Summary
Porous metal has been introduced to obtain biological fixation and improve longevity of orthopedic implants. The new generation of porous metal has intriguing characteristics that allows bone healing and high osteointegration of the metallic implants. This article gives an overview about biomaterials properties of the contemporary class of highly porous metals and about the clinical use in orthopaedic surgery.
Keywords: porous metals, metallic scaffold, orthopedics implants
Introduction
In the last two decades, a variety of porous metallic materials have been used to support biological fixation of implants. For the long term success of cementless implants is crucial bone ingrowth around and with the porous surfaces. Over time, the porous biomaterials field continue to increase as first-generation ingrowth surfaces have given way to developed a new generation of metallic foams with the potential to expand cementless technology in all facets of orthopaedic surgery (1). This article gives an overview about biomaterials properties of the contemporary class of highly porous metals and about the current propositions in orthopaedic surgery.
Ideal porous metal
The main characteristics for an ideal porous metal are:
Biocompatibility: it should support normal cellular activity without any local and systemic toxic effects to the host tissue; it must be osteoconductive and osteoinductive and be able to induce blood vessels formation within or around the implant. Furthermore it should be non-immunogenic (2).
Mechanical properties: it should offer mechanical properties similar to the host bone with a sufficient mechanical strength. Bone responds to the absence and presence of physical load. In response to these loads, the body either resorbs or forms bone (3). Given this principle, it is important to design a matrix that possesses mechanical properties that are similar to the tissue in the immediate surrounding area of the defect (4). An over engineered matrix may results in bone resorption around the implant site, while an under engineered matrix may fail as a mechanical support to the skeleton.
Pore size: scaffolds should have macro- (pore size >100 mm) and micro-porosity (pore size < 20 mm) and pores must be interconnected. Multi-scale porous scaffolds involving both micro and macro porosities can perform better than only one dimensional porosity scaffold (5). Unfortunately, porosity reduces mechanical properties such as compressive strength and resistance to corrosion (6).
Physical properties: initial strength for safe handling during sterilizing, packaging, transportation to surgery, as well as survival through physical forces in vivo and sterile environment for cell seeding.
The material should be reproducibly processable into three-dimensional structure and it must tolerate sterilisation according to the required international standards for clinical use. In addition, the ideal porous metal would be able to stand alone as an independent structure, rather than solely as a porous coating.
Moreover, manufacturing costs of these materials should ideally be reasonable and implantation also should be relatively simple, precise, and reproducible (1).
Currently, no “perfect” coating material exists that satisfies all these criteria.
Limitation of metallic scaffolds
Compared to other biomaterials like ceramics and polymers, the metallic biomaterials offer a wider range of mechanical properties such as high strength, ductility, fracture toughness, hardness, formability, as well as corrosion resistance, and bio-compatibility. These are the required properties for most load-bearing applications in joint arthroplasty and bone replacement (7).
However there are many disadvantages of metallic biomaterials as bone scaffold:
The main disadvantage of metallic biomaterials is their lack of biological recognition on the material surface. To overcome this restraint, surface coating or surface modification presents a way to preserve the mechanical properties of established biocompatible metals improving the surface biocompatibility. Moreover, in order to enhance communication between cells, facilitating their organization within the porous scaffold; it is desired to integrate cell-recognizable ligands and signaling growth factors on the surface of the scaffolds. Indeed, biofactors that influence cell proliferation, differentiation, migration, morphologies and gene expression can be incorporated in the scaffold design and fabrication to enhance cell growth rate and direct cell functions.
Another limitation of the current metallic biomaterials is the possible release of toxic metallic ions and/or particles through corrosion or wear possible that lead to inflammatory cascades and allergic reactions, which reduce the bio-compatibility and cause tissue loss. A proper treatment of the material surface may help to avoid this problem and create a direct bonding with the tissue.
The magnitude of elastic modulus for bulk metallic implant materials surpasses that of cortical bone by far and results in a failed stress transmission from biomaterial to bone, the so-called stress-shielding effect. The stress shielding may lead to bone resorption or even fretting, due to micro motions occurring at the bone/implant interface. The ideal porous metal coating would have an open-cell structure, high porosity, and microstructure resembling that of cancellous bone. Additionally, it would possess a similar modulus of elasticity and high frictional characteristics. Thus, it would be more biologically compatible and result in earlier and increased levels of bone ingrowth into the implant (7).
Porous metals
A major classification of porous metals, or metal foams, is between open-cell and closed-cell. In closed-cell foams each cell is completely enclosed by a thin wall or membrane of metal, whilst in open-cell foams the individual cells are interconnected, allowing tissue to infiltrate the foam and anchor it into position (8).
It is recognised that there are three distinct types of porous implants:
partly or fully porous-coated solid substrates
fully porous materials
porous metal segment joined to a solid metallic part (8).
These porous materials are best suited for use as coatings since they do not readily have the required mechanical properties that would allow them to be used as bulk structural materials for implants, bone augmentation, or substitutes for bone graft (9). Moreover, these porous coated Ti alloy implants show 50 to 75% lower fatigue strength compared to their equivalent fully dense materials, which arises due to highly stress concentrated regions at particle substrate neck regions acting as crack initiation sites. Other perceived limitations of these porous metals and processing routes include: the desire for controlled porosity characteristics, relatively high modulus of coatings, difficult to make stand-alone structures and limited part geometries and sizes (9). Some of these limitations have been addressed by developing a manufacturing process that involve creating a reticulated skeleton with deposition of a metal onto the surface.
To date there are several biocompatible metallic materials that are frequently used as implanting materials in orthopaedic surgery. In general, compared to cobalt-chromium alloys (Co-Cr) and stainless steels, titanium, some of its alloys and tantalum are the more suitable porous metallic materials used for orthopaedic applications.
Stainless steel
Stainless steels were the first reliable metals used as prosthesis in orthopaedics. The basic elements in steels are iron and carbon and may usually contain chromium, nickel, and molybdenum as additional elements. The most common stainless steel in orthopaedics is designated 316L. Compared to other metallic implants, stainless steels exhibit lower strength and much higher corrosion resistance, but possess greater ductility and lower production. Their high stiffness makes them inferior to Ti in bone replacement applications (7).
Cobalt-chromium alloys
In the early 1970s, Welsh et al. focused on porous cobalt-chromium surfaces due to its attractive properties of inertness/biocompatibility and mechanical durability (1). The CoCr beads producted from spherical cobalt chrome metal are sintered to the implant substrate to create a macro and micro porous surface for bone ingrowth. The CoCr beaded porous coatings are applied in acetabular cups, femoral stems and total knee arthroplasty components.
In a study of 72 hips with a CoCr beaded acetabular cup the incidence of aseptic loosening was found to be 4% at an average of 8.5 years follow up. CoCr beaded femoral stems have garnered better clinical success with regards to survival and fixation. Sakalkale et al. reported 95% stable ingrowth of CoCr beaded stems at an average of 11.4 years.
However ingrowth analysis shows minimal