Abstract
RATIONALE
Ayahuasca is a psychoactive tea prepared from a combination of plants that contain a hallucinogenic tryptamine and monoamine oxidase inhibitors (MAOIs). Behavioral Pattern Monitor (BPM) experiments demonstrated that the combination of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) and a behaviorally inactive dose of an MAOA inhibitor such as harmaline or clorgyline induces biphasic effects on locomotor activity in rats, initially reducing locomotion and then increasing activity as time progresses.
OBJECTIVES
The present study investigated whether the biphasic locomotor profile induced by the combination of 5-MeO-DMT and an MAOI is a consequence of a reduction in the rate of 5-MeO-DMT metabolism. This hypothesis was tested using a deuterated derivative of 5-MeO-DMT (α,α,β,β-tetradeutero-5-MeO-DMT) that is resistant to metabolism by MAO.
RESULTS
Confirming our previous findings, 1.0 mg/kg 5-MeO-DMT (s.c.) had biphasic effects on locomotor activity in rats pretreated with a behaviorally inactive dose of the nonselective MAOI pargyline (10 mg/kg). Administration of 5-MeO-DMT alone, even at doses greater than 1.0 mg/kg, produced only reductions in locomotor activity. Although low doses of α,α,β,β-tetradeutero-5-MeO-DMT (0.3 and 1.0 mg/kg, s.c.) produced only hypoactivity in the BPM, a dose of 3.0 mg/kg induced a biphasic locomotor profile similar to that produced by the combination of 5-MeO-DMT and an MAOI. Receptor binding studies demonstrated that deuterium substitution had little effect on the affinity of 5-MeO-DMT for a wide variety of neurotransmitter binding sites.
CONCLUSIONS
The finding with α,α,β,β-tetradeutero-5-MeO-DMT indicates that the hyperactivity induced by 5-MeO-DMT after MAO inhibition is a consequence of reduced metabolism of 5-MeO-DMT, leading to prolonged occupation of central serotonin receptors. These results demonstrate that deuterated tryptamines may be useful in behavioral and pharmacological studies to mimic the effects of tryptamine/MAOI combinations.
Keywords: Ayahuasca; hallucinogen; kinetic isotope effect; locomotor activity; 5-methoxy-N,N-dimethyltryptamine; monoamine oxidase; deutero-5-MeO-DMT
The serotonergic hallucinogens are a class of agents capable of producing a complex syndrome of mental and perceptual alterations, including profound distortions of perceptual processes, increased intensity and lability of affective responses, and changes in thought and cognition (Nichols 2004). Structurally, classical hallucinogens can be divided into two classes of compounds: (1) indoleamines such as N,N-dimethyltryptamine (DMT) and lysergic acid diethylamide (LSD), which bind non-selectively to serotonin (5-HT) receptors, and (2) phenylalkylamines such as mescaline and 2,5-dimethoxy-4-bromoamphetamine (DOB), which bind selectively to 5-HT2A and 5-HT2C receptors. There is extensive evidence, from both human and animal studies, that the characteristic effects of the indoleamine and phenylalkylamine hallucinogens are mediated by activation of the 5-HT2A receptor (for reviews, see: Nichols 2004; Halberstadt and Geyer 2011). For example, most of the effects of psilocybin in human volunteers are blocked by the 5-HT2A antagonist ketanserin (Vollenweider et al. 1998; Carter et al. 2005, 2007).
A variety of animal behavioral paradigms, including drug discrimination, prepulse inhibition of startle, and head twitch response, have been used to study hallucinogen effects in rodents (reviewed by: Halberstadt and Geyer 2011). We have also used the Behavioral Pattern Monitor (BPM) to test the effects of hallucinogens (Halberstadt and Geyer 2011). The BPM is a combination of activity and holeboard chambers that assesses both the quantity and quality of unconditioned locomotor and investigatory responding, and can characterize drug effects on spatiotemporal patterns of activity and responsiveness to environmental stimuli (Geyer et al. 1986; Geyer 1990). When phenylalkylamine and indoleamine hallucinogens are tested in rats in a novel BPM environment, they produce a characteristic behavioral profile that includes reductions of locomotor activity and investigatory behavior, and increased avoidance of the center of the BPM chamber (Geyer et al. 1979; Adams and Geyer 1985a; Wing et al. 1990; Halberstadt and Geyer 2011). LSD has similar effects on investigatory behavior and center avoidance (Adams and Geyer 1985b), but it produces a biphasic locomotor profile where activity is initially reduced and then increases over time (Mittman and Geyer 1991). Importantly, most of the effects of phenylalkylamine hallucinogens in the BPM are blocked by pretreatment with 5-HT2A antagonists (Wing et al. 1990; Krebs-Thomson et al. 1998).
Ayahuasca is a hallucinogenic beverage used as a sacrament by indigenous populations throughout the Amazon basin of South America, as well as by syncretic religious groups in Brazil and New Mexico. Ayahuasca is prepared from the jungle liana Banisteriopsis caapi, which contains β-carboline alkaloids such as harmaline and harmine, in combination with DMT-containing plants such as Psychotria viridis or Diplopterys cabrerana (Schultes and Hofmann 1980; McKenna et al. 1984; Schultes and Raffauf 1990). DMT by itself is not orally active due to extensive first-pass metabolism, but harmaline and harmine are MAOA inhibitors that block DMT catabolism (Agurell et al. 1968). Hence, by mixing extracts of one plant containing DMT with another plant containing β-carbolines, DMT becomes active orally in the form of an infusion or decoction.
We have used the BPM to test whether there are behavioral interactions between Ayahuasca constituents. Because of the very short-acting nature of DMT in rats, it is a difficult drug to use in extended behavioral studies. The hallucinogen 5-methoxy-DMT (5-MeO-DMT), which is also found in some Ayahuasca preparations and in many other plant extracts used in ritual settings (Holmstedt et al. 1980; Schultes and Raffauf 1995), has pharmacology similar to DMT (Glennon et al. 1982) and is easier to use in animal studies because it is longer-acting (Krebs-Thomson et al. 2006). Hence, our previous studies used a combination of 5-MeO-DMT and an MAO inhibitor as an approximation of Ayahuasca (Halberstadt et al. 2008). 5-MeO-DMT produces a short-lived decrease in exploratory behavior in rats in the BPM (Krebs-Thomson et al. 2006). However, after pretreatment with a behaviorally inactive dose of an MAOA inhibitor, 5-MeO-DMT induces biphasic effects on locomotor activity, with activity initially reduced and then elevated as time progresses (Halberstadt et al. 2008). The hyperactivity is accompanied by a reduction of the measure spatial d, indicating an increase in the smoothness of the locomotor pattern. As was noted above, this behavioral profile was previously observed only with the hallucinogen LSD (Mittman and Geyer 1991; Krebs-Thomson et al. 1998; Grailhe et al. 1999). As was found with LSD (Mittman and Geyer 1991; Ouagazzal et al. 2001), the delayed hyperactivity produced by 5-MeO-DMT in combination with an MAOA inhibitor is blocked by a selective 5-HT2A antagonist (MDL 11,939) (Halberstadt et al. 2008).
The primary route of 5-MeO-DMT metabolism is oxidative deamination by MAOA (Agurell et al. 1969; Sitaram et al. 1987b), and there is evidence that MAO inhibitors alter 5-MeO-DMT pharmacokinetics (Squires 1975; Sitaram et al. 1987a). Thus, it is possible that the ability of 5-MeO-DMT to produce delayed hyperactivity in the presence of MAO inhibitors is a consequence of a reduction in the rate of 5-MeO-DMT deamination by MAOA. It is well established that α-deutero substitution in the ethylamine side-chain of tryptamines induces resistance to metabolism by MAO via the kinetic isotope effect (Beaton et al. 1982; Barker et al. 1982, 1984; Dyck and Boulton 1986). Indeed, after α,α,β,β-tetradeuteration of DMT, higher brain levels are achieved and clearance time is increased (Barker et al. 1982). Hence, we tested whether a deuterated derivative of 5-MeO-DMT (α,α,β,β-tetradeutero-5-MeO-DMT; Shaw et al. 1977) can reproduce the behavioral profile produced by 5-MeO-DMT and an MAO inhibitor. Receptor binding studies were also conducted to compare the affinities of 5-MeO-DMT and α,α,β,β-tetradeutero-5-MeO-DMT for a variety of neurotransmitter receptors and transporters. The structures of 5-MeO-DMT and α,α,β,β-tetradeutereo-5-MeO-DMT are shown in Figure 1.
Figure 1.
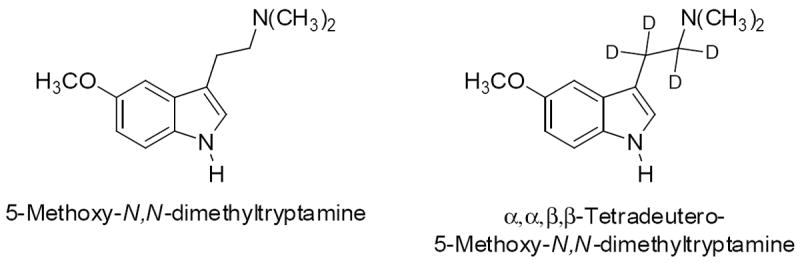
Chemical structures of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT, left) and α,α,β,β-tetradeutereo-5-MeO-DMT (right).
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats (Harlan Industries, Indianapolis, IN, USA; initial weight 250 to 275 g) were housed in pairs in a temperature- and humidity-controlled vivarium under a 12-h reverse light–dark cycle (lights off at 0700 hours). Food and water were available ad libitum. Animals were acclimatized for approximately 1 week after arrival prior to behavioral testing and maintained in American Association for Accreditation of Laboratory Animal Care-approved facilities that meet all federal and state guidelines. Procedures were approved by the University of California San Diego (UCSD) institutional animal care and use committee. Principles of laboratory animal care were followed as well as specific laws of the United States.
Apparatus
Locomotor activity and patterns were measured in the Behavioral Pattern Monitor (BPM), a 30.5 × 61.0 × 28.0 cm black Plexiglas chamber. The animal’s position in an X–Y plane was detected by a 4 × 8 grid of infrared photobeams. A computer continuously monitored the status of the photobeams and stored the data for subsequent off-line analysis. For a more detailed description of the BPM, see: (Geyer et al. 1986). The BPM also records investigatory behaviors such as rearing and holepokes, but those data are not reported herein because there is no specific interaction between 5-MeO-DMT and MAO inhibitors for those behavioral measures (see: Halberstadt et al. 2008).
Procedure
One day prior to testing in the BPM, rats were taken to the testing room, weighed, handled briefly, placed in a clear Plexiglas box (24 × 46 cm) for approximately 30 s, and then returned to their home cages in the animal colony. On the testing day, animals were brought to the testing room and allowed to sit for 60 min before receiving injections. Injections were administered in the testing room under red lights. Animals were tested during the dark phase in darkness. Animals were placed in the BPM chambers 10 min after treatment with 5-MeO-DMT or α,α,β,β-tetradeutero-5-MeO-DMT, and behavior monitored for 60 min. In experiment 1, rats (n=7–8, 61 total) were treated with the nonselective MAO inhibitor pargyline (0 or 10 mg/kg) 20 min before administration of 5-MeO-DMT (0, 0.01, 0.1, or 1.0 mg/kg). In experiment 2, rats (n=7–8, 31 total) were treated with α,α,β,β-tetradeutero-5-MeO-DMT (0, 0.3, 1.0, or 3.0 mg/kg. In experiment 3, rats (n=6–7, 27 total) were treated with 5-MeO-DMT (0, 0.3, 1.0, or 3.0 mg/kg). The animals tested for these experiments were all naïve to the BPM chambers.
Analysis
The raw data were reduced to the X and Y coordinates of the rat in the chamber. Further analyses produced specific measures of behavior (Geyer et al. 1986). Locomotor activity was quantified by the number of crossings between eight equal square sectors within the BPM. Analysis of the spatial structure of locomotor paths was performed by calculating a descriptive statistic, spatial d. The statistic spatial d is based conceptually on fractal geometry and calculated using scaling arguments (for a detailed description, see: Paulus and Geyer 1991). Changes in spatial d reflect smoother (decreases in spatial d) or rougher (increases in spatial d) locomotor paths. Locomotor data were examined in 10-min time blocks, and spatial d data were examined in 30-min time blocks. Data were analyzed using two- or three-way analysis of variance with pretreatment and treatment as between-subject factors and time as a repeated measure. Specific post hoc comparisons between selected groups were done using Tukey’s studentized range method. Significance was demonstrated by surpassing an α level of 0.05.
Drugs
5-Methoxy-N, N-dimethyltryptamine oxalate (5-MeO-DMT) and pargyline hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO, USA). α,α,β,β-Tetradeutero-5-methoxy-N,N-dimethyltryptamine (2:1) fumarate (α,α,β,β-tetradeutero-5-MeO-DMT) was prepared in the laboratory of Dr. David Nichols by reduction of 2-(5-methoxy-3-indolyl)-N,N-dimethylglyoxylamide with lithium aluminum deuteride. The synthetic material met analytical criteria for 1H NMR, mass spectrum, and elemental analysis. Doses of pargyline are expressed as the salt form of the drug, and doses of 5-MeO-DMT and α,α,β,β-tetradeutero-5-MeO-DMT refer to the freebase. 5-MeO-DMT, α,α,β,β-tetradeutero-5-MeO-DMT, and pargyline were dissolved in isotonic saline. All drugs were administered subcutaneously in a volume of 1 ml/kg.
RESULTS
Experiment 1
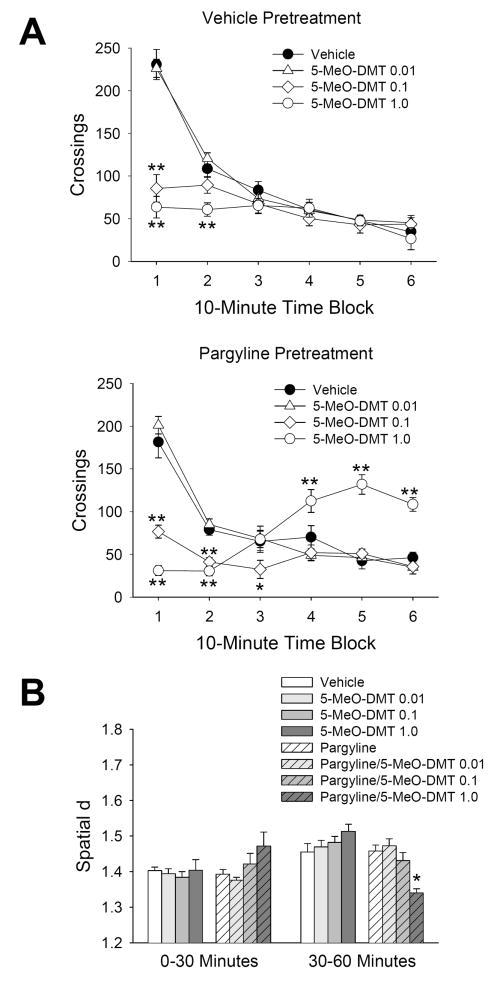
As shown in Fig. 2a, treatment with 0.1 and 1.0 mg/kg 5-MeO-DMT reduced crossings in vehicle-pretreated animals, leading to a main effect of treatment [F(3,53)=13.54, p<0.0001], and an interaction between treatment and time [F(15,265)=42.92, p<0.0001]. Consistent with our previous findings (Halberstadt et al., 2008), there was an interaction of pargyline pretreatment and 5-MeO-DMT treatment [F(3,53)=5.36, p=0.0027], and a three-way interaction between pretreatment, treatment, and time [F(15,265)=3.62, p<0.0001]. Indeed, in animals pretreated with pargyline, 1.0 mg/kg 5-MeO-DMT altered crossings in a biphasic manner, reducing locomotor activity during the first half-hour of the session and then increasing activity during the second half-hour (p<0.01, Tukey’s test; Fig. 2a). By contrast, the combination of pargyline and 0.1 mg/kg 5-MeO-DMT reduced crossings during the first half-hour, but did not increase activity during the second half-hour. There was an interaction of pargyline pretreatment and time [F(5,265)=18.32, p<0.0001], but post hoc analysis failed to confirm this effect for any 10-min time block.
Figure 2.
Modification of the behavioral response to 5-MeO-DMT by pargyline pretreatment. (a) Effect of vehicle (●), 0.01 mg/kg (△), 0.1 mg/kg (◇), or 1.0 mg/kg (○) 5- MeO-DMT on crossings in animals pretreated with vehicle (top panel) or 10 mg/kg pargyline (bottom panel). (b) Effect on spatial d. Data are expressed as group means±SEM for successive 10 min intervals (a), or group means±SEM (b). Drug doses are given in mg/kg. *p<0.05, **p<0.01, significant difference from vehicle-vehicle control group.
Pargyline [pretreatment × time: F(1,55)=18.14, p=0.0001] and 5-MeO-DMT [treatment × time: F(3,53)=5.62, p=0.002] altered spatial d, a measure of the complexity of locomotor paths, but post hoc analysis failed to confirm this for any specific 30-min block. More importantly, there was a significant three-way interaction between pargyline pretreatment, 5-MeO-DMT treatment, and time [F(3,53)=12.36, p<0.0001]. As illustrated in Fig. 2b, 1.0 mg/kg 5-MeO-DMT significantly reduced spatial d during the second half-hour of the session in animals pretreated with pargyline (p<0.01, Tukey’s test). The 0.01 and 0.1 mg/kg doses of 5-MeO-DMT had no effect on spatial d in pargyline-pretreated animals.
Experiment 2
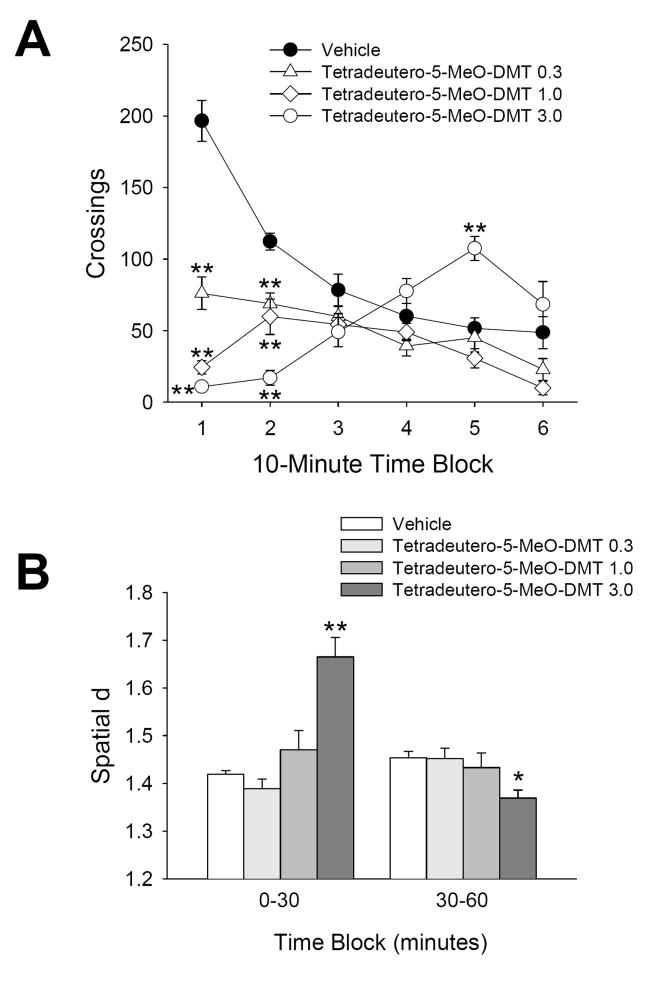
α,α,β,β-Tetradeutero-5-MeO-DMT treatment had a significant main effect on crossings [F(3,27)=14.68, p<0.0001], and interacted with time [F(15,135)=23.93, p<0.0001]. Post-hoc analysis demonstrated that the 0.3, 1.0, and 3.0 mg/kg doses of tetradeutero-5-MeO-DMT significantly reduced crossings during the first 20 min of the session (Fig. 3a). Confirming the primary hypothesis, the 3.0 mg/kg dose of tetradeutero-5-MeO-DMT significantly increased crossings during the fifth 10-min time block (p<0.01, Tukey’s test). Rats treated with 3.0 mg/kg of deuterated-5-MeO-DMT displayed more than twice the amount of locomotor activity exhibited by animals treated with vehicle (107.5±8.5 crossings (mean±S.E.M.) versus 51.8±7.3 crossings, respectively) during that time block. Neither of the lower doses tested (0.3 and 1.0 mg/kg) induced a delayed increase in activity.
