Abstract
Background
An extensive clinical literature has noted gender differences in the etiology and clinical characteristics of individuals with alcohol dependence (AD). Despite this knowledge, many important questions remain.
Methods
Using the 2001 to 2002 National Epidemiologic Survey on Alcohol and Related Conditions (n = 43,093), we examined differences in sociodemographic characteristics, psychiatric and medical comorbidities, clinical correlates, risk factors, and treatment-utilization patterns of men (N = 2,974) and women (N = 1,807) with lifetime AD.
Results
Men with lifetime AD were more likely than women to be diagnosed with any substance use disorder and antisocial personality disorder, whereas women were more likely to have mood and anxiety disorders. After adjusting for sociodemographic characteristics and gender differences in psychiatric comorbidity in the general population, AD was associated with externalizing disorders and any mood disorder among women only. Men with AD met more criteria, had longer episodes, and were younger at the age of first drink. There were no gender differences in remission rates. Women with AD were more likely to have a family and a spouse with history of alcohol use disorders. Treatment rates were low for both genders, and women were more likely to report social stigmatization as a treatment barrier.
Conclusions
There are important gender differences in the psychiatric comorbidities, risk factors, clinical characteristics, and treatment-utilization patterns among individuals with lifetime AD.
Keywords: NESARC, Alcohol Dependence, Gender Differences, Epidemiology
Recent studies have suggested that the prevalence of alcohol dependence (AD) among women is rising while remaining relatively constant among men (Grant et al., 2004a; Grucza et al., 2008a, 2008b). This finding has increased public concern over gender differences in AD and disparities in its treatment. Although the gender gap in alcohol problems is narrowing (Keyes et al., 2011), an extensive literature has noted gender differences in the etiology and clinical characteristics of individuals with AD (Nolen-Hoeksema, 2004). Despite this knowledge, several important questions remain unanswered.
First, epidemiological studies have consistently documented that most individuals with AD have at least 1 comorbid psychiatric disorder (Hasin et al., 2007; Kessler et al., 1997). Women with AD are more likely to have comorbid mood or anxiety disorders, whereas men with AD are more likely to have a history of conduct disorder and antisocial personality disorder (ASPD; Dawson et al., 2010). To date, however, no study has examined whether these patterns simply reflect gender differences in the prevalence of psychiatric disorders in the general population, or whether AD moderates the relationship between gender and psychiatric comorbidity.
Second, earlier clinical and epidemiological studies found that men tend to start drinking at younger ages than women (Johnston et al., 1996), but this gender gap has decreased in more recent cohorts (Chen et al., 2009). There is a need to compare the course of AD in men and women in more recent cohorts. Third, some clinical and epidemiological studies have suggested that women are more likely than men to drink excessively to alleviate negative emotional states (Rubonis et al., 1994) and that women with alcohol use disorders are more likely to marry men with alcohol-related disorders (Roberts and Leonard, 1997). In addition, family and marital problems strongly affect the risk of both developing and relapsing from AD, especially in women (Connors et al., 1998). Besides investigations concerning the heritability of AD, we know of few epidemiological investigations with large samples that directly investigated gender differences in the risk factors for AD (Hasin et al., 2007).
Fourth, women are consistently underrepresented in alcohol treatment settings, and clinical studies have reported that stigma is a significant barrier in the treatment for AD (Copeland, 1997). Several epidemiologic samples have suggested that the most common treatment-seeking barrier for both men and women is a lack of motivation to seek treatment (Grant, 1997; Wells et al., 2007). However, to date, only 1 epidemiological study has compared gender differences in the treatment barriers for AD (Grant, 1997). It found that women were more likely to report an inability to arrange childcare, and the belief that their drinking was a symptom of another problem/situation as their primary barriers to treatment (Grant, 1997). As a result of this scarcity of data, gender effects in treatment-seeking patterns of individuals with AD in the community remain poorly characterized. Given that only a small percentage of people seek formal treatment for AD (Grant, 1997), a better characterization of gender differences in treatment-seeking may help develop gender-sensitive treatments for AD.
In spite of rich clinical and epidemiological data, many questions still remain on the gender differences in AD. We sought to address these gaps in knowledge by drawing on data from the National Epidemiological Survey on Alcohol and Related Conditions (NESARC), a nationally representative community sample of U.S. adults (n = 43,093). The specific goals of the present study are as follows: (i) to compare the socio-demographic characteristics of men and women with AD; (ii) to compare the rates and pattern of psychiatric comorbidity among men and women with AD; (iii) to examine gender differences in the course, clinical presentation and risk factors in men and women with AD; and (iv) to compare gender differences in treatment-utilization patterns for AD among individuals with the disorder.
MATERIALS AND METHODS
Sample
The 2001 to 2002 NESARC is a nationally representative sample of the U.S. population that has been described in detail elsewhere (Grant et al., 2004b). The target population was aged 18 years and older, and included those residing in households and group quarters. Face-to-face interviews were conducted with 43,093 respondents. The survey response rate was 81%. Blacks, Hispanics, and young adults (ages 18 to 24 years) were oversampled with data adjusted for oversampling and nonresponse. The weighted data were adjusted to represent the U.S. civilian population based on the 2000 census (Grant et al., 2004b).
Assessment
The diagnostic interview was the Alcohol Use Disorder and Associated Disabilities Interview Schedule–DSM-IV Version (AUDADIS-IV; Grant et al., 2001), a structured interview designed for professional lay interviewers (Grant et al., 2004b).
AUDADIS-IV Version lifetime AD (hereafter referred to as AD) diagnoses required that subjects meet 3 or more of the 7 DSM-IV dependence criteria in the last 12 months or during any previous 12-month period. For prior diagnoses of AD, 3 or more criteria must have occurred within a 1-year period following the DSM-IV clustering criterion. The reliability of the AUDADIS-IV alcohol diagnoses is documented in clinical and general population samples (Grant et al., 2003b; Hasin et al., 1997) with test–retest reliability ranging from good to excellent (K = 0.70 to 0.84). Convergent, discriminant, and construct validity of AUDADISIV dependence criteria and diagnoses are good to excellent (Hasin et al., 2003) and included the World Health Organization/National Institutes of Health International Study on Reliability and Validity (Nelson et al., 1999), where clinical reappraisals documented good validity of DSM-IV AD diagnoses (K = 0.60 to 0.76).
We assessed the following sociodemographic variables: race/ethnicity, nativity, age, education, individual income, employment status, marital status, urbanicity (e.g., urban or rural), U.S. region, and insurance.
Mood disorders included DSM-IV primary major depressive disorder, bipolar I, bipolar II, and dysthymia. Anxiety disorders included DSM-IV primary panic disorder, social anxiety disorder, specific phobia, and generalized anxiety disorder. AUDADIS-IV methods to diagnose these disorders are described in detail elsewhere (Grant et al., 2004b). In the DSM-IV, “primary” excludes substance-induced disorders or those due to medical conditions; major depressive disorder diagnoses also ruled out bereavement. Personality disorders, assessed on a lifetime basis, included DSM-IV avoidant, dependent, obsessive-compulsive, paranoid, schizoid, histrionic, and ASPDs. The test–retest reliability for AUDADIS-IV mood, anxiety, and personality diagnoses in the general population and clinical settings were fair to good (K = 0.40 to 0.62; Grant et al., 2003b; Ruan et al., 2008). Test–retest reliabilities of AUDA-DIS-IV personality disorders (not measured in prior surveys) compare favorably with those in patient samples using semistructured personality interviews (Zimmerman, 1994). Convergent validity was good to excellent for all affective and anxiety diagnoses, and selected diagnoses showed good agreement (K = 0.64 to 0.68) with psychiatrist reappraisals (Hasin et al., 1997, 2005).
Measures of the clinical course of AD included the age at first drink, age of onset at heavy drinking, age at onset of AD, total number of episodes, total number of diagnostic criteria met, duration of the longest episode, age at remission, and the percentage of individuals who remitted. Number of drinks was determining by asking individuals: “counting all types of alcohol combined, how many drinks did you usually have on days when you drank during the period you drank the most?” Heavy drinking was defined as: 5+ drinks in a single day, with a frequency ranging from every day to 1 to 2 times per year. To examine the progression across the landmark stages of AD (“telescoping”; Randall et al., 1999), we measured 3 time points and the intervals between them: (i) the age at first drink to age at heavy drinking; (ii) the age at heavy drinking to the age of onset of AD; and (iii) the age at first drink to the age at onset of AD.
Previously described risk factors for AD (Prescott and Kendler, 1999) and general psychopathology were measured. These included a family history of alcohol use disorders, marriage to a spouse with an alcohol use disorder, a vulnerable family environment (operationalized as parental absence/separation from a biological parent before age 18), parental loss before age 18, and a family history of depression. In addition to risk factors, we included measures such as use of alcohol or drugs to relieve mood or anxiety symptoms, and self-reported medical conditions (liver disease/cirrhosis, hypertension/ angina, and ulcers) that were the subject of previous research on gender differences in AD (Tuyns and Pequignot, 1984).
Treatment-Utilization for Alcohol Dependence
To be consistent with other reports (Kessler et al., 2005), we divided treatment-utilization into professional treatment and non-professional treatment (treatment provided by human service professionals) among individuals who sought treatment. Professional treatment included the following: (i) outpatient visits to a physician, psychologist, or any other professional; (ii) inpatient treatment in a drug detoxification or rehabilitation unit, or hospital ward; and (iii) treatment in an emergency department. Human service professionals included members of the clergy, employee assistance programs, family and social services, halfway houses, therapeutic communities, crisis centers, and self-help groups.
Among individuals with AD who did not seek treatment, we categorized reasons for not seeking help into 4 categories: (i) logistical barriers (e.g., financial difficulties, lack of time, no childcare, or transportation); (ii) lack of motivation (e.g., wanted to keep using alcohol); (iii) social stigma (e.g., was embarrassed by the problem); and (iv) low perceived need (e.g., thought that treatment was not necessary because they already handled the problem).
Statistical Analyses
Weighted means and percentages were computed to determine gender differences in the sociodemographic correlates, prevalence of psychiatric comorbidities, clinical course and characteristics, risk factors, and treatment-utilization behaviors among respondents with lifetime DSM-IVAD. Logistic regression analyses yielded odds ratios (ORs), indicating measures of association among: (i) lifetime AD and psychiatric comorbidities; (ii) lifetime AD and risk factors; (iii) lifetime AD and clinical characteristics; and (iv) lifetime AD and treatment-utilization behaviors.
To ensure that gender differences in the risk of psychiatric comorbidities were not due to sociodemographic correlates or to gender differences in the distribution of psychiatric disorders in the general population, the association between gender, AD, and comorbidity was examined using additional logistic regression models. These logistic regression models used each psychiatric disorder as the outcome variable, and included gender, lifetime AD, and their interaction as predictors. These models also adjusted for sociodemographic characteristics. Similarly, models examining treatment-utilization behaviors also adjusted for sociodemographic characteristics, to ensure that the gender differences in treatment-utilization patterns were not due to the differential distribution of sociodemographic characteristics across genders.
Due to the cross-sectional nature of the study, both unadjusted and adjusted ORs are used as measures of association without implying any causal association. We focus most of our analyses on outcomes that are most easily observable by the clinician, that is, unadjusted ORs. We focus on adjusted ORs in the case of comorbidity to be able to fully account for the effect of gender by disorder interactions. We also focus on adjusted ORs when examining treatment- utilization behaviors, to account for sociodemographic variables (e.g., insurance) that may influence access to care. We consider 2 percentages to be different if the 95% confidence interval of their ORs does not include 1.0. All standard errors and 95% confidence intervals were estimated using SUDAAN version 9.0 software (RTI International, Research Triangle Park, NC) to adjust for the design characteristics of the NESARC. Women (and individuals without AD, when modeling interactions) were considered the reference group for all analyses.
RESULTS
Sociodemographic Characteristics
Previous data from the NESARC estimates the prevalence of lifetime AD to be 17.4% among men and 8.0% among women (Hasin et al., 2007). Table 1 shows that men with lifetime AD were significantly more likely than women to be foreign-born, aged 45 or older, to have a high school education or less, to have an income >$20,000, and to be uninsured. Men with AD were less likely than women to be unemployed, and widowed, separated, or divorced.
Table 1.
Sociodemographic Characteristics Among Individuals with Lifetime DSM-IV Alcohol Dependence by Gender
| Characteristic | Male n = 2,974 |
Femalea n = 1,807 |
OR | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | ||||||
| Race/Ethnicity | |||||||||
| Whitea | 77.81 | 74.38 | 80.91 | 79.12 | 76.39 | 81.60 | 1.00 | 1.00 | 1.00 |
| Black | 7.39 | 6.26 | 8.71 | 7.52 | 6.31 | 8.94 | 1.00 | 0.81 | 1.23 |
| Native American | 3.19 | 2.45 | 4.14 | 3.86 | 2.64 | 5.60 | 0.84 | 0.54 | 1.30 |
| Asian | 2.41 | 1.59 | 3.65 | 1.49 | 0.92 | 2.40 | 1.65 | 0.97 | 2.82 |
| Hispanic | 9.20 | 6.91 | 12.14 | 8.03 | 6.23 | 10.28 | 1.17 | 0.92 | 1.47 |
| Nativity | |||||||||
| U.S.-borna | 92.52 | 90.21 | 94.32 | 96.00 | 94.59 | 97.05 | 1.00 | 1.00 | 1.00 |
| Foreign-born | 7.48 | 5.68 | 9.79 | 4.00 | 2.95 | 5.41 | 1.94 | 1.43 | 2.63 |
| Age | |||||||||
| 18–29a | 29.18 | 27.09 | 31.36 | 32.18 | 29.39 | 35.09 | 1.00 | 1.00 | 1.00 |
| 30–44 | 36.91 | 34.92 | 38.94 | 40.11 | 37.49 | 42.79 | 1.01 | 0.86 | 1.19 |
| 45–64 | 28.78 | 26.88 | 30.76 | 24.77 | 22.62 | 27.05 | 1.28 | 1.08 | 1.52 |
| 65+ | 5.14 | 4.39 | 6.00 | 2.94 | 2.15 | 4.01 | 1.93 | 1.35 | 2.75 |
| Education | |||||||||
| Less than high school | 14.23 | 12.48 | 16.19 | 9.84 | 8.21 | 11.75 | 1.65 | 1.32 | 2.06 |
| High school | 29.24 | 27.03 | 31.55 | 25.61 | 22.85 | 28.57 | 1.30 | 1.11 | 1.54 |
| Collegea | 56.53 | 54.05 | 58.97 | 64.55 | 61.51 | 67.48 | 1.00 | 1.00 | 1.00 |
| Individual income | |||||||||
| 0–19Ka | 36.29 | 33.94 | 38.70 | 55.81 | 52.71 | 58.87 | 1.00 | 1.00 | 1.00 |
| 20–34K | 25.59 | 23.67 | 27.62 | 23.65 | 21.15 | 26.34 | 1.66 | 1.37 | 2.02 |
| 35–69K | 28.78 | 26.72 | 30.93 | 16.85 | 14.64 | 19.32 | 2.63 | 2.14 | 3.22 |
| >70K | 9.34 | 8.00 | 10.87 | 3.69 | 2.78 | 4.88 | 3.89 | 2.86 | 5.30 |
| Employment status | |||||||||
| Employeda | 76.61 | 74.69 | 78.43 | 67.83 | 65.11 | 70.42 | 1.00 | 1.00 | 1.00 |
| Unemployed | 23.39 | 21.57 | 25.31 | 32.17 | 29.58 | 34.89 | 0.64 | 0.55 | 0.75 |
| Marital status | |||||||||
| Marrieda | 55.41 | 53.37 | 57.44 | 50.95 | 47.79 | 54.11 | 1.00 | 1.00 | 1.00 |
| Widowed/Separated/Divorced | 15.12 | 13.57 | 16.80 | 21.40 | 19.39 | 23.55 | 0.65 | 0.55 | 0.77 |
| Never married | 29.47 | 27.58 | 31.43 | 27.65 | 24.85 | 30.63 | 0.98 | 0.83 | 1.16 |
| Urbanicity | |||||||||
| Urbana | 77.24 | 73.07 | 80.93 | 80.02 | 75.68 | 83.75 | 1.00 | 1.00 | 1.00 |
| Rural | 22.76 | 19.07 | 26.93 | 19.98 | 16.25 | 24.32 | 1.18 | 0.98 | 1.42 |
| Region | |||||||||
| Northwest | 16.54 | 11.70 | 22.86 | 16.86 | 12.06 | 23.06 | 1.03 | 0.83 | 1.28 |
| Midwest | 27.01 | 21.42 | 33.44 | 29.30 | 22.83 | 36.72 | 0.97 | 0.78 | 1.20 |
| South | 30.37 | 24.94 | 36.40 | 26.47 | 20.99 | 32.79 | 1.20 | 0.98 | 1.47 |
| Westa | 26.09 | 19.85 | 33.47 | 27.38 | 20.80 | 35.10 | 1.00 | 1.00 | 1.00 |
| Insurance | |||||||||
| Public | 10.48 | 9.22 | 11.90 | 12.20 | 10.52 | 14.11 | 0.89 | 0.72 | 1.10 |
| Privatea | 65.97 | 63.59 | 68.26 | 68.00 | 64.95 | 70.91 | 1.00 | 1.00 | 1.00 |
| No insurance | 23.55 | 21.54 | 25.68 | 19.80 | 17.46 | 22.37 | 1.23 | 1.01 | 1.49 |
Reference group.
Bolded: p-value < 0.05.
Psychiatric Comorbidity
Table 2 shows that after adjusting for sociodemographic characteristics, men with AD were less likely to have any psychiatric disorder, any Axis I disorder, most mood disorders, all anxiety disorders, and avoidant and paranoid personality disorder when compared to women with AD. Men with AD were significantly more likely to have any substance use disorder, any drug use disorder, nicotine dependence, conduct disorder, pathological gambling, psychotic disorder, and ASPD. The likelihood of drug dependence, bipolar I, any personality disorder, and dependent, obsessive-compulsive, schizoid, and histrionic personality disorder did not differ between men and women with AD.
Table 2.
Prevalence and Interaction of Lifetime DSM-IV Alcohol Dependence (AD) and Other Psychiatric Disorders by Gender
| Characteristic | Male N = 2,974 |
Womena N = 1,807 |
Sex main effect
|
Interaction effect
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | SE | % | SE | AORb | 95% CI | AORb | 95% CI | |||
| Any psychiatric disorder | 79.17 | 0.88 | 85.09 | 1.00 | 0.85 | 0.80 | 0.90 | 0.89 | 0.73 | 1.08 |
| Any Axis I disorder | 75.52 | 0.96 | 84.17 | 1.03 | 0.81 | 0.76 | 0.86 | 0.82 | 0.67 | 0.99 |
| Any substance use disorderc | 64.33 | 1.11 | 62.55 | 1.30 | 1.48 | 1.36 | 1.60 | 0.85 | 0.73 | 1.00 |
| Any drug use disorderd | 41.59 | 1.15 | 37.60 | 1.44 | 2.00 | 1.76 | 2.26 | 0.72 | 0.59 | 0.88 |
| Drug abuse | 27.31 | 0.95 | 22.41 | 1.16 | 2.05 | 1.80 | 2.33 | 0.73 | 0.60 | 0.90 |
| Drug dependence | 14.27 | 0.77 | 15.19 | 0.96 | 1.34 | 1.00 | 1.79 | 0.86 | 0.62 | 1.19 |
| Nicotine dependence | 48.57 | 1.14 | 49.51 | 1.41 | 1.29 | 1.19 | 1.40 | 0.84 | 0.71 | 0.99 |
| Any mood disorder | 29.12 | 1.07 | 53.13 | 1.33 | 0.53 | 0.49 | 0.57 | 0.77 | 0.65 | 0.91 |
| Major depressive disorder | 16.79 | 0.82 | 33.09 | 1.22 | 0.46 | 0.42 | 0.50 | 0.97 | 0.81 | 1.17 |
| Bipolar I | 9.21 | 0.66 | 13.81 | 1.05 | 0.94 | 0.78 | 1.13 | 0.84 | 0.62 | 1.12 |
| Bipolar II | 2.36 | 0.34 | 4.54 | 0.57 | 0.72 | 0.52 | 0.99 | 0.74 | 0.45 | 1.23 |
| Dysthymia | 4.50 | 0.43 | 10.19 | 0.87 | 0.52 | 0.43 | 0.63 | 0.92 | 0.64 | 1.32 |
| Any anxiety disorder | 26.24 | 0.94 | 44.31 | 1.35 | 0.49 | 0.45 | 0.52 | 1.01 | 0.85 | 1.19 |
| Panic disorder | 8.23 | 0.63 | 17.27 | 0.98 | 0.45 | 0.38 | 0.53 | 1.12 | 0.84 | 1.48 |
| Social anxiety disorder | 8.83 | 0.69 | 14.98 | 1.17 | 0.72 | 0.62 | 0.82 | 0.84 | 0.64 | 1.10 |
| Specific phobia | 13.05 | 0.77 | 26.79 | 1.31 | 0.43 | 0.39 | 0.47 | 1.05 | 0.85 | 1.29 |
| Generalized anxiety disorder | 6.49 | 0.57 | 13.54 | 0.89 | 0.49 | 0.42 | 0.58 | 1.01 | 0.75 | 1.36 |
| Conduct disorder | 1.59 | 0.31 | 1.47 | 0.32 | 2.52 | 1.90 | 3.35 | 0.45 | 0.24 | 0.88 |
| Pathological gambling | 1.79 | 0.29 | 1.30 | 0.32 | 3.50 | 2.12 | 5.75 | 0.42 | 0.18 | 0.98 |
| Psychotic disorder | 2.14 | 0.36 | 3.09 | 0.47 | 1.47 | 1.01 | 2.14 | 0.64 | 0.35 | 1.17 |
| Any personality disorder | 33.26 | 1.11 | 36.53 | 1.32 | 1.04 | 0.96 | 1.14 | 0.92 | 0.78 | 1.08 |
| Avoidant | 4.63 | 0.51 | 8.47 | 0.81 | 0.69 | 0.56 | 0.85 | 0.87 | 0.60 | 1.27 |
| Dependent | 1.10 | 0.24 | 1.60 | 0.32 | 0.67 | 0.35 | 1.29 | 1.46 | 0.66 | 3.22 |
| Obsessive-compulsive | 14.38 | 0.76 | 18.83 | 1.04 | 0.95 | 0.85 | 1.06 | 0.81 | 0.66 | 1.00 |
| Paranoid | 9.70 | 0.71 | 15.50 | 1.02 | 0.75 | 0.65 | 0.88 | 0.91 | 0.68 | 1.21 |
| Schizoid | 6.86 | 0.58 | 7.89 | 0.71 | 1.04 | 0.89 | 1.22 | 0.93 | 0.70 | 1.25 |
| Histrionic | 5.52 | 0.50 | 7.51 | 0.73 | 0.94 | 0.74 | 1.20 | 0.85 | 0.61 | 1.21 |
| Antisocial | 16.58 | 0.85 | 9.78 | 0.84 | 2.98 | 2.46 | 3.61 | 0.73 | 0.53 | 1.01 |
Reference group.
The adjusted odds ratio (AORs) from the logistic regression model that controls for gender, AD, the interaction of AD and gender, and socioeconomic factors such as ethnicity, nativity, age, education, individual income, employment status, marital status, urbanicity, and region.
Except AD/alcohol abuse.
Any drug use disorder does not count nicotine dependence, whereas any substance use disorder does.
Bolded: p-value < 0.05.
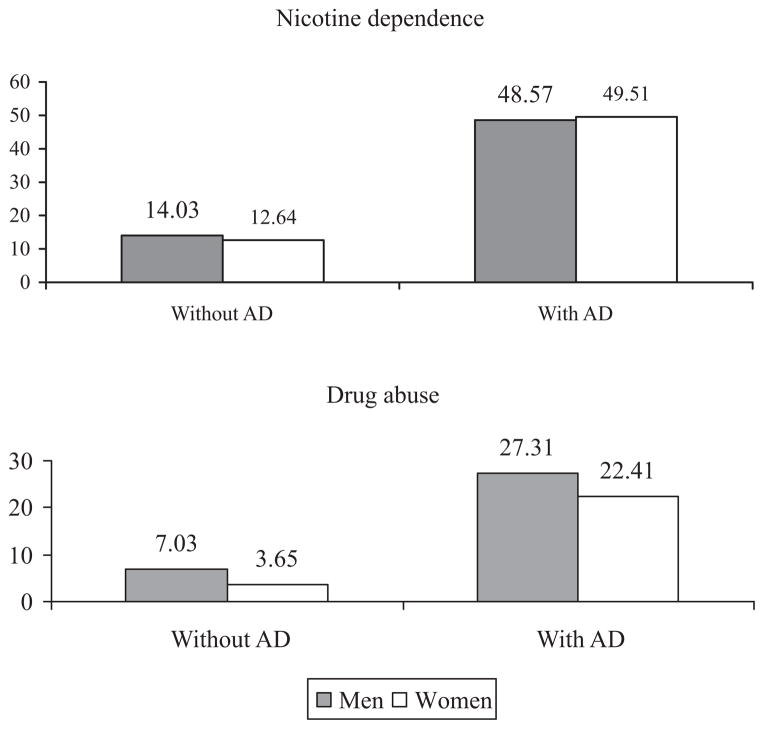
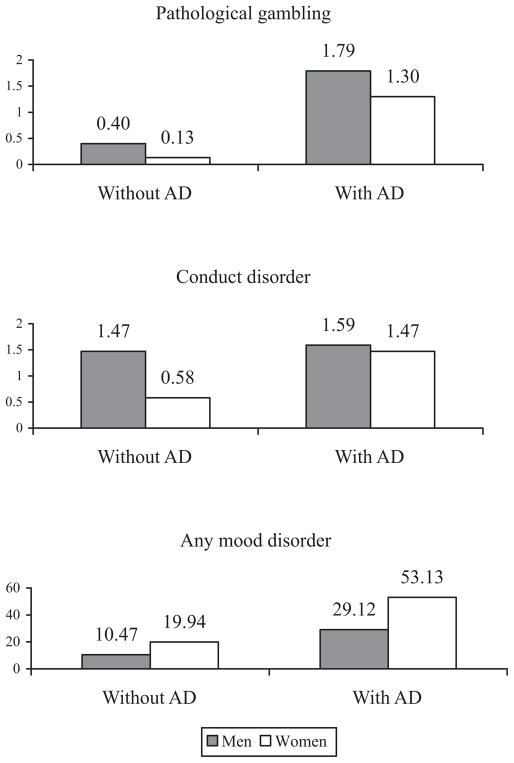
As indicated by the gender by AD interactions, after adjusting for the gender differences in sociodemographic characteristics and in the prevalence of psychiatric disorders in the general population, men with lifetime AD were less likely than women with lifetime AD to exhibit any Axis I disorder, any drug use disorder, drug abuse, nicotine dependence, any mood disorder, conduct disorder, and pathological gambling. For example, the interaction term indicated that whereas AD was associated with an almost 4-fold increase in the prevalence of drug abuse in men (from7.03 to 27.31%), it was associated with a 6-fold increase in women (from 3.65 to 22.41%; Fig. 1). The interaction term for nicotine dependence indicated that, although the prevalence of nicotine dependence was higher in men with AD than in those without AD (14.03 vs. 48.57%), the difference in prevalence was even larger among women (12.64 vs. 49.51%; Fig. 1). Similarly, although the prevalence of conduct disorder was slightly higher in men with AD than those without AD (1.47 vs. 1.59%), the difference in prevalence was even larger among women (0.58 vs. 1.47%; Fig. 2). Overall, the statistical significance of the interaction terms indicated that after adjusting for gender differences in sociodemographic characteristics and in the prevalence of psychiatric disorders in the general population, women with AD had a higher risk than men with AD for most externalizing disorders.
Fig. 1.
Lifetime prevalence of nicotine dependence and drug abuse in men and women with and without alcohol dependence.
Fig. 2.
Lifetime prevalence of pathological gambling, conduct disorder, and any mood disorder in men and women with and without alcohol dependence.
Clinical Course
Table 3 shows that men with AD were significantly younger at the age of first drink, had more episodes of the disorder, had longer episodes of the disorder, met significantly more criteria for the disorder, and were older at remission than women. The interval between the age at first drink and the age at heavy drinking was significantly longer in men. However, the age at onset of heavy drinking, age at onset of AD, time from age at first drink to age at onset of the AD, time from age at heavy drinking to onset of AD did not differ between men and women. Similarly, rates of remission among individuals with AD did not differ between genders.
Table 3.
The Clinical Course of Lifetime DSM-IV Alcohol Dependence (AD) Among Individuals by Gender
| Characteristic | Male n = 2,974 |
Femalea N = 1,807 |
F-test | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |||||
| Age at first drink, years | 16.57 | 16.42 | 16.72 | 17.40 | 17.14 | 17.66 | 33.60 | <0.0001 |
| Age of onset of heavy drinking, yearsb | 23.47 | 23.08 | 23.86 | 23.57 | 23.08 | 24.05 | 0.13 | 0.7245 |
| Time from age at first drink to age of heavy drinking, years | 6.94 | 6.57 | 7.32 | 6.33 | 5.91 | 6.74 | 5.77 | 0.0192 |
| Time from age of heavy drinking to age at onset of AD, years | 0.36 | 0.09 | 0.62 | 0.35 | 0.01 | 0.69 | <0.01 | 0.9803 |
| Time from age at first drink to age of onset of AD, years | 7.31 | 6.93 | 7.69 | 6.89 | 6.43 | 7.35 | 2.22 | 0.1411 |
| Age at onset of AD, years | 23.90 | 23.50 | 24.30 | 24.36 | 23.84 | 24.87 | 2.23 | 0.1402 |
| Age at remission of AD, yearsc | 31.82 | 31.06 | 32.58 | 29.59 | 28.76 | 30.41 | 17.04 | 0.0001 |
| Number of Episodes | 2.28 | 2.02 | 2.54 | 1.87 | 1.69 | 2.06 | 6.67 | 0.0121 |
| Total number of criteria | 4.78 | 4.72 | 4.84 | 4.63 | 4.55 | 4.72 | 8.42 | 0.0051 |
| Duration of longest episode, month | 42.13 | 38.79 | 45.46 | 29.69 | 27.22 | 32.15 | 38.34 | <0.0001 |
| % | 95% CI | % | 95% CI | OR | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| Remission | 42.77 | 40.47 | 45.11 | 42.78 | 40.00 | 45.60 | 1.00 | 0.87 | 1.15 |
Reference group.
Among heavy drinkers.
Among individuals who remitted.
Bolded: p-value < 0.05.
Risk Factors, Use of Medication to Relieve Mood or Anxiety Symptoms, and Medical Morbidities
Table 4 shows that men with AD were less likely than women to have a family history of alcohol use disorders, to have grown up in a vulnerable family environment, and to have a family history of depression. In addition, men with AD were less likely to have ever been married to a spouse with alcohol use disorders and were significantly less likely to use alcohol or drugs to relieve mood or anxiety symptoms when compared to women with AD. Men with AD were more likely to have liver disease or cirrhosis than women, but these associations were not significant after controlling for the total number of drinks consumed. Men with AD were more likely to have hypertension/angina than women with AD; this relationship persisted even after controlling for total number of drinks.
Table 4.
Prevalence of Risk Factors, Use of Medication to Relieve Mood or Anxiety Symptoms, and Medical Morbidities Among Individuals with Lifetime DSM-IV Alcohol Dependence by Gender
| Characteristic | Male n = 2,974 |
Femalea n = 1,807 |
OR | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | ||||||
| Risk factors | |||||||||
| Family history of alcohol use disorders | 50.47 | 47.99 | 52.94 | 60.21 | 57.34 | 63.01 | 0.67 | 0.57 | 0.79 |
| Ever married to a spouse with an alcohol use disorder | 13.34 | 11.94 | 14.88 | 38.80 | 35.95 | 41.72 | 0.24 | 0.20 | 0.29 |
| Vulnerable Family environment | 36.69 | 34.54 | 38.89 | 40.96 | 38.34 | 43.62 | 0.84 | 0.74 | 0.95 |
| Family History of Depression | 40.42 | 38.15 | 42.74 | 59.76 | 56.75 | 62.70 | 0.46 | 0.39 | 0.53 |
| Parental Loss before age 18 | 10.11 | 8.82 | 11.56 | 9.53 | 7.94 | 11.40 | 1.07 | 0.84 | 1.36 |
| Use of medication to relieve mood or anxiety symptoms | |||||||||
| Alcohol | 26.96 | 24.98 | 29.03 | 35.86 | 33.30 | 38.49 | 0.66 | 0.57 | 0.77 |
| Drugs | 10.75 | 9.44 | 12.22 | 13.41 | 11.70 | 15.32 | 0.78 | 0.64 | 0.95 |
| Medical morbidities | |||||||||
| Liver disease/Cirrhosis | 2.06 | 1.52 | 2.79 | 1.00 | 0.61 | 1.66 | 2.08 | 1.14 | 3.79 |
| Liver disease/Cirrhosisb | 2.06 | 1.52 | 2.79 | 1.00 | 0.61 | 1.66 | 1.66 | 0.90 | 3.05 |
| HTN OR angina | 25.01 | 23.17 | 26.95 | 19.71 | 17.51 | 22.12 | 1.36 | 1.15 | 1.61 |
| HTN OR anginab | 25.01 | 23.17 | 26.95 | 19.71 | 17.51 | 22.12 | 1.25 | 1.03 | 1.51 |
| Gastric ulcers | 4.24 | 3.44 | 5.21 | 5.16 | 4.05 | 6.57 | 0.81 | 0.59 | 1.12 |
Reference group.
Controlling for total number of drinks.
Bolded: p-value < 0.05.
Treatment-Utilization Behaviors
Table 5 shows that men with AD were significantly more likely than women to seek any type of treatment, which included any professional treatment and treatment by human service professionals. Regardless of gender, a lack of motivation was the most common reason for not seeking help. Men with AD were less likely than women with AD to endorse stigmatization as a reason for not seeking treatment. There were no significant gender differences in logistical or motivational barriers for AD. These relationships remained significant after adjusting for sociodemographic characteristics.
Table 5.
Rates of Treatment-Seeking and Reasons for Not Seeking Treatment Among Individuals with Lifetime DSM-IV Alcohol Dependence by Gender
| Characteristic | Male n = 2,974 |
Femalea n = 1,807 |
OR | 95% CI | AORb | 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | |||||||||
| Treatment type | ||||||||||||
| Any treatment | 26.30 | 24.48 | 28.20 | 19.87 | 17.81 | 22.09 | 1.44 | 1.22 | 1.70 | 1.64 | 1.36 | 1.97 |
| Professional treatment | 21.31 | 19.60 | 23.14 | 16.20 | 14.33 | 18.26 | 1.40 | 1.18 | 1.67 | 1.61 | 1.32 | 1.96 |
| Outpatient treatment | 15.12 | 13.60 | 16.78 | 12.61 | 10.89 | 14.57 | 1.23 | 1.01 | 1.51 | 1.42 | 1.14 | 1.78 |
| Inpatient treatment | 15.74 | 14.22 | 17.38 | 11.27 | 9.720 | 13.03 | 1.47 | 1.22 | 1.77 | 1.81 | 1.45 | 2.26 |
| Emergency room | 8.17 | 7.07 | 9.42 | 6.16 | 4.98 | 7.59 | 1.36 | 1.03 | 1.78 | 1.71 | 1.25 | 2.32 |
| Human service professional treatmentc | 23.97 | 22.23 | 25.79 | 17.45 | 15.54 | 19.54 | 1.49 | 1.26 | 1.76 | 1.74 | 1.44 | 2.09 |
| Reasons for not getting helpd | ||||||||||||
| Logisticale | 30.80 | 25.98 | 36.07 | 36.76 | 29.64 | 44.50 | 0.77 | 0.51 | 1.16 | 0.95 | 0.63 | 1.43 |
| Lack of motivation | 78.49 | 73.38 | 82.84 | 83.79 | 78.69 | 87.86 | 0.71 | 0.46 | 1.08 | 0.68 | 0.44 | 1.05 |
| Stigma | 28.92 | 24.74 | 33.50 | 42.25 | 35.01 | 49.83 | 0.56 | 0.39 | 0.79 | 0.61 | 0.43 | 0.88 |
| Decreased perceived need | 20.86 | 16.89 | 25.47 | 27.92 | 21.56 | 35.32 | 0.68 | 0.45 | 1.02 | 0.71 | 0.46 | 1.10 |
AOR, adjusted odds ratio.
Reference group.
Adjusted for ethnicity, nativity, age, education, individual income, employment status, marital status, urbanicity, region, and insurance.
Clergy member, employee assistance program, social services.
Among those who did not seek treatment.
Subject experienced financial/health insurance problems, afraid to lose job, no transport, did not speak English fluently.
Bolded: p-value < 0.05.
DISCUSSION
There were several gender differences in the psychiatric comorbidities, clinical characteristics, risk factors, and treatment- utilization patterns among a nationally representative sample of U.S. adults with lifetime AD. We highlight 4 major findings: (i) After adjusting for gender differences in sociodemographic characteristics and in the prevalence of psychiatric disorders in the general population, women with AD had a higher risk than men with AD for most externalizing disorders; (ii) Although men with AD started to drink at earlier ages than women, and women tended to remit earlier than men, the course and clinical presentation of AD was otherwise similar in men and women; (iii) Women endorsed more risk factors for AD than men; and (iv) Treatment-utilization rates were low, especially for women. Furthermore, stigmatization was more likely to be endorsed by women as a reason not to seek treatment.
Consistent with prior studies, women with AD had higher rates than men of mood, anxiety, and most personality disorders (Kessler et al., 1997), whereas men with AD were more likely to have comorbid substance use disorders and ASPD, reflecting broad gender patterns in the general population (Kessler et al., 1994). The interaction terms in the logistic regression analysis showed that women with AD did not have a higher risk than men with AD for any specific internalizing disorder. However, the interaction terms in the logistic regression analyses did yield the novel finding that women with AD had a higher risk than men with AD for most externalizing disorders. The mechanisms underlying this counter-intuitive result are unknown. One possibility, however, may involve the fact women with AD are more likely than men to have a family history of alcohol use disorders, as found in this study and others (Dawson and Grant, 1998). Individuals with a family history of alcohol use disorders are at greater risk of manifesting externalizing disorders (Hill et al., 2008). Conduct disorder in particular has been consistently associated with AD (Heath et al., 1997; Slutske et al., 1998) and may more strongly influence the risk of AD in women than in men (Heath et al., 1997; Slutske et al., 1998). Shared genetic factors account for much of the association between conduct disorder and adult AD, and there is evidence that these genetic factors may influence the heritability of these disorders more strongly in women (Slutske et al., 1998). Individuals with conduct disorder may be more likely to exhibit other externalizing disorders because of a genetic vulnerability to substance use disorders (Slutske et al., 1998), and because of a tendency to participate in environments that promote substance use.
Contrasting with results from clinical studies (Randall et al., 1999), but consistent with recent studies using community samples, we generally found few gender differences in the course of AD in men and women (Keyes et al., 2010). One exception was the interval between the age at first drink and the age at heavy drinking, which occurred at a slightly shorter period in women. This may represent a period of gender- specific vulnerability. In spite of this, there appeared to be few other gender differences in the clinical course of AD. In the past, there was evidence that women had an accelerated clinical course of AD, but these results were derived from treatment samples (Randall et al., 1999). Telescoping may be demonstrated in treatment samples because they are more likely to feature individuals with the most severe manifestations of AD. In contrast, the NESARC sample is not restricted to the most severe cases of AD, but rather is representative of the population of individuals with AD as a whole. The differences in severity of AD between treatment samples and the NESARC sample may be why the telescoping findings do not hold true when examined by a community sample such as the NESARC. In addition, the lack of gender differences in the course of AD may be due to the documented increases in the rates of both alcohol use and AD in community samples (Alvanzo et al., 2011), especially among women (Grucza et al., 2008a, b; Keyes et al., 2008).
In accord with previous reports, men tended to have their first drink at an earlier age than women (Johnston et al., 1996). Greater levels of impulsivity and sensation seeking among men (Petry et al., 2002) and social norms (Nolen-Hoeksema, 2004) may influence men to continue to drink at earlier ages. Furthermore, although there were no gender differences in the percentage of individuals that remitted from AD, women remitted at slightly younger ages than men. Women may remit earlier because heavy alcohol use might prompt them to seek treatment for the psychosocial consequences of their heavy use, such as marital problems or emotional problems (Duckert, 1987). Our data also suggest that women exhibit less severe forms of AD than men, which may also facilitate remission.
Women endorsed more risk factors for AD than men. Although a large percentage of men with AD reported a parental history of alcohol use disorders and vulnerable family environments, these risk factors were more common among women with AD. In addition to endorsing a family history of alcohol use disorders, women were substantially more likely to be married to a spouse with an alcohol use disorder, in line with data from previous studies (Roberts and Leonard, 1997). In women, family and marital problems strongly affect the risk of both developing and relapsing from AD (Connors et al., 1998). Addressing co-occurring interpersonal problems may be an especially important treatment need of women with AD. Coping skills training and 12-step facilitation may specifically benefit women married to individuals with an alcohol use disorder by decreasing the overall rates of their spouse’s drinking (Rychtarik and McGillicuddy, 2005). Last, although recent studies have indicated that the same amount of average alcohol consumption is related to a higher risk of liver cirrhosis in women than men (Rehm et al., 2010), we found that men and women with AD had no significant difference in rates of liver disease/cirrhosis after controlling for the total number of drinks.
Treatment-utilization rates for AD were low regardless of gender, but were especially low for women, consistent with prior studies (Chartier and Caetano, 2011; Grant, 1997). There were both general and gender-specific barriers to treatment. General barriers tended to be logistical or due to a lack of motivation. Logistical barriers in accessing treatment have been documented in previous studies and include difficulties getting time off work, distance to treatment, and financial barriers to treatment (Grant, 1997). Supplemental monitoring and counseling via telephone contact may help decrease those logistical barriers, although recent studies examining the efficacy of such telephone-based follow-ups have had mixed results on alcohol use outcomes and abstinence (Hubbard et al., 2007; McKay et al., 2011).
Social stigmatization was a gender-specific barrier to treatment- seeking for AD and had a stronger effect among women. Our findings are in accord with results from other studies, which noted that women endorsed an increased fear of stigmatization (Beckman and Amaro, 1986) as a common obstacle to treatment. Although some gender-sensitive treatment programs have been developed in response to the characteristics, needs, and clinical course of AD (especially among women), results from these types of interventions have been mixed (Greenfield et al., 2007). Programs that simply changed treatment from mixed gender to women-only (but kept all other treatment-related variables the same) had little effect on changing substance use outcomes (Greenfield et al., 2007). On the other hand, programs were most effective when they responded to the special needs of substance abusing women, such as women who had children (Luthar and Suchman, 2000).
Given the high prevalence of mood and anxiety disorders among individuals with AD, targeting both disorders may also be a key component in meeting the treatment needs of this population. There is evidence that sertraline plus naltrexone (Pettinati et al., 2010) may be effective in reducing depressive symptoms and alcohol consumption among depressed individuals with AD. In addition, there is evidence that cognitive-behavioral therapy is useful in treating the combination of depression and AD (Brown et al., 1997).
Our study has limitations common to most large-scale surveys. First, information on alcohol consumption, medical conditions, and other clinical characteristics was based on self-report and not confirmed by collateral informants. As a result, recall bias is a potential limitation. Second, general population surveys like the NESARC may fail to capture some individuals with AD because individuals with substance use disorders are less likely to live in households, the sampling frame of most general population surveys (Grant et al., 2003a). The NESARC, however, also sampled from shelters and group homes; this strategy increases the representation of individuals with AD within the sample and makes the underrepresentation of this population less likely. Because of this sampling strategy, NESARC estimates of prevalence, risk, comorbidity, and treatment-utilization behaviors are more likely to be representative of individuals with AD. Third, although the NESARC provides the most extensive assessment of psychiatric disorders among men and women with AD, some disorders such as obsessive-compulsive disorder were not assessed in this study. Fourth, the cross-sectional design of our study precludes examination of the causal relationship between AD and other measures included in our study. Fifth, our assessment of motivation was limited, as it was assessed by a single question that asked whether the respondent “wanted to keep using alcohol.” Sixth, it was challenging to assess gender differences in the physical comorbidities of individuals with AD, because our data were based on self-report and not confirmed with medical data and diagnoses. In addition, this study provided a brief and limited assessment of the gender differences in medical comorbidities among individuals with AD.
Despite these limitations, the NESARC constitutes the largest nationally representative survey to date to include information on psychiatric disorders, clinical course, risk factors, and treatment-utilization patterns among individuals with AD. Overall, these findings suggest that there may be gender-specific pathways that distinctly influence the development, course, and treatment for AD. It appears that after adjusting for population characteristics, women with AD had a higher risk for externalizing disorders than men, have more risk factors for AD, and are less likely to seek treatment for AD. Developing strategies to reduce the effects of stigmatization and a lack of motivation are important steps in improving the access to treatment for AD.
Acknowledgments
Work on this manuscript was supported by NIH grants DA019606-S3 and DA020783-S4 (SK), DA019606, DA020783, DA023200, DA023973, MH076051, MH082773, and CA133050 (CB) and AA014223 and AA018111 (DSH), a grant from the American Foundation for Suicide Prevention (CB), the New York State Psychiatric Institute (CB, DSH), and by Spanish Ministry of Education grant PR2010- 0501 (RS-V).
References
- Alvanzo AA, Storr CL, La Flair L, Green KM, Wagner FA, Crum RM. Race/ethnicity and sex differences in progression from drinking initiation to the development of alcohol dependence. Drug Alcohol Depend. 2011;118:375–382. doi: 10.1016/j.drugalcdep.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman LJ, Amaro H. Personal and social difficulties faced by women and men entering alcoholism treatment. J Stud Alcohol. 1986;47:135–145. doi: 10.15288/jsa.1986.47.135. [DOI] [PubMed] [Google Scholar]
- Brown RA, Evans DM, Miller IW, Burgess ES, Mueller TI. Cognitive- behavioral treatment for depression in alcoholism. J Consult Clin Psychol. 1997;65:715–726. doi: 10.1037//0022-006x.65.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier KG, Caetano R. Trends in alcohol services utilization from 1991–1992 to 2001–2002: ethnic group differences in the U.S. population. Alcohol Clin Exp Res. 2011;35:1485–1497. doi: 10.1111/j.1530-0277.2011.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Yi H, Williams GD, Faden VB. Surveillance Report #86—Trends in Underage Drinking in the United States, 1991–2007. National Institutes of Health; Bethesda, MD: 2009. [Google Scholar]
- Connors GJ, Maisto SA, Zywiak WH. Male and female alcoholics’ attributions regarding the onset and termination of relapses and the maintenance of abstinence. J Subst Abuse. 1998;10:27–42. doi: 10.1016/s0899-3289(99)80138-2. [DOI] [PubMed] [Google Scholar]
- Copeland J. A qualitative study of barriers to formal treatment among women who self-managed change in addictive behaviours. J Subst Abuse Treat. 1997;14:183–190. doi: 10.1016/s0740-5472(96)00108-0. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Moss HB, Li TK, Grant BF. Gender differences in the relationship of internalizing and externalizing psychopathology to alcohol dependence: likelihood, expression and course. Drug Alcohol Depend. 2010;112:9–17. doi: 10.1016/j.drugalcdep.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Grant BF. Family history of alcoholism and gender: their combined effects on DSM-IV alcohol dependence and major depression. J Stud Alcohol. 1998;59:97–106. doi: 10.15288/jsa.1998.59.97. [DOI] [PubMed] [Google Scholar]
- Duckert F. Recruitment into treatment and effects of treatment for female problem drinkers. Addict Behav. 1987;12:137–150. doi: 10.1016/0306-4603(87)90020-7. [DOI] [PubMed] [Google Scholar]
- Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol. 1997;58:365–371. doi: 10.15288/jsa.1997.58.365. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson D, Hasin D. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-DSM-IV Version (AUDADIS-IV) National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2001. [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004a;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV(AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003b;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Moore T, Shepard J, Kaplan K. Series Source and Accuracy Statement: Wave 1 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2003a. Source and Accuracy Statement: Wave 1 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004b;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, Lincoln M, Hien D, Miele GM. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend. 2007;86:1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Bucholz KK, Rice JP, Bierut LJ. Secular trends in the lifetime prevalence of alcohol dependence in the United States: a re-evaluation. Alcohol Clin Exp Res. 2008a;32:763–770. doi: 10.1111/j.1530-0277.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Norberg K, Bucholz KK, Bierut LJ. Correspondence between secular changes in alcohol dependence and age of drinking onset among women in the United States. Alcohol Clin Exp Res. 2008b;32:1493–1501. doi: 10.1111/j.1530-0277.2008.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Carpenter KM, McCloud S, Smith M, Grant BF. The alcohol use disorder and associated disabilities interview schedule (AUDA-DIS): reliability of alcohol and drug modules in a clinical sample. Drug Alcohol Depend. 1997;44:133–141. doi: 10.1016/s0376-8716(97)01332-x. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Schuckit MA, Martin CS, Grant BF, Bucholz KK, Helzer JE. The validity of DSM-IV alcohol dependence: what do we know and what do we need to know? Alcohol Clin Exp Res. 2003;27:244–252. doi: 10.1097/01.ALC.0000060878.61384.ED. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke-Wellman J, Matthews AG, McDermott M. Psychopathology in offspring from multiplex alcohol dependence families with and without parental alcohol dependence: a prospective study during childhood and adolescence. Psychiatry Res. 2008;160:155–166. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RL, Leimberger JD, Haynes L, Patkar AA, Holter J, Liepman MR, Lucas K, Tyson B, Day T, Thorpe EA, Faulkner B, Hasson A. Telephone enhancement of long-term engagement (TELE) in continuing care for substance abuse treatment: a NIDA clinical trials network (CTN) study. Am J Addict. 2007;16:495–502. doi: 10.1080/10550490701641678. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. National Survey Results on Drug Use from the Monitoring the Future Study, 1975–1995. National Institute on Drug Abuse; Rockville, MD: 1996. [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008;93:21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Li G, Hasin DS. Birth cohort effects and gender differences in alcohol epidemiology: a review and synthesis. Alcohol Clin Exp Res. 2011;35:2101–2112. doi: 10.1111/j.1530-0277.2011.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Martins SS, Blanco C, Hasin DS. Telescoping and gender differences in alcohol dependence: new evidence from two national surveys. Am J Psychiatry. 2010;167:969–976. doi: 10.1176/appi.ajp.2009.09081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar SS, Suchman NE. Relational Psychotherapy Mothers’ Group: a developmentally informed intervention for at-risk mothers. Dev Psychopathol. 2000;12:235–253. doi: 10.1017/s0954579400002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Van Horn D, Oslin DW, Ivey M, Drapkin ML, Coviello DM, Yu Q, Lynch KG. Extended telephone-based continuing care for alcohol dependence: 24-month outcomes and subgroup analyses. Addiction. 2011;106:1760–1769. doi: 10.1111/j.1360-0443.2011.03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CB, Rehm J, Ustun TB, Grant B, Chatterji S. Factor structures for DSM-IV substance disorder criteria endorsed by alcohol, cannabis, cocaine and opiate users: results from the WHO reliability and validity study. Addiction. 1999;94:843–855. doi: 10.1046/j.1360-0443.1999.9468438.x. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Petry N, Kirby K, Kranzler H. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol. 2002;63:83–90. [PubMed] [Google Scholar]
- Pettinati HM, Oslin DW, Kampman KM, Dundon WD, Xie H, Gallis TL, Dackis CA, O’Brien CP. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatry. 2010;167:668–675. doi: 10.1176/appi.ajp.2009.08060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60:252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J, Roerecke M. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 2010;29:437–445. doi: 10.1111/j.1465-3362.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, Leonard KE. Gender Differences and Similarities in the Alcohol and Marriage Relationship. Rutgers Center of Alcohol Studies; Piscataway, NJ: 1997. [Google Scholar]
- Ruan WJ, Goldstein RB, Chou SP, Smith SM, Saha TD, Pickering RP, Dawson DA, Huang B, Stinson FS, Grant BF. The alcohol use disorder and associated disabilities interview schedule-IV (AUDADISIV): reliability of new psychiatric diagnostic modules and risk factors in a general population sample. Drug Alcohol Depend. 2008;92:27–36. doi: 10.1016/j.drugalcdep.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD. Alcohol cue reactivity and mood induction in male and female alcoholics. J Stud Alcohol. 1994;55:487–494. doi: 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- Rychtarik RG, McGillicuddy NB. Coping skills training and 12-step facilitation for women whose partner has alcoholism: effects on depression, the partner’s drinking, and partner physical violence. J Consult Clin Psychol. 2005;73:249–261. doi: 10.1037/0022-006X.73.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol. 1998;107:363– 374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- Tuyns AJ, Pequignot G. Greater risk of ascitic cirrhosis in females in relation to alcohol consumption. Int J Epidemiol. 1984;13:53–57. doi: 10.1093/ije/13.1.53. [DOI] [PubMed] [Google Scholar]
- Wells JE, Horwood LJ, Fergusson DM. Reasons why young adults do or do not seek help for alcohol problems. Aust N Z J Psychiatry. 2007;41:1005–1012. doi: 10.1080/00048670701691218. [DOI] [PubMed] [Google Scholar]
- Zimmerman M. Diagnosing personality disorders. A review of issues and research methods. Arch Gen Psychiatry. 1994;51:225–245. doi: 10.1001/archpsyc.1994.03950030061006. [DOI] [PubMed] [Google Scholar]