Abstract
Background
BK virus (BKV), a human polyomavirus, causes BKV nephritis, which often leads to graft loss after renal transplantation. Currently, the only efficient therapy against BKV nephritis seems to be a reduction or change of immunosuppressive agents, but this may increase the inherent risk of rejection. Here, we report the ability of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitor (statin), which is routinely used to treat hypercholesterolemia, to repress BKV entry pathways in human renal proximal tubular epithelial cells (HRPTEC) and, correspondently, prevent BKV infection.
Methods
HRPTEC were co-incubated with BKV and pravastatin. Then the percentage of HRPTEC infected with BKV by immunofluorescent analysis and large T-antigen expression which suggested BKV infection by Western blots was assessed in the absence and presence of pravastatin. The distribution of purified and labeled BKV particles in the presence and absence of pravastatin was also investigated.
Results
Both the percentage of BKV infected cells and the large T-antigen expression were significantly decreased in HRPTEC pretreated and co-incubated with pravastatin. However, when pravastatin was added 72 hr after BKV infection it failed to decrease percentage of BKV infected cells. It is likely, that pravastatin's inhibitory effect is explained by depletion of caveolin-1, a critical element of caveolae. BKV enters HRPTEC by caveolar-mediated endocytosis. We provide evidence that pravastatin dramatically decreased caveolin-1 expression in HRPTEC and interfered with internalization of labeled BKV particles.
Conclusions
Our data suggest that pravastatin, acting through depletion of caveolin-1, prevented caveolar-dependent BKV internalization and repressed BKV infection of HRPTEC.
Keywords: BK virus, Statins, Human renal proximal tubular epithelial cells
BK virus (BKV) belongs to the family polyomaviridae and received its name from the initials of the first patient in which it was identified. The primary BKV infection occurs during childhood causing about 80% of the adult population to be seropositive to BKV. BKV remains latent, without any manifestation of BKV-induced disease, and progresses to BKV nephritis only in immunocompromised patients, particularly in renal transplant recipients. In this decade, BKV nephritis has evolved to become a major cause of renal dysfunction and graft loss after renal transplantation because of the appearance of potent immunosuppressive agents such as tacrolimus and mycophenolae mofetil. The most serious problem with BKV nephritis is that efficient antiviral therapies have not been established yet, whereas about 10% of renal transplant recipients are developing BKV nephritis and about half of them are losing their graft (1–5). Reduction or alteration of treatment with immunosuppressive agents has been recognized as the only efficient therapy against BKV nephritis, however, this strategy has increased the risk of rejection (1–5). Other therapies employing such pharmaceutical agents as cidofovir (6, 7), leflunomide (8, 9), quinolone antibiotics (10), and intravenous immunoglobulin (11), have been used to treat the progression of BKV to clinical polyomavirus nephropathy, however, these therapies have not reached a consensus (12–14). Therefore, alternative efficient anti-BKV therapy is required without delay.
3-Hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors (statins) are frequently used as cholesterol lowering agents in a number of clinical situations. They act by impeding synthesis of cholesterol in the liver through the mevalonate pathway and their beneficial effects include vasodilative function, antithrombotic action and anti-inflammatory actions. As to the reduction of cardiovascular disease, these beneficial effects of statins have been reported to be beyond the cholesterol lowering effect (15–17). In vitro studies showed that statins prevented monocyte adherance to endothelial cells and protected from oxidation of low-density lipoprotein (LDL) in mesangial cells (18, 19). In vivo studies indicated that statins suppressed mesangial cell proliferation, mesangial matrix expansion, and glomerular macrophage infiltration, and prevented tubular atrophy and interstitial fibrosis (18, 19). Clinical studies reported that statins decreased proteinuria, improved renal function and slowed the rate of progression of kidney disease (20, 21). Although statins have been reported to have a beneficial effect on kidney disease, they have also decreased the expression of caveolin-1, the principal scaffolding proteins of caveolae, both in vitro (22) and in vivo (23).
We have reported that BKV entered into human renal proximal tubular epithelial cells (HRPTEC) through the caveolar endocytosis pathway (24), moved along microtubules, and reached the endoplasmic reticulum before reaching nuclei, but bypassed or transiently passed the Golgi apparatus (25). Our report that BKV entered through caveolae in HRPTEC (24) and other reports that statins decreased the expression of caveolin-1 (22, 23) allowed us to hypothesize that statins interfered with BKV internalization by disrupting the caveolae. In this study we investigated whether pravastatin, one of the hydrophilic statins currently used for treatment of the patients of hypercholesterolemia, decreased BKV infection in HRPTEC.
MATERIALS AND METHODS
Cells and Viruses
HRPTEC were purchased from Cambrex Bio Science Inc. (Walkersville, MD) and cultured in renal epithelial cell basal medium (REBM) (Cambrex Bio Science Inc.) containing 0.5% or 10% fetal bovine serum (FBS), renal epithelial growth media SingleQuots (human epidermal growth factor, insulin, hydrocortisone, gentamicin or amphotericin-B-1000, epinephrine, triiodothyronine, transferrin) (Cambrex Bio Science Inc.) and 100 units/mL penicillin-G (Invitrogen, Carlsbad, CA). REBM with 10% FBS was used to grow HRPTEC until passage 5, and REBM with 0.5% FBS was used at actual experiment with HRPTEC at passage 6.
BKV was purchased from ATCC (Manassas, VA) and viral titers were calculated as fluorescence forming units (FFU) per milliliter using fluorescent focus assay as previously described (24, 25). BKV was purified by cesium chloride density gradient ultracentrifuge and labeled by Alexa Fluor 488 Microscale Protein Labeling Kit (Molecular Probes, Eugene, OR) as previously described (24, 25). Viral titer of labeled purified virus was recalculated and viral viability confirmed.
Infection and Treatment
In experiments presented in Figure 1, HRPTEC were preincubated with pravastatin sodium (pravastatin) (Sigma-Aldrich, St. Louis, MO) for 1 hr before addition of BKV (multiplicity of infection [MOI]=0.5 FFU/cell) and co-incubated for another 72 hr. Cells were washed and incubated with fresh medium containing pravastatin for another 48 hr. This protocol was established to induce most frequent BKV infection in HRPTEC according to our previous report (24). In experiments presented in Figure 2, HRPTEC were incubated with 50 μM of pravastatin for 6, 12, 24, and 48 hr. In experiments presented in Figure 3, HRPTEC were incubated with pravastatin 50 μM for 1 hr before co-incubation with BKV (MOI=5 FFU/cell) and pravastatin for another 6, 12, 24, and 48 hr. This protocol was established to identify the ability of pravastatin to interfere with caveolae endocytosis of BKV based on our previously reported time course of BKV particles entry in HRPTEC (24, 25). In experiments presented in Figure 4, HRPTEC were incubated with BKV for 72 hr. Cells were washed and incubated with fresh medium containing pravastatin for another 48 hr. Untreated HRPTEC were used as negative control, and HRPTEC incubated with BKV alone were used as positive control. The concentrations of pravastatin which did not affect cell viability were determined by cytotoxic assay (CytoTox 96 Non-Radioactive Cytotoxicity Assay, Promega, Madison, WI) according to the manufacture's protocol and 5 to 50 μM of pravastatin have been frequently used to treat human cells by in vitro experiments (26–28).
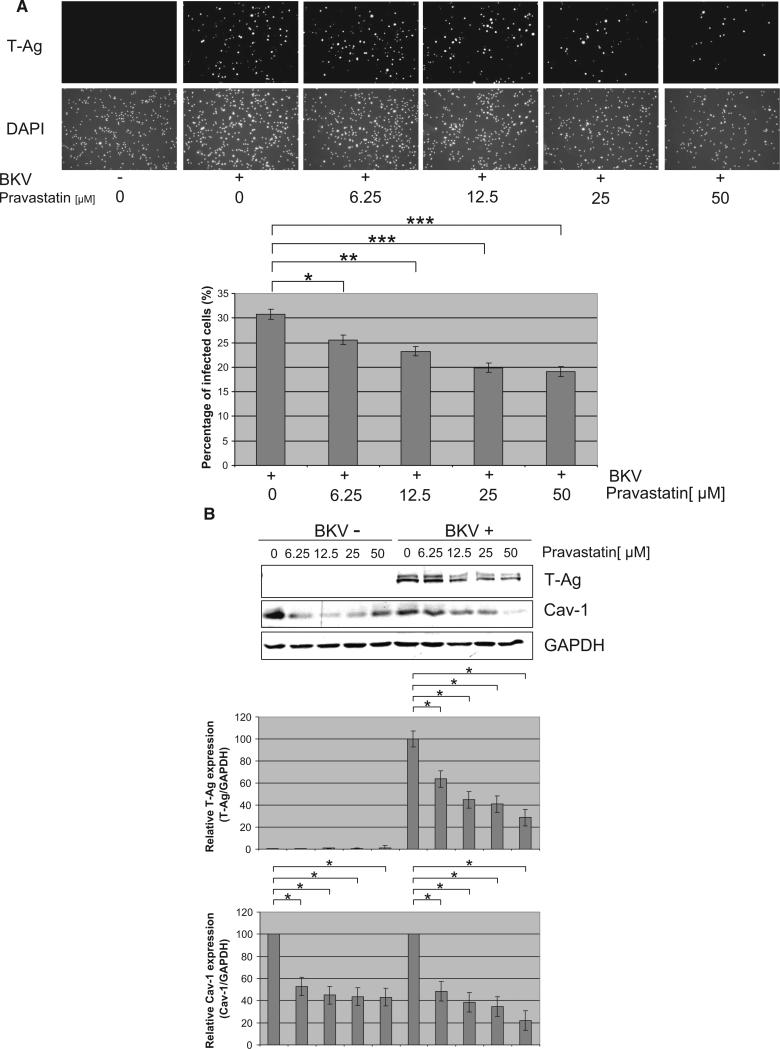
FIGURE 1.