Figure 3.
Behavioral response to α,α,β,β-tetradeutero-5-MeO-DMT. (a) Effect of vehicle (●), 0.3 mg/kg (△), 1.0 mg/kg (◇), or 3.0 mg/kg (○) α,α,β,β-tetradeutero-5-MeO-DMT on crossings. (b) Effect on spatial d. Data are expressed as group means±SEM for successive 10 min intervals (a), or group means±SEM (b). Drug doses are given in mg/kg. *p<0.05, **p<0.01, significant difference from vehicle control group.
For spatial d, there was a significant main effect of treatment with α,α,β,β-tetradeutero-5-MeO-DMT [F(3,27)=5.14, p<0.007], and a significant interaction between treatment and time [F(3,27)=21.92, p<0.0001]. As shown in Figure 3b, specific comparisons revealed that compared to vehicle treatment 3.0 mg/kg α,α,β,β-tetradeutero-5-MeO-DMT significantly increased spatial d during the first 30 min of the session (p<0.01, Tukey’s test) and significantly reduced spatial d during the second 30 min of the session (p<0.01).
Experiment 3
We previously reported (Krebs-Thompson et al. 2006) that administration of 5-MeO-DMT at doses of 0.01-1.0 mg/kg produces a short-lived reduction in locomotor activity in the BPM. To examine the behavioral response to higher doses of 5-MeO-DMT, we tested the drug at doses ranging from 0.3 to 3.0 mg/kg. As expected, there was a significant main effect of treatment [F(3,23)=5.73, p<0.005] and a significant interaction between treatment and time [F(15,115)=8.39, p<0.0001]. As we found previously, 5-MeO-DMT significantly reduced crossings during the first 10 min of testing (p<0.01, Tukey’s test; Fig. 4a). Importantly, 5-MeO-DMT did not induce biphasic effects on locomotor activity, even when tested at 3 mg/kg.
Figure 4.
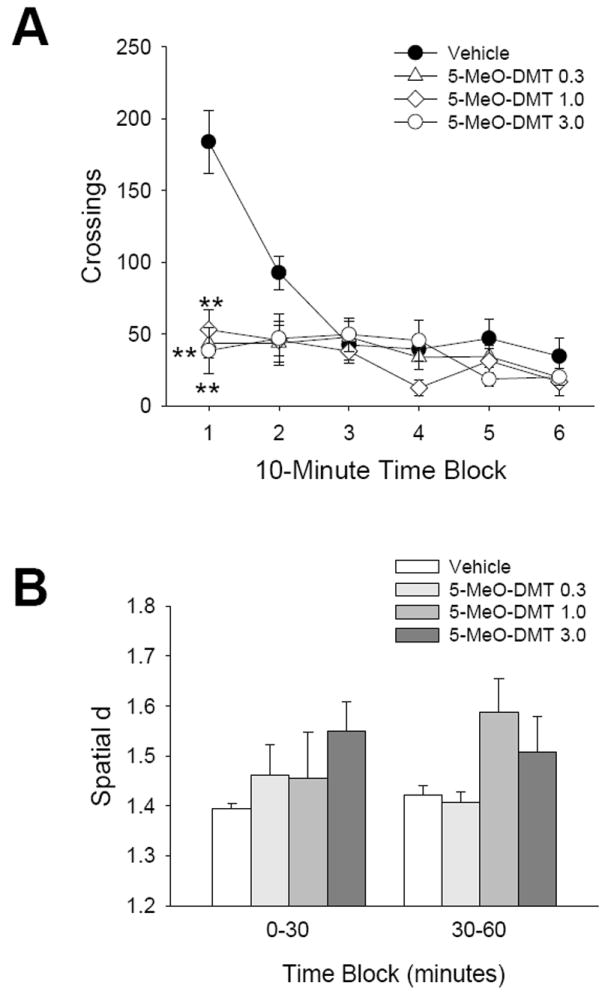
Behavioral response to 5-MeO-DMT. (a) Effect of vehicle (●), 0.3 mg/kg (△), 1.0 mg/kg (◇), or 3.0 mg/kg (○) 5-MeO-DMT on crossings. (b) Effect on spatial d. Data are expressed as group means±SEM for successive 10 min intervals (a), or group means±SEM (b). Drug doses are given in mg/kg. **p<0.01, significant difference from vehicle control group.
There was an increase in spatial d after administration of 5-MeO-DMT, yielding an interaction between treatment and time that approached but did not reach significance [F(3,23)=2.41, p<0.1]. However, post hoc analysis revealed that 5-MeO-DMT failed to alter spatial d significantly during either the first or the second half-hour of testing (Fig. 4b).
Radioligand binding experiments
Receptor binding studies were performed by the NIMH Psychoactive Drug Screening Program (NIMH PDSP). The binding affinities of 5-MeO-DMT and α,α,β,β-tetradeutero-5-MeO-DMT for a variety of receptor sites are listed in Table 1. 5-MeO-DMT displays moderate to high affinity for most 5-HT receptors, and has moderate affinity for α- and β-adrenergic receptor subtypes, the 5-HT transporter (SERT), and sigma-2 binding sites. 5-MeO-DMT binds with lower affinity to dopamine receptors, histamine receptors, opiate receptors, dopamine (DAT) and norepinephrine (NET) transporters, and sigma-1 binding sites. The effect of tetradeuteration on the binding profile of 5-MeO-DMT is generally unremarkable except for serotonergic 5-HT1B and adrenergic α1A receptors where the deuterium-substituted compound binds with approximately 4-fold higher affinity than proteo-5-MeO-DMT.
Table 1.
Receptor binding data for 5-MeO-DMT and α,α,β,β-tetradeutero-5-MeO-DMT
| Receptor | Radioligand | Speciesa | 5-MeO-DMT Ki nM (±SEM)b | α,α,β,β-Tetradeutero-5-MeO-DMT Ki nM (±SEM)b |
|---|---|---|---|---|
| 5-HT1A | [3H]8-OH-DPAT | Human | 3.0 (0.2) | 3.0 (0.4) |
| 5-HT1B | [3H]GR125743 | Human | 14.0 (2) | 3.5 (0.6) |
| 5-HT1D | [3H]GR125743 | Human | 2.3 (0.3) | 1.7 (0.3) |
| 5-HT1E | [3H]5-HT | Human | 376 (67) | 483 (90) |
| 5-HT2A | [3H]ketanserin | Human | 907 (170) | 1292 (243) |
| 5-HT2B | [3H]LSD | Human | 36 (6) | 90 (14) |
| 5-HT2C | [3H]mesulergine | Rat | 418 (35) | 286 (25) |
| 5-HT5A | [3H]LSD | Human | 505 (72) | 151 (20) |
| 5-HT6 | [3H]LSD | Human | 6.5 (1.2) | 10.0 (1) |
| 5-HT7 | [3H]LSD | Human | 4.5 (0.5) | 2.6 (0.4) |
| α1A | [3H]prazocin | Human | 4373 (354) | 1013 (92) |
| α1B | [3H]prazocin | Human | 2188 (219) | 841 (67) |
| α1D | [125I]HEAT | Human | 439 (52) | 202 (17) |
| α2A | [3H]clonidine | Human | 938 (56) | 1871 (187) |
| α2B | [3H]clonidine | Human | 430 (40) | 513 (48) |
| α2C | [3H]clonidine | Human | 206 (14) | 274 (16) |
| β2 | [3H]CGP12177 | Human | 2679 (238) | 4560 (365) |
| D3 | [3H]N-methyl-spiperone | Rat | > 10000 | 7087 (1125) |
| H1 | [3H]pyrilamine | Human | 7580 (1024) | > 10000 |
| H2 | [3H]tiotidine | Human | > 10000 | 7165 (853) |
| SERT | [3H]citalopram | Human | 3603 (559) | 2761 (330) |
| Sigma-2 | [3H]DTG | Rat | 3689 (336) | 7171 (1156) |
The experiments were performed using cloned receptors.
5-MeO-DMT and α,α,β,β-tetradeutero-5-MeO-DMT bound to the following sites with Ki values > 10,000 nM: 5-HT3, rat brain benzodiazepine site, Ca2+ channels, D1, D2, D4, D5, DAT, NET, β1, β3, DOR, MOR, KOR, EP3, EP4, GABAA, H3, H4, M1, M2, M3, M4, M5, and sigma-1.
DISCUSSION
We previously reported that administration of 1 mg/kg 5-MeO-DMT in combination with an MAOA inhibitor such as clorgyline or harmaline induced a biphasic locomotor profile where activity was initially reduced and then increased as time progressed (Halberstadt et al. 2008). The delayed hyperactivity was accompanied by a decrease in spatial d, suggesting the animals made smoother locomotor paths. The present studies have confirmed those earlier findings by showing that the combination of 5-MeO-DMT and the nonselective MAO inhibitor pargyline also induces a biphasic locomotor profile and a reduction in spatial d. The experiments demonstrated that the interaction between 5-MeO-DMT and MAO inhibitors is extremely dose dependent, occurring with 1 mg/kg 5-MeO-DMT but not with 0.3 mg/kg. Furthermore, we found that 5-MeO-DMT alone does not induce delayed hyperactivity, even if administered at doses > 1 mg/kg.
The dose of pargyline used in the present investigation (10 mg/kg, s.c.) non-selectively inhibits MAO (78% MAOA inhibition vs. 92% MAOB inhibition; Ortmann et al. 1984). We previously demonstrated that treatment with either the MAOA inhibitor harmaline or a low dose of the selective MAOA inhibitor clorgyline transforms the effect of 5-MeO-DMT to a biphasic locomotor profile, whereas the selective MAOB inhibitor (–)-deprenyl is ineffective (Halberstadt et al. 2008). Given those earlier findings, it is likely that MAOA inhibition by pargyline is responsible for the interaction with 5-MeO-DMT. Although there is some evidence that pargyline can bind to sites other than MAOA and MAOB, it is unlikely that those effects contribute to the interaction with 5-MeO-DMT. Clorgyline, harmaline, and pargyline have been shown to bind to imidazoline I2 receptors (Lione et al. 1996; Husbands et al. 2001; Miralles et al. 2005). However, binding to I2 receptors probably does not play a role in the interaction because (–)-deprenyl binds to I2 receptors with roughly the same affinity as clorgyline (Lione et al. 1996) yet fails to interact with 5-MeO-DMT. Likewise, it has been reported that pargyline and clorgyline bind to σ receptors (Itzhak and Kassim, 1990) and to an MAOI-displaceable quinpirole binding site (MQB; Levant et al. 1996), but (–)-deprenyl also binds to these sites with moderately high affinity.
As discussed previously (Halberstadt et al. 2008), there are at least two potential explanations for the behavioral interactions between 5-MeO-DMT and MAOA inhibitors. First, 5-MeO-DMT pharmacokinetics are altered by pretreatment with a MAO inhibitor (Squires 1975; Sitaram et al. 1987a; Shen et al. 2010a,b), so it is possible that the ability of 5-MeO-DMT to induce delayed hyperactivity when administered in combination with a MAO inhibitor is due to a reduction in the rate of 5-MeO-DMT metabolism by MAOA. Second, MAO inhibition could alter 5-MeO-DMT pharmacodynamics, including the downstream neurochemical response to the drug. For example, 5-MeO-DMT can enhance the firing of dopaminergic neurons (Christoph et al. 1977; White and Wang 1983), and this effect could be altered by MAOI-induced changes in dopamine metabolism. We hypothesized that if the ability of MAO inhibitors to alter the behavioral effects of 5-MeO-DMT is due to a pharmacokinetic interaction, then α,α,β,β-tetradeutero-5-MeO-DMT should produce a similar behavioral profile because it is more resistant to metabolism by MAO as a result of a kinetic isotope effect. Importantly, we found that α,α,β,β-tetradeutero-5-MeO-DMT alone produces a biphasic locomotor profile and a delayed reduction of spatial d. Because α,α,β,β-tetradeutero-5-MeO-DMT does not inhibit MAO, this finding completely eliminates the possibility that neurochemical changes subsequent to MAO inhibition are responsible for the delayed hyperactivity. Instead, this finding supports the hypothesis that the biphasic behavioral profile is a consequence of altered 5-MeO-DMT pharmacokinetics.
Like other indoleamine hallucinogens, 5-MeO-DMT is an agonist at 5-HT1A and 5-HT2A receptors (Halberstadt & Geyer 2011). Our previous experiments have shown that the hyperactivity induced by 5-MeO-DMT in combination with an MAO inhibitor is blocked by the highly selective 5-HT2A antagonist MDL 11,939 but unaffected by the selective 5-HT1A antagonist WAY-100,635 (Halberstadt et al. 2008). Based on those findings, we have concluded that the hyperactivity is mediated by 5-HT2A receptor activation. The fact that the binding profiles of 5-MeO-DMT and α,α,β,β-tetradeutero-5-MeO-DMT at 5-HT receptors are very similar indicates that differences in receptor affinity are unlikely to be responsible for the behavioral differences between these two compounds.
As was noted earlier, there is strong support for a link between 5-HT2A receptor activation and hallucinogenic effects (Nichols 2004; Halberstadt & Geyer 2011). Given that 5-MeO-DMT is a potent hallucinogenic agent that is active in humans at parenteral doses of 2-3 mg (Shulgin and Shulgin 1997), it is surprising that this compound displayed relatively low (~ 0.9 μM) affinity for the 5-HT2A receptor. It is important to note, however, that 5-HT2A receptors exist in high-affinity and low-affinity agonist binding conformations depending on whether they are coupled to G proteins. 5-HT2A antagonists such as [3H]ketanserin label both states with equal affinity, whereas radiolabeled agonists such as [3H]DOB bind selectively to the G protein-coupled, high-affinity state of the receptor (Glennon et al. 1988; Lyon et al. 1987). The binding affinity of agonists for the 5-HT2A receptor varies depending on whether the receptor is radiolabeled with an agonist or an antagonist, and agonists generally display 10-100-fold higher affinity for agonist-labeled 5-HT2A receptors compared with antagonist-labeled receptors (Titeler et al. 1988; Glennon et al. 1992, 1994). Since the 5-HT2A binding data listed in Table I were obtained using [3H]ketanserin, they likely underestimate the affinity of 5-MeO-DMT and α,α,β,β-tetradeutereo-5-MeO-DMT for the high-affinity state of the receptor. Indeed, it was previously reported that 5-MeO-DMT binds to [3H]DOB-labeled 5-HT2A receptors with a Ki of 90 nM (Egan et al. 2000), which is 10-fold greater than the value reported herein.
We have previously demonstrated that the time course of the biphasic locomotor effects induced by the combination of MAO inhibitors and 5-MeO-DMT is dependent on the time delay between injection and testing (Halberstadt et al. 2008). This finding indicates that the biphasic effects reflect distinct temporal phases of drug action, as opposed to an interaction of the drug and the amount of time spent in the BPM chamber. The major route of 5-MeO-DMT metabolism is oxidative deamination by MAOA (Agurell et al. 1969; Sitaram et al. 1987b), but 5-MeO-DMT is also O-demethylated to bufotenine (5-hydroxy-DMT) by cytochrome P450 2D6 (Agurell et al. 1969; Yu et al. 2003). Importantly, bufotenine is a highly efficacious 5-HT2A agonist with 10-fold higher affinity for the 5-HT2A receptor than 5-MeO-DMT (Roth et al. 1997; Egan et al. 2000). It was demonstrated recently that conversion of 5-MeO-DMT to bufotenine is markedly increased in mice pretreated with the MAOA inhibitor harmaline (Shen et al. 2010a,b). In light of that finding, we hypothesize that the delayed hyperactive phase induced by 5-MeO-DMT in the presence of an MAOA inhibitor is a consequence of conversion of 5-MeO-DMT to bufotenine, which produces hyperactivity via 5-HT2A receptor activation. Although 5-HT2A agonists typically reduce activity in rats (Wing et al. 1990; Krebs-Thomson et al. 1998; Hameleers et al. 2007), there is some evidence that high doses of 5-HT2A agonists can increase locomotor activity (Yamamoto and Ueki 1975; Pálenícek et al. 2008). Bufotenine is deaminated by MAO (Gessner et al. 1960), and high levels could potentially accumulate in the brain after MAO inhibition. Studies are in progress to determine whether the time course of hyperactivity produced by 5-MeO-DMT and an MAO inhibitor is temporally correlated with bufotenine brain levels. Many tryptamine derivatives are metabolized by 6-hydroxylation (Szara and Axelrod 1959; Kopin et al. 1961; Szara 1961; Szara et al. 1962), and thus it is also possible that 6-hydroxy-5-methoxy-DMT may contribute to the hyperactivity produced by 5-MeO-DMT and an MAOA inhibitor. However, 6-hydroxy-5-methoxy-DMT has yet to be conclusively identified as a metabolite of 5-MeO-DMT in rats (Agurell et al. 1969). Although the route for metabolism of α,α,β,β-tetradeutero-5-MeO-DMT has not been characterized, it is anticipated that the biotransformation of that compound would be very similar to that of 5-MeO-DMT in the presence of an MAO inhibitor. Thus, it is possible that the hyperactivity induced by α,α,β,β-tetradeutero-5-MeO-DMT could be a consequence of O-demethylation to α,α,β,β-tetradeutero-bufotenine. Future studies will compare the metabolism of 5-MeO-DMT and α,α,β,β-tetradeutero-5-MeO-DMT.
In summary, we have shown that the behavioral interaction between 5-MeO-DMT and MAO inhibitors is likely a consequence of altered 5-MeO-DMT pharmacokinetics. Indeed, a similar behavioral profile is produced by a deuterated derivative of 5-MeO-DMT that is resistant to metabolism by MAO. The results obtained with α,α,β,β-tetradeutero-5-MeO-DMT indicate that deuterated tryptamine derivatives may be useful as single-drug approximations of Ayahuasca. Furthermore, because deuteration has little apparent effect on receptor binding, these compounds may have utility in studies where it is desirable to use tryptamine derivatives that are not metabolically labile and thus have long-lasting pharmacological and behavioral effects. Given the complex pharmacokinetic and behavioral interactions that occur between tryptamine derivatives and MAO inhibitors, the present results indicate that interactions between Ayahuasca constituents need to be studied systematically in order to understand fully the mechanism of action of the botanical preparation. It is also important to note that 5-MeO-DMT has recently become popular as a “designer drug”, often obtained from online vendors, and is now controlled in the United States as a Schedule I hallucinogen by the Drug Enforcement Administration (Anonymous 2010). There have been anecdotal reports that 5-MeO-DMT is sometimes ingested in combination with MAO inhibitors (Shulgin and Shulgin 1997; Ott 2001; Brush et al. 2004). Our findings indicate that these drug combinations may have distinct behavioral and toxicological effects compared with 5-MeO-DMT taken alone. Indeed, it has been reported that ingestion of a high dose of 5-MeO-DMT in combination with an MAO inhibitor resulted in a fatality (Sklerov et al. 2005). Studies are currently in progress to examine whether behavioral and pharmacokinetic interactions occur between MAO inhibitors and a variety of tryptamine derivatives.
Acknowledgments
Supported by National Institute on Drug Abuse Awards R01 DA002925 and F32 DA025412, and the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center. Receptor binding data were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is directed by Dr. Bryan Roth at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA. For experimental details, please refer to the PDSP web site: http://pdsp.med.unc.edu/
References
- Adams L, Geyer MA. Effects of DOM and DMT in a proposed animal model of hallucinogenic activity. Prog Neuropsychopharmacol Biol Psychiatry. 1985a;9:121–132. doi: 10.1016/0278-5846(85)90074-0. [DOI] [PubMed] [Google Scholar]
- Adams L, Geyer MA. A proposed animal model for hallucinogens based on LSD’s effects on patterns of exploration in rats. Behav Neurosci. 1985b;99:881–900. doi: 10.1037//0735-7044.99.5.881. [DOI] [PubMed] [Google Scholar]
- Ahlborg U, Holmstedt B, Lindgren JE. Fate and metabolism of some hallucinogenic indolealkylamines. Adv Pharmacol. 1968;6(Pt. B):213–229. doi: 10.1016/s1054-3589(08)60320-8. [DOI] [PubMed] [Google Scholar]
- Agurell S, Holmstedt B, Lindgren JE. Alkaloid content of Banisteriopsis rusbyana. Am J Pharmacy Sci Supporting Publ Health. 1968;140:148–151. [PubMed] [Google Scholar]
- Agurell S, Holmstedt B, Lindgren JE. Metabolism of 5-methoxy-N,-N dimethyltryptamine-14C in the rat. Biochem Pharmacol. 1969;18:2771–2781. doi: 10.1016/0006-2952(69)90185-3. [DOI] [PubMed] [Google Scholar]
- Anonymous. Schedules of controlled substances: placement of 5-methoxy-N,N-dimethyltryptamine into Schedule I of the Controlled Substances Act. Final rule Fed Regist. 2010;75:79296–300. [PubMed] [Google Scholar]
- Barker SA, Beaton JM, Christian ST, Monti JA, Morris PE. Comparison of the brain levels of N,N-dimethyltryptamine and α,α,β,β-tetradeutero-N,N-dimethyltryptamine following intraperitoneal injection. The in vivo kinetic isotope effect. Biochem Pharmacol. 1982;31:2513–2516. doi: 10.1016/0006-2952(82)90062-4. [DOI] [PubMed] [Google Scholar]
- Barker SA, Beaton JM, Christian ST, Monti JA, Morris PE. In vivo metabolism of α,α,β,β-tetradeutero-N,N-dimethyltryptamine in rodent brain. Biochem Pharmacol. 1984;33:1395–400. doi: 10.1016/0006-2952(84)90404-0. [DOI] [PubMed] [Google Scholar]
- Beaton JM, Barker SA, Liu WF. A comparison of the behavioral effects of proteo- and deutero-N, N-dimethyltryptamine. Pharmacol Biochem Behav. 1982;16:811–814. doi: 10.1016/0091-3057(82)90240-4. [DOI] [PubMed] [Google Scholar]
- Brush DE, Bird SB, Boyer EW. Monoamine oxidase inhibitor poisoning resulting from Internet misinformation on illicit substances. J Toxicol Clin Toxicol. 2004;42:191–195. doi: 10.1081/clt-120030949. [DOI] [PubMed] [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. 2005;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- Carter OL, Hasler F, Pettigrew JD, Wallis GM, Liu GB, Vollenweider FX. Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology (Berl) 2007;195:415–424. doi: 10.1007/s00213-007-0930-9. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Kuhn DM, Jacobs BL. Electrophysiological evidence for a dopaminergic action of LSD: depression of unit activity in the substantia nigra of the rat. Life Sci. 1977;21:1585–1596. doi: 10.1016/0024-3205(77)90235-1. [DOI] [PubMed] [Google Scholar]
- Dyck LE, Boulton AA. Effect of deuterium substitution on the disposition of intraperitoneal tryptamine. Biochem Pharmacol. 1986;35:2893–2896. doi: 10.1016/0006-2952(86)90482-x. [DOI] [PubMed] [Google Scholar]
- Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors. Synapse. 2000;35:144–150. doi: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gessner PK, Khairallah PA, McIsaac WM, Page IH. The relationship between the metabolic fate and pharmacological actions of serotonin, bufotenine and psilocybin. J Pharmacol Exp Ther. 1960;130:126–133. [PubMed] [Google Scholar]
- Geyer MA. Approaches to the characterization of drug effects on locomotor activity in rodents. In: Adler MW, Cowan A, editors. Modern methods in pharmacology: testing and evaluation of drugs of abuse. Wiley-Liss; New York: 1990. pp. 81–99. [Google Scholar]
- Geyer MA, Light RK, Rose GJ, Petersen LR, Horwitt DD, Adams LM, Hawkins RL. A characteristic effect of hallucinogens on investigatory responding in rats. Psychopharmacology. 1979;65:35–40. doi: 10.1007/BF00491975. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Dukat M, El-Bermawy M, Law H, De Los Angeles J, Teitler M, King A, Herrick-Davis K. Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines. J Med Chem. 1994;37:1929–1935. doi: 10.1021/jm00039a004. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Raghupathi R, Bartyzel P, Teitler M, Leonhardt S. Binding of phenylalkylamine derivatives at 5-HT1C and 5-HT2 serotonin receptors: evidence for a lack of selectivity. J Med Chem. 1992;35:734–740. doi: 10.1021/jm00082a014. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Rosecrans JA, Young R. The use of the drug discrimination paradigm fpr studying hallucinogenic agents. A review. In: Colpaert FC, Slangen JL, editors. Drug Discrimination: Applications in CNS Pharmacology. Elsevier; Amsterdam: 1982. pp. 69–96. [Google Scholar]
- Glennon RA, Seggel MR, Soine W, Davis KH, Lyon RA, Titeler M. 125I-2,5-Dimethoxy-4-iodophenyl-2-aminopropane (DOI): An iodinated radioligand that specifically labels the agonist high affinity state of the 5HT2 serotonin receptor. J Med Chem. 1988;31:5–7. doi: 10.1021/jm00396a003. [DOI] [PubMed] [Google Scholar]
- Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D, Geyer MA, Hen R. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron. 1999;22:581–591. doi: 10.1016/s0896-6273(00)80712-6. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology. 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Hallucinogens. In: Koob G, Thompson RM, Le Moal M, editors. Encyclopedia of Behavioral Neuroscience. Vol. 2. London: Academic Press; 2010. pp. 12–20. [Google Scholar]
- Halberstadt AL, Geyer MA. Multiple receptors mediate the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameleers R, Blokland A, Steinbusch HW, Visser-Vandewalle V, Temel Y. Hypomobility after DOI administration can be reversed by subthalamic nucleus deep brain stimulation. Behav Brain Res. 2007;185:65–67. doi: 10.1016/j.bbr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Holmstedt B, Lindgren JE, Plowman T, Rivier L, Schultes RE, Tovar O. Indole alkaloids in Amazonian myrisicaceae: Field and laboratory research. Harvard University Bot Museum Leaflets. 1980;28:215–234. [Google Scholar]
- Husbands SM, Glennon RA, Gorgerat S, Gough R, Tyacke R, Crosby J, Nutt DJ, Lewis JW, Hudson AL. β-Carboline binding to imidazoline receptors. Drug Alcohol Depend. 2001;64:203–208. doi: 10.1016/s0376-8716(01)00123-5. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Kassim CO. Clorgyline displays high affinity for σ binding sites in C57BL/6 mouse brain. Eur J Pharmacol. 1990;176:107–108. doi: 10.1016/0014-2999(90)90139-w. [DOI] [PubMed] [Google Scholar]
- Kopin IJ, Pare CM, Axelrod J, Weissbach H. The facte of melatonin in animals. J Biol Chem. 1961;236:3072–3075. [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology. 2006;189:319–329. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- Levant B, Moehlenkamp JD, Morgan KA, Leonard NL, Cheng CC. Modulation of [3H]quinpirole binding in brain by monoamine oxidase inhibitors: evidence for a potential novel binding site. J Pharmacol Exp Ther. 1996;278:145–153. [PubMed] [Google Scholar]
- Lione LA, Nutt DJ, Hudson AL. [3H]2-(2-benzofuranyl)-2-imidazoline: a new selective high affinity radioligand for the study of rabbit brain imidazoline I2 receptors. Eur J Pharmacol. 1996;304:221–229. doi: 10.1016/0014-2999(96)00131-8. [DOI] [PubMed] [Google Scholar]
- Lyon RA, Davis KH, Titeler M. 3H-DOB (4-bromo-2,5-dimethoxyphenylisopropylamine) labels a guanyl nucleotide-sensitive state of cortical 5-HT2 receptors. Mol Pharmacol. 1987;31:194–199. [PubMed] [Google Scholar]
- McKenna DJ, Towers GHN, Abbott F. Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and β-carboline constituents of ayahuasca. J Ethnopharmacol. 1984;10:195–223. doi: 10.1016/0378-8741(84)90003-5. [DOI] [PubMed] [Google Scholar]
- Miralles A, Esteban S, Sastre-Coll A, Moranta D, Asensio VJ, Garcia-Sevilla JA. High-affinity binding of β-carbolines to imidazoline I2B receptors and MAO-A in rat tissues: norharman blocks the effect of morphine withdrawal on DOPA/noradrenaline synthesis in the brain. Eur J Pharmacol. 2005;518:234–242. doi: 10.1016/j.ejphar.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Dissociation of multiple effects of acute LSD on exploratory behavior in rats by ritanserin and propranolol. Psychopharmacology. 1991;105:69–76. doi: 10.1007/BF02316866. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ortmann R, Schaub M, Felner A, Lauber J, Christen P, Waldmeier PC. Phenylethylamine-induced stereotypies in the rat: a behavioral test system for assessment of MAO-B inhibitors. Psychopharmacology. 1984;84:22–27. doi: 10.1007/BF00432018. [DOI] [PubMed] [Google Scholar]
- Ortmann R, Waldmeier PC, Radeke E, Felner A, Delini-Stula A. The effects of 5-HT uptake- and MAO-inhibitors on L-5-HTP-induced excitation in rats. Naunyn Schmiedebergs Arch Pharmacol. 1980;311:185–192. doi: 10.1007/BF00510258. [DOI] [PubMed] [Google Scholar]
- Ott J. Pharmepéna-Psychonautics: human intranasal, sublingual and oral pharmacology of 5-methoxy-N,N-dimethyl-tryptamine. J Psychoactive Drugs. 2001;33:403–407. doi: 10.1080/02791072.2001.10399925. [DOI] [PubMed] [Google Scholar]
- Ouagazzal A, Grottick AJ, Moreau J, Higgins GA. Effect of LSD on prepulse inhibition and spontaneous behavior in the rat. A pharmacological analysis and comparison between two rat strains. Neuropsychopharmacology. 2001;25:565–575. doi: 10.1016/S0893-133X(01)00282-2. [DOI] [PubMed] [Google Scholar]
- Pálenícek T, Balíková M, Bubeníková-Valesová V, Horácek J. Mescaline effects on rat behavior and its time profile in serum and brain tissue after a single subcutaneous dose. Psychopharmacology. 2008;196:51–62. doi: 10.1007/s00213-007-0926-5. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology. 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Roth BL, Choudhary MS, Khan N, Uluer AZ. High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model. J Pharmacol Exp Ther. 1997;280:576–583. [PubMed] [Google Scholar]
- Schultes RE, Hofmann A. The Botany and Chemistry of Hallucinogens. Charles C Thomas Springfield; 1980. [Google Scholar]
- Schultes RE, Raffauf RF. Medicinal and Toxic Plants of the Northwest Amazonia. Dioscorides Press; Portland: 1990. The Healing Forest. [Google Scholar]
- Shaw GJ, Wright GJ, Milne GWA. Mass spectra of some specifically deuterated tryptamines. Biomed Mass Spectrometry. 1977;4:348–353. doi: 10.1002/bms.1200040605. [DOI] [PubMed] [Google Scholar]
- Shen HW, Jiang XL, Winter JC, Yu AY. Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Current Drug Metab. 2010a;11:659–666. doi: 10.2174/138920010794233495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Wu C, Jiang XL, Yu AM. Effects of monoamine oxidase inhibitor and cytochrome P450 2D6 status on 5-methoxy-N,N-dimethyltryptamine metabolism and pharmacokinetics. Biochem Pharmacol. 2010b;80:122–128. doi: 10.1016/j.bcp.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin AT, Shulgin A. TIHKAL: The Continuation. Transform Press; Berkeley: 1997. [Google Scholar]
- Sitaram BR, Lockett L, Talomsin R, Blackman GL, McLeod WR. In vivo metabolism of 5-methoxy-N,N-dimethyltryptamine and N,N-dimethyltryptamine in the rat. Biochem Pharmacol. 1987a;6:1509–1512. doi: 10.1016/0006-2952(87)90118-3. [DOI] [PubMed] [Google Scholar]
- Sitaram BR, Talomsin R, Blackman GL, McLeod WR. Study of metabolism of psychotomimetic indolealkylamines by rat tissue extracts using liquid chromatography. Biochem Pharmacol. 1987b;36:1503–1508. doi: 10.1016/0006-2952(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Sklerov J, Levine B, Moore KA, King T, Fowler D. A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation. J Anal Toxicol. 2005;29:838–841. doi: 10.1093/jat/29.8.838. [DOI] [PubMed] [Google Scholar]
- Squires RF. Evidence that 5-methoxy-N,N-dimethyltryptamine is a specific substrate for MAO-A in the rat: implications for the indoleamine dependent behavioral syndrome. J Neurochem. 1975;24:47–50. doi: 10.1111/j.1471-4159.1975.tb07626.x. [DOI] [PubMed] [Google Scholar]
- Szara S. 6-Hydroxylation: an important metabolic route for α-methyltryptamine. Experentia. 1961;17:76–77. doi: 10.1007/BF02171429. [DOI] [PubMed] [Google Scholar]
- Szara S, Axelrod J. Hydroxylation and N-demethylation of N.N-dimethyltryptamine. Experientia. 1959;15:216–217. doi: 10.1007/BF02158111. [DOI] [PubMed] [Google Scholar]
- Szara S, Hearst E, Putney F. Metabolism and behavioural action of psychotropic tryptamine homologues. Int J Neuropharm. 1962;1:111–117. [Google Scholar]
- Titeler M, Lyon LA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropyl amine hallucinogens. Psychopharmacology. 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MFI, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Comparision of the effects of LSD and lisuride on A10 dopamine neurons in the rat. Neuropharmacology. 1983;22:669–676. doi: 10.1016/0028-3908(83)90089-8. [DOI] [PubMed] [Google Scholar]
- Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology. 1990;100:417–425. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ueki S. Behavioral effects of 2,5-dimethoxy-4-methylamphetamine (DOM) in rats and mice. Eur J Pharmacol. 1975;32:156–162. doi: 10.1016/0014-2999(75)90278-2. [DOI] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Herraiz T, Kupfer A, Gonzalez FJ. Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. Pharmacogenetics. 2003;13:307–319. doi: 10.1097/01.fpc.0000054094.48725.b7. [DOI] [PubMed] [Google Scholar]