joint showing of C3 pilon fracture (b) Anteroposterior and lateral X-rays of ankle joint showing internal fixation of the fibula and application of a medial external fixator (c) Preoperative clinical photograph showing the anterolateral incision and a second, medially incision used for percutaneous medial plate or screw application in the second stage (d) Fluoroscopic view showing reduction of pilon fracture through the anterolateral approach with applying external fixator and percutaneous medial plate application after reduction (e) Anteroposterior and lateral X-ray of the pilon fracture showing internal fixation through the anterolateral and a second, medial incision
RESULTS
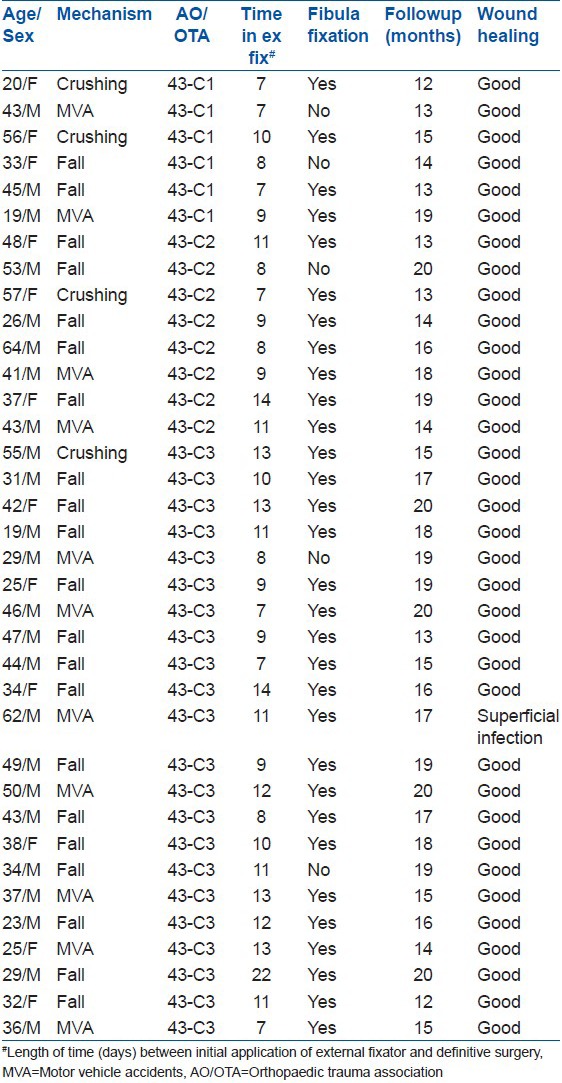
36 patients with pilon fractures were operated on by a single surgeon (HX) using this technique. The characteristics of patients are shown in Table 1. The average time from external fixator placement to definitive ORIF was 10 days (range 7-22 days). All patients who underwent an anterolateral approach were treated using the no touch technique during definitive ORIF. Ten patients required percutaneous medial incision. Patients in the study had NPWT applied to their wound for a mean of 60 h (range 20-219 h). All patients received preoperative intravenous antibiotics and postoperative intravenous antibiotics for 24-48 h. Only one patient developed superficial infection and resolved with antibiotics and local wound care. None of the patients developed deep infection and pin tract infection.
Table 1.
Clinical details of patients
DISCUSSION
Historically, surgical treatment of pilon fractures has been fraught with wound complications and many authors have reported poor results particularly when primary ORIF was used. Teeny and Wiss3 evaluated 60 plafond fractures over 2.5 years. They noted skin slough in 27% of their cases and a 37% infection rate in their high-energy fractures. Seven secondary procedures to obtain soft tissue coverage were required in patient population. Similarly, in a study by McFerran and Smith4 evaluating complications encountered in the treatment of plafond fractures, the local complication rate was 54%. Of these patients, 13 had wound breakdown, 3 had inadequate soft tissue coverage, 9 had a deep soft tissue infection/osteomyelitis and 6 had superficial wound infections. Eighteen soft tissue coverage procedures were required in addition to multiple debridements and closure attempts. This growing body of literature led to the concept that the soft tissue injury can be more severe than the fracture classification implies, leading to an underestimation of the wound complication risk. Subsequently, a staged approach to management for high-energy plafond fractures was developed.
During the first stage and by means of immediate external fixation, the fracture was provisionally stabilized, allowing soft tissue edema to resolve before formal ORIF. Definitive fixation was delayed for 7-22 days until swelling had diminished, fracture blisters had resolved and the skin displayed “wrinkling.” Our average time from injury until fixation was 10 days. This delay was identical to the findings of both Sirkin et al. and Patterson and Cole.7,8 Furthermore, the frame was initially removed and flashed for sterility so that it can be reused intraoperatively as a distractor during the definitive surgery. The reason for this was that the fixator could help maintain length and the definitive plating could then take place with less manipulation of the soft tissues.
Incision placement affects the surgeon's ability to see, reduce and stabilize bony and articular fragments and can further traumatize overlying soft tissues. The use of the anterolateral approach to the distal tibia and ankle has become accepted for ORIF of pilon fractures. This is because of improved understanding of pilon fracture patterns, availability of precontoured anterolateral plates and most importantly because it provides an extensile exposure of the distal tibia and tibiotalar joint. Several reports14,15 have presented evidence that pilon fracture patterns are predictable and that most comminution occurs in the anterolateral aspect of the distal tibia. Herscovici et al.13 described the use of an anterolateral surgical approach to the ankle and stated that this approach has the advantage of providing not only good exposure of the distal tibia, but also easy access to distal anterolateral (Chaput) fracture fragments, thereby allowing direct reduction of the articular surface. Another potential advantage of this approach is that it provides greater soft tissue coverage over the implants when compared with the traditional anteromedial approach. We found that the anterolateral approach provided excellent exposure to the distal tibia for intraarticular pilon fracture reduction and fixation. As a result of the location of the anterolateral incision, a second, medially based incision can be used for reduction of separate medial malleolar fractures and for percutaneous medial plate or screw application.
However, to adequately visualize the fracture during fixation, oftentimes the soft tissues are retracted for a significant amount of time. The retraction of compromised soft tissues and swelling that is often present at the time of fixation may lead to complications. The swelling may be decreased from the initial injury with staged protocol, but is still present at fixation and can contribute to a poor outcome. With the no-touch technique, there is no retraction of the soft tissues, with the exception of the initial placement of the K-wires. Thus, the use of the no-touch technique resulted in a low complication rate.
Although, the concept of the application of NPWT to open and dehisced wounds is well-established in the literature, there is little literature regarding the potential application over incisions to prevent complications in high risk wounds. A recently published study by Timmers et al.16 applied NPWT to patients’ forearms with an intact soft tissue envelope. These data suggest a statistically significant increase in microvascular blood flow to the skin.16 A number of clinical experience and animal data also suggest increased blood flow as a result of application of NPWT.17,18,19,20,21,22,23 The mechanism of action of NPWT to augment wound healing is not completely understood. There are at least three proposed mechanisms: Increased blood flow through the capillary vessels; edema reduction; and mechanical stretching of cells leading to cell growth and expansion.17,24 Based on the results of the above study, application of NPWT to surgical incisions immediately after surgical fixation and closure should be considered after high-energy pilon fractures to decrease wound complications; although, good closure was achieved.25
The limitations of the study are: this is a retrospective study. It does not provide a statistical comparison with a control group for operative treatment of complex pilon fractures. The length of followup for this study was only 8 weeks. The focus of the study was on wound healing and soft tissue complications; therefore, this duration of followup was deemed to be sufficient as opposed to complications arising at a later time caused by nonunion, loss of fixation, malunion or late osteomyelitis. Further study is required to examine these late complications and whether they may be related to the use of the strategy of “soft tissue control.” Finally, our sample size is relatively small and it is therefore difficult to predict trends that may occur with a larger cohort of patients with pilon fractures treated using the strategy of soft tissue control.
To conclude, this study represents a case series of AO/OTA (Orthopaedic Trauma Association) type C pilon fractures treated with the strategy of “soft tissue control.” All fractures reviewed were AO/OTA (Orthopaedic Trauma Association) type C fractures with 61% being type C3. We feel that this played a large role in our ability to manage the soft tissue postoperatively and limit a relatively low incidence of soft tissue complications despite being at a high risk for complication. As successful management of pilon fractures relies in part on avoidance of complications, this series provides evidence that the strategy of “soft tissue control” can reliably be used for fixation of these complex injuries.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Rüedi TP, Allgöwer M. The operative treatment of intraarticular fractures of the lower end of the tibia. Clin Orthop Relat Res. 1979;138:105–10. [PubMed] [Google Scholar]
- 2.Ovadia DN, Beals RK. Fractures of the tibial plafond. J Bone Joint Surg Am. 1986;68:543–51. [PubMed] [Google Scholar]
- 3.Teeny SM, Wiss DA. Open reduction and internal fixation of tibial plafond fractures. Variables contributing to poor results and complications. Clin Orthop Relat Res. 1993;292:108–17. [PubMed] [Google Scholar]
- 4.McFerran MA, Smith SW, Boulas HJ, Schwartz HS. Complications encountered in the treatment of pilon fractures. J Orthop Trauma. 1992;6:195–200. doi: 10.1097/00005131-199206000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Bonar SK, Marsh JL. Tibial plafond fractures: Changing principles of treatment. J Am Acad Orthop Surg. 1994;2:297–305. doi: 10.5435/00124635-199411000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Thordarson DB. Complications after treatment of tibial pilon fractures: Prevention and management strategies. J Am Acad Orthop Surg. 2000;8:253–65. doi: 10.5435/00124635-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Sirkin M, Sanders R, DiPasquale T, Herscovici D., Jr A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 1999;13:78–84. doi: 10.1097/00005131-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Patterson MJ, Cole JD. Two-staged delayed open reduction and internal fixation of severe pilon fractures. J Orthop Trauma. 1999;13:85–91. doi: 10.1097/00005131-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Liporace FA, Mehta S, Rhorer AS, Yoon RS, Reilly MC. Staged treatment and associated complications of pilon fractures. Instr Course Lect. 2012;61:53–70. [PubMed] [Google Scholar]
- 10.Liporace FA, Yoon RS. Decisions and staging leading to definitive open management of pilon fractures: Where have we come from and where are we now? J Orthop Trauma. 2012;26:488–98. doi: 10.1097/BOT.0b013e31822fbdbe. [DOI] [PubMed] [Google Scholar]
- 11.Crist BD, Khazzam M, Murtha YM, Della Rocca GJ. Pilon fractures: Advances in surgical management. J Am Acad Orthop Surg. 2011;19:612–22. doi: 10.5435/00124635-201110000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Ketz J, Sanders R. Staged posterior tibial plating for the treatment of orthopaedic trauma association 43C2 and 43C3 tibial pilon fractures. J Orthop Trauma. 2012;26:341–7. doi: 10.1097/BOT.0b013e318225881a. [DOI] [PubMed] [Google Scholar]
- 13.Herscovici D, Jr, Sanders RW, Infante A, DiPasquale T. Bohler incision: An extensile anterolateral approach to the foot and ankle. J Orthop Trauma. 2000;14:429–32. doi: 10.1097/00005131-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Topliss CJ, Jackson M, Atkins RM. Anatomy of pilon fractures of the distal tibia. J Bone Joint Surg Br. 2005;87:692–7. doi: 10.1302/0301-620X.87B5.15982. [DOI] [PubMed] [Google Scholar]
- 15.Tornetta P, 3rd, Gorup J. Axial computed tomography of pilon fractures. Clin Orthop Relat Res. 1996;323:273–6. doi: 10.1097/00003086-199602000-00037. [DOI] [PubMed] [Google Scholar]
- 16.Timmers MS, Le Cessie S, Banwell P, Jukema GN. The effects of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann Plast Surg. 2005;55:665–71. doi: 10.1097/01.sap.0000187182.90907.3d. [DOI] [PubMed] [Google Scholar]
- 17.Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: A new method for wound control and treatment: Animal studies and basic foundation. Ann Plast Surg. 1997;38:553–62. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Chen SZ, Li J, Li XY, Xu LS. Effects of vacuum-assisted closure on wound microcirculation: An experimental study. Asian J Surg. 2005;28:211–7. doi: 10.1016/S1015-9584(09)60346-8. [DOI] [PubMed] [Google Scholar]
- 19.Lindstedt S, Malmsjö M, Ingemansson R. Blood flow changes in normal and ischemic myocardium during topically applied negative pressure. Ann Thorac Surg. 2007;84:568–73. doi: 10.1016/j.athoracsur.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 20.Petzina R, Gustafsson L, Mokhtari A, Ingemansson R, Malmsjö M. Effect of vacuum-assisted closure on blood flow in the peristernal thoracic wall after internal mammary artery harvesting. Eur J Cardiothorac Surg. 2006;30:85–9. doi: 10.1016/j.ejcts.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Wackenfors A, Gustafsson R, Sjögren J, Algotsson L, Ingemansson R, Malmsjö M. Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann Thorac Surg. 2005;79:1724–30. doi: 10.1016/j.athoracsur.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 22.Wackenfors A, Sjögren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjö M. Effects of vacuum-assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen. 2004;12:600–6. doi: 10.1111/j.1067-1927.2004.12602.x. [DOI] [PubMed] [Google Scholar]
- 23.Argenta LC, Morykwas MJ. Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann Plast Surg. 1997;38:563–76. [PubMed] [Google Scholar]
- 24.Mendonca DA, Papini R, Price PE. Negative-pressure wound therapy: A snapshot of the evidence. Int Wound J. 2006;3:261–71. doi: 10.1111/j.1742-481X.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stannard JP, Volgas DA, McGwin G, 3rd, Stewart RL, Obremskey W, Moore T, et al. Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma. 2012;26:37–42. doi: 10.1097/BOT.0b013e318216b1e5. [DOI] [PubMed] [Google Scholar]