Pravastatin interfered with BKV infection. HRPTEC were preincubated with pravastatin (6.25, 12.5, 25, and 50 μM) for 1 hr before co-incubation with BKV (MOI=0.5 FFU/cell) and pravastatin for 72 hr. Medium was removed and cells were incubated for another 48 hr with fresh medium containing pravastatin. (A) After incubation, cells were analyzed by indirect immunofluorescent analysis (magnification ×20). (upper) T-Ag positive cells were counted as BKV infected cells and the percentage was calculated against DAPI stained nuclei (lower) as total cells. *P<0.01, **P<0.005, ***P<0.001. (B) After incubation, cells were analyzed by Western blot. Relative T-Ag and Cav-1 expression was detected as graph bars by measurement of the intensity by Odyssey. Intensity of T-Ag and Cav-1 expression was revised by the intensity of GAPDH as loading control. *P<0.001.
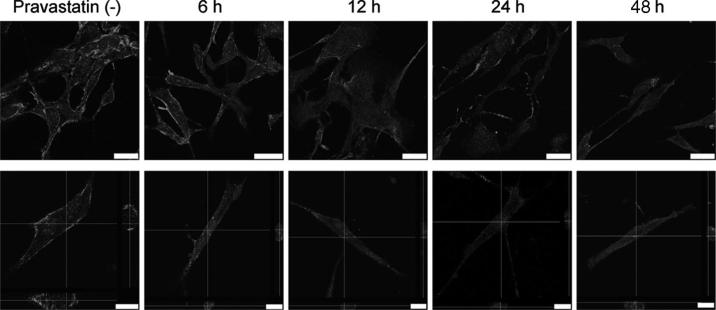
FIGURE 2.
Pravastatin decreases the expression of Cav-1. HRPTRC were incubated with 50 μM pravastatin for 0, 6, 12, 24 and 48 hr. After incubation, cells were immunostained for Cav-1 and observed by confocal microscope with 63× objective lens (upper). Cross-section confocal images of the interior of cells after caveolin-1 staining are also shown (lower). Horizontal and vertical lines indicate the X and Y planes through which the cross-section images were made. Bars are 25 μM (upper) and 10 μM (lower).
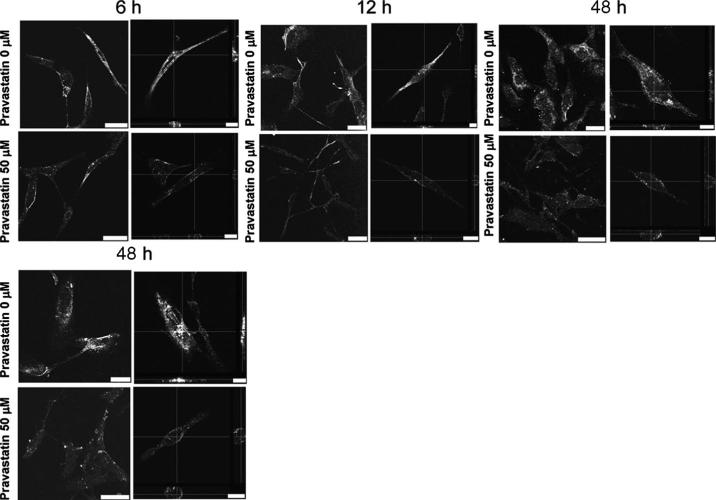
FIGURE 3.
Pravastatin interfered with BKV entry into HRPTEC. (upper) HRPTEC were incubated with BKV (MOI=5 FFU/cell) alone for 6, 12, 24, and 48 hr. (lower) HRPTEC were preincubated with 50 μM of pravastatin for 1 hr before another 6, 12, 24, and 48 hr co-incubation with labeled BKV (MOI=5 FFU/cell) and 50 μM of pravastatin. After incubation, Alexa Fluor 488 labeled BKV particles were observed by confocal microscope with 63× objective lens. Cross-section confocal images of the interior of cells incubated with labeled BKV are shown for each incubation time (right). Horizontal and vertical lines indicate the X and Y planes through which the cross-sectioned images were made. Bars are 25 μM (left) and 10 μM (right).
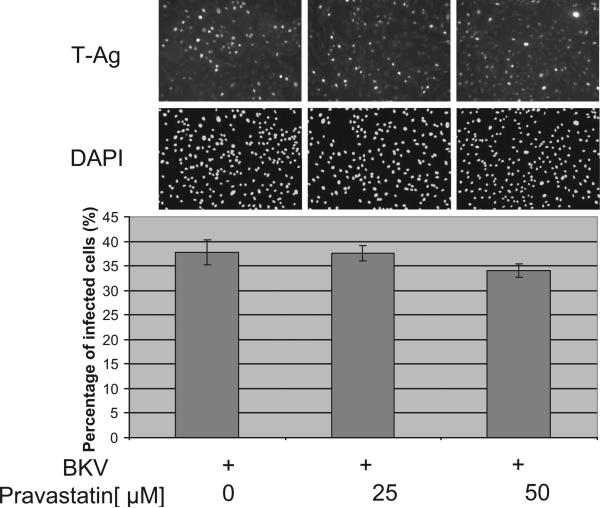
FIGURE 4.
The effect of pravastatin after BKV infection was established. HRPTEC were incubated with BKV (MOI=0.5 FFU/cell) for 72 hr. Medium was removed and cells were incubated for another 48 hr with pravastatin (25 and 50 μM). After 5 days incubation, cells were analyzed by indirect immunofluorescent analysis (magnification ×20). (upper) T-Ag positive cells were counted as BKV infected cells and the percentage was calculated against DAPI stained nuclei (lower) as total cells.
Western Blot Analysis and Indirect Immunofluorescent Analysis
Western blot analysis was performed as previously described (24, 25). BKV large T-antigen (T-Ag) was detected by anti-SV40 T antigen mouse monoclonal antibody purchased from Calbiochem (San Diego, CA), Cav-1 was detected by anti-Cav-1 rabbit polyclonal antibody purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was detected by rabbit polyclonal antibodies to GAPDH purchased from Abcam (Cambridge, MA). To detect bands by Odyssey infrared imaging system (LI-COR Inc., Lincoln, NE), Alexa flour 680 goat anti-mouse IgG (H+L), (Molecular Probes), and IRDye 800 conjugated affinity purified anti-rabbit IgG (H&L) (Rockland Immunochemicals Inc., Gilbertsville, PA) were used as secondary antibody. Integrated intensity of each signal was measured by Odyssey, and relative intensity of T-Ag and Cav-1 revised by GAPDH was expressed as graph bars. Means and SE were calculated from three independent experiments.
Indirect immunofluorescent (IF) analysis was also performed as previously described (24, 25). After fixation and blockage, cells were incubated with primary antibody (1 μg/mL mouse monoclonal antibody), and then incubated with secondary antibody, 1:200 dilution of Alexa flour 488 goat anti-mouse IgG (H+L) purchased from Molecular Probes. To stain nuclei, cells were incubated with 300 nM of 4′, 6-diamidino-2-phenylindole (DAPI) dilactate purchased from Molecular Probes.
To detect the percentage of infected cells, a fluorescent microscope (Nikon Eclipse E600) and SPOT version 4.0.9 (Diagnostic instruments, Scotland, UK) were used to capture images. The number of T-Ag positive cells as BKV infected cells and the number of DAPI stained nuclei as total cell numbers were counted automatically by the MetaVue Imaging System (Sunnyvale, CA), and the percentage of infected cells was calculated by using formula shown below. At least 500 cells were counted from each three independent cover slips and means and SE were calculated from two independent experiments.
Percentage of infected cells=number of T-Ag positive cells/number of DAPI stained nuclei×100.
A confocal microscope (Leica TCS SP5) was used to observe labeled BKV (MOI=5 FFU/cell) and caveolin-1 expression with or without co-incubation of pravastatin and Leica application suite advanced fluorescence was used to capture images.
Statistical Analysis
All experiments were repeated at least twice. Mean±SE was calculated from repeated data. We used a factorial analysis of variance with Dunnett's multiple comparison's correction, and linear (polynomial) contrasts were used for trend in dose.
RESULTS
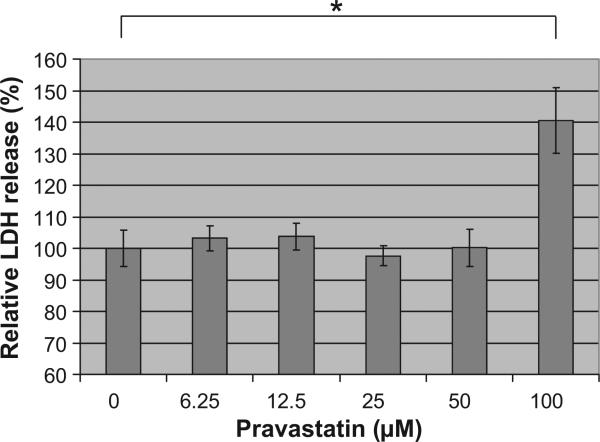
To determine the appropriate dose of pravastatin, we investigated the cytotoxicity of pravastatin by measuring released lactate dehydrogenase from cells. When HRPTEC were incubated with pravastatin at 6.25, 12.5, 25, and 50 μM, there was no significant difference in comparison to untreated cells. However, when HRPTEC were incubated with 100 μM pravastatin, lactate dehydrogenase release was significantly increased in comparison with untreated cells (P<0.01) indicating drug induced cytotoxicity (Fig. 5). Thus, the appropriate dose of pravastatin was determined to be below 50 μM.
FIGURE 5.
Cytotoxic assay of pravastatin. HRPTEC were incubated with pravastatin (6.25, 12.5, 25, 50, and 100 μM) for 5 days. After that, supernatants and cell lysates were used for measuring released lactate dehydrogenase. Lactate dehydrogenase was measured in five different wells for each dose. Means and SE were calculated from the results of 10 different wells from two independent examinations. *P<0.01.
To examine the effect of pravastatin, HRPTEC were pretreated and co-incubated with pravastatin and BKV. In IF analysis, there were no T-Ag positive cells in untreated cells (Fig. 1A) and cells incubated with pravastatin alone (data not shown). The percentage of infected cells were 30.77%±0.99%, when HRPTEC were incubated with BKV alone. However, when HRPTEC were incubated with BKV and several doses of pravastatin, the percentage of infected cells was significantly decreased in comparison to HRPTEC incubated with BKV alone (6.25 μM, P<0.01; 12.5 μM, P<0.005; 25 and 50 μM, P<0.001), and there was also a significant decreasing trend with increasing dose of pravastatin (P<0.0001) (Fig. 1A). As expected, T-Ag expression was not detected by western blotting in untreated HRPTEC and HRPTEC incubated with pravastatin alone. However, in HRPTEC incubated with BKV, T-Ag signal was prominent and relative T-Ag expression exceeded significantly the T-Ag expression level in HRPTEC incubated with BKV and several doses of pravastatin (6.25, 12.5, 25, and 50 μM, P<0.001). There was also a significant decreasing trend with dose of pravastatin (P<0.0001). Importantly, Cav-1 expression was also significantly decreased by the treatment of pravastatin. Decreased level of Cav-1 expression in cells treated with pravastatin was observed regardless of the infection with BKV (without BKV: 6.25, 12.5, 25, and 50 μM, P<0.001; with BKV: 6.25, 12.5, 25, and 50 μM, P<0.001) (Fig. 1B).
Effect of pravastatin on Cav-1 expression was also visible using immunofluorescence with anti Cav-1 antibodies (Fig. 2). In untreated HRPTEC, Cav-1 expression is significant. When HRPTEC were incubated with pravastatin from 6 to 48 hr, Cav-1 expression was dramatically decreased at all time points.
The distribution of purified and labeled BKV particles in HRPTEC after 6, 12, 24, and 48 hr incubation was significantly affected by pravastatin pretreatment and co-incubation (Fig. 3). When HRPTEC were incubated with labeled BKV alone, almost of all BKV particles were located in the cytoplasm at each time point. However, when BKV infected HRPTEC were pre- and co-incubated with pravastatin, most of BKV particles remained to locate on the cell surface indicating that BKV entry pathway was attenuated by pravastatin treatment.
Next, we examined whether the inhibitory effect of pravastatin on BKV infection was detected when pravastatin was added 72 hr after BKV infection. Large T-Ag was detected 36 hr after infection and peaked 7 days after infection (29). We incubated HRPTEC with pravastatin for 48 hr starting 72 hr after infection. The percentage of infected cells in HRPTEC incubated with pravastatin after BKV infection was not significantly decreased in comparison with HRPTEC incubated with BKV alone (Fig. 4).
These results suggest that pravastatin interfered with BKV internalization which occurs through caveolae-mediated endocytosis and repressed BKV infection in HRPTEC perhaps by affecting the function of caveolae at the cell surface, but was not capable to suppress BKV infection when added after virus entered HRPTEC.
DISCUSSION
BKV infection is asymptomatic and has a high prevalence during childhood. After primary infection from upper respiratory route, BKV mainly persists in the urogenital tract and enters latent phase in the renal tubular epithelial and urothelial cells. Latent BKV is transported with donor kidney to recipients who receive intense immunosuppressive therapy, becomes activated in these immunocompromised hosts and undergoes replication in the renal tubular cells. Daughter viruses are delivered to other cells and infection expands. Thus latent BKV progresses to BKV nephritis in the renal transplant patients. After renal transplantation, 35% to 60% of patients have BKV viruria, 5% to 30% of patients have BKV viremia, the prevalence of BKV nephropathy is about 10% of renal transplant patients, and about half of them have been reported to progress irreversible allograft failure within 1 year after diagnosis (1–5). Anti-BKV therapies without reduction or alteration of immunosuppressive agent have been proposed in recent years. However, none of the therapies resulted in establishing efficient strategies to prevent BKV infection, because there was no prospective large sized randomized controlled study, and also the protocols to remit BKV activity are still unclear. Because of this, many clinical centers still treat BKV infection with reduction or alteration of immunosuppressive agents.
We have reported that BKV entered into HRPTEC through caveolae which are membrane invaginations abundant in free cholesterol, and that methyl beta cyclodextrin, membrane cholesterol-depleting agent, and nystatin, known to sequester plasma membrane cholesterol, interfered with BKV internalization as detected by IF analysis and decreased BKV infection in HRPTEC as detected by Western blot and IF analysis (24). Previous reports indicated that atorvastatin decreased the Cav-1 expression regardless of the level of extracellular LDL-cholesterol in bovine aortic endothelial cells (22) and rosuvastatin decreased the Cav-1 expression in apolipoprotein E–/–, dyslipidemic mice (23). Accordingly, we hypothesized that statins which are actually used in a clinical situation as cholesterol lowering agents might decrease caveolae, interfering with BKV internalization and decreasing BKV infection in HRPTEC. We selected pravastatin, because its interaction with cytochrome P450 3A4 metabolism which was affected by calcineurin inhibitors in transplant patients was lowest among the statins (30, 31).
Our results showed that pravastatin pretreatment significantly decreased BKV infection in HRPTEC (Fig. 1A,B) and also decreased the level of Cav-1 (Fig. 1B). Moreover pravastatin treatment dramatically decreased Cav-1 expression as detected by IF analysis on the cell surface (Fig. 2), and also changed distribution of labeled BKV causing retention of BKV particles on the cell surface, even though labeled BKV particles in untreated cell were mostly detected in the cytoplasm (Fig. 3). However, after BKV infection was established, pravastatin treatment did not decrease BKV infection in HRPTEC (Fig. 4). These results indicate that the treatment with pravastatin interferes with BKV entry into HRPTEC which occurs through caveolae. Pravastatin decreases the level of caveolae on the cell surface and impedes the spread of BKV infection in HRPTEC. However, pravastatin did not have any ability to suppress BKV infection after infection is established in cultured HRPTEC.
Statins are most frequently used to treat hypercholesterolemia, and are also used to treat renal transplant patients with hypercholesterolemia, as hypercholesterolemia is one of the side effects of calcineurin inhibitors (31–33). But to the best of our knowledge, there is no report which showed the relationship between statins and BKV infection either in basic or clinical studies. Our report shows the potential of statins to prevent BKV spreading before latent BKV progresses to BKV nephropathy. Statins are likely to be less efficient against progressed BKV nephritis, because the effect of pravastatin on BKV infection seemed to depend on the interference with BKV internalization through caveolae, and pravastatin was not able to repress established BKV infection in HRPTEC. Therefore, we believe that the treatment with statins should be started as soon as possible after finding BKV viruria or viremia and it may be too late when BKV nephritis is diagnosed by renal biopsy, because at the time of diagnosis, BKV infection would be already established and BKV will spread to other cells.
However, we have to mention several limitations to this study. The peak serum concentration of pravastatin in human is about 0.1 μM (30, 34) and there are no reports about the concentration in human kidney tissue, but it was reported that the tissue concentration of rat kidney was about three times higher than serum concentration (35). Though there is no data about the concentration of pravastatin in human kidney tissue, our experimental dose was higher than serum concentration in human and might be higher than the human kidney tissue. However, it must be taken into consideration that because pravastatin can be bound by serum factors, the real level of remaining free active pravastatin could be significantly less than the concentration initially added to cultured cells. In any case it is the fact that statins have some possibility to abolish BKV internalization into HRPTEC which are one of the main natural targets of BKV nephritis and they are actually used against a lot of transplant patients with hypercholesterolemia. The details of the relationship between the treatment of statins and BKV nephritis, such as the type and dose of statins, the time onset of statins therapy, the level of LDL, hyper density lipoprotein, and total cholesterol, the level of BKV viruria and viremia, renal function, histological findings and the outcome, should be analyzed in the large sized clinical study to elucidate the beneficial effect of statins on BKV nephritis.
ACKNOWLEDGMENTS
The authors thank Dr. Raymond Hoffmann (Medical College of Wisconsin [MCW]) for help with statistical analysis and Dr. Tetsuro Wakatsuki (MCW) for assistance with technical use of confocal microscope. The authors are grateful to Dr. Simon Prosser (MCW) for critical reading and correcting this article.
This work was supported by Program Development Grant from Department of Medicine, Medical College of Wisconsin (A.S.) and NIH R01 grants HL022563 and DK041684 (A.S.).
REFERENCES
- 1.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch HH. BK virus: Opportunity makes a pathogen. Clin Infect Dis. 2005;41:354. doi: 10.1086/431488. [DOI] [PubMed] [Google Scholar]
- 3.Nickeleit V, Mihatsch MJ. Polyomavirus nephropathy in native kidneys and renal allografts: An update on an escalating threat. Transpl Int. 2006;19:960. doi: 10.1111/j.1432-2277.2006.00360.x. [DOI] [PubMed] [Google Scholar]
- 4.Randhawa P, Brennan DC. BK virus infection in transplant recipients: An overview and update. Am J Transplant. 2006;6:2000. doi: 10.1111/j.1600-6143.2006.01403.x. [DOI] [PubMed] [Google Scholar]
- 5.Trofe J, Hirsch HH, Ramos E. Polyomavirus-associated nephropathy: Uptake of clinical management in kidney transplant patients. Transpl Infect Dis. 2006;8:76. doi: 10.1111/j.1399-3062.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- 6.Kadambi PV, Josephson MA, Williams J, et al. Treatment of refractory BK virus-associated nephropathy with cidofovir. Am J Transplant. 2003;3:186. doi: 10.1034/j.1600-6143.2003.30202.x. [DOI] [PubMed] [Google Scholar]
- 7.Kuypers DR, Vandooren AK, Lerut E, et al. Adjuvant low-dose cidofovir therapy for BK polyomavirus interstitial nephritis in renal transplant recipients. Am J Transplant. 2005;5:1997. doi: 10.1111/j.1600-6143.2005.00980.x. [DOI] [PubMed] [Google Scholar]
- 8.Josephson MA, Gillen D, Javaid B, et al. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplant. 2006;81:704. doi: 10.1097/01.tp.0000181149.76113.50. [DOI] [PubMed] [Google Scholar]
- 9.Williams JW, Javaid B, Kadambi PV, et al. Leflunomide for polyomavirus type BK nephropathy. N Engl J Med. 2005;352:1157. doi: 10.1056/NEJM200503173521125. [DOI] [PubMed] [Google Scholar]
- 10.Leung AY, Chan MT, Yuen KY, et al. Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogenic hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:528. doi: 10.1086/427291. [DOI] [PubMed] [Google Scholar]
- 11.Sener A, House AA, Jevnikar AM, et al. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: One-year follow-up of renal allograft recipients. Transplant. 2006;81:117. doi: 10.1097/01.tp.0000181096.14257.c2. [DOI] [PubMed] [Google Scholar]
- 12.Josephson MA, Williams JW, Chandraker A, et al. Polyomavirus-associated nephropathy: Update on antiviral strategies. Transpl Infect Dis. 2006;9:95. doi: 10.1111/j.1399-3062.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 13.Roskopf J, Trofe J, Stratta RJ, et al. Pharmacotherapeutic options for the management of human polyomaviruses. Adv Exp Med Biol. 2006;577:228. doi: 10.1007/0-387-32957-9_17. [DOI] [PubMed] [Google Scholar]
- 14.Trofe J, Hirsch HH, Ramos E. Polyomavirus-associated nephropathy: Update of clinical management in kidney transplant patients. Transpl Infect Dis. 2005;8:76. doi: 10.1111/j.1399-3062.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- 15.Kinlay S. Potential vascular benefits of statins. Am J Med. 2005;118(suppl 12A):62. doi: 10.1016/j.amjmed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 2005;96:24F. doi: 10.1016/j.amjcard.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massy ZA, Guijarro C. Statins: Effects beyond cholesterol lowering. Nephrol Dial Transplant. 2001;16:1738. doi: 10.1093/ndt/16.9.1738. [DOI] [PubMed] [Google Scholar]
- 18.Campese VM, Nadim MK, Epstein M. Are 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors renoprotective? J Am Soc Nephrol. 2005;16:S11. doi: 10.1681/asn.2004110958. [DOI] [PubMed] [Google Scholar]
- 19.Campese VM, Park J. HMG-CoA reductase inhibitors and the kidney. Kidney Int. 2007;71:1215. doi: 10.1038/sj.ki.5002174. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748. doi: 10.1016/j.amjcard.2005.09.110. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R, Curley TM. The role of statins in chronic kidney disease. Am J Med Sci. 2005;330:69. doi: 10.1097/00000441-200508000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Feron O, Dessy C, Desager JP, et al. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103:113. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- 23.Pelat M, Dessy C, Massion P, et al. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E−/− mice in vivo. Circulation. 2003;107:2480. doi: 10.1161/01.CIR.0000065601.83526.3E. [DOI] [PubMed] [Google Scholar]
- 24.Moriyama T, Marquez JP, Wakatsuki T, et al. Caveolar endocytosis is critical for BK virus infection of human renal proximal tubular epithelial cells. J Virol. 2007;81:8552. doi: 10.1128/JVI.00924-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriyama T, Sorokin A. Intracellular trafficking pathway of BK virus in human renal proximal tubular epithelial cells. Virology. 2008;371:336. doi: 10.1016/j.virol.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bessler H, Salman H, Bergman M, et al. In vitro effect of statins on cytokine production and mitogen response of human peripheral blood mononuclear cells. Clin Immunol. 2005;117:73. doi: 10.1016/j.clim.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Tornio A, Pasanen MK, Laitila J, et al. Comparison of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) as inhibitors of cytochrome P450 2C8. Basic Clin Pharmacol Toxicol. 2005;97:104. doi: 10.1111/j.1742-7843.2005.pto_134.x. [DOI] [PubMed] [Google Scholar]
- 28.Kanno T, Abe K, Yabuki M, et al. Selective inhibition of formylmethionyl-leucyl-phenylalanine (fMLF)-dependent superoxide generation in neutrophils by pravastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. Biochem Pharmacol. 1999;58:1975. doi: 10.1016/s0006-2952(99)00260-9. [DOI] [PubMed] [Google Scholar]
- 29.Low J, Humes HD, Szczypla M, et al. BKV and SV40 infection of human kidney tubular epithelial cells in vitro. Virology. 2004;323:182. doi: 10.1016/j.virol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Lilja JJ, Kivistö KT. Neuvonen PJ. Grapefruit juice increases serum concentrations of atorvastatin and has no effect on pravastatin. Clin Phamacol Ther. 1999;66:118. doi: 10.1053/cp.1999.v66.100453001. [DOI] [PubMed] [Google Scholar]
- 31.Christians U, Jacobsen W, Floren LC. Metabolism and drug interactions of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in transplant patients: Are the statins mechanically similar? Pharmacol Ther. 1998;80:1. doi: 10.1016/s0163-7258(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 32.Lentine KL, Brennan DC. Statin use after renal transplantation: A systematic quality review of trial-based evidence. Nephrol Dial Transplant. 2004;19:2378. doi: 10.1093/ndt/gfh385. [DOI] [PubMed] [Google Scholar]
- 33.Navaneethan SD, Pansini F, Strippoli GFM. Statins in patients with chronic kidney disease: Evidence from systematic reviews and randomized clinical trials. PLoS Med. 2006;3:e123. doi: 10.1371/journal.pmed.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pentikainen PJ, Saraheimo M, Schwartz JI, et al. Comparative pharmacokinetics of lovastatin, simvastatin and pravastatin in humans. J Clin Pharmacol. 1992;32:136. doi: 10.1002/j.1552-4604.1992.tb03818.x. [DOI] [PubMed] [Google Scholar]
- 35.Komai T, Kawai K, Toui T, et al. Disposition and metabolism of pravastatin sodium in rats, dogs and monkeys. Eur J Drug Metab Pharmacokinet. 1992;17:103. doi: 10.1007/BF03188778. [DOI] [PubMed] [Google Scholar]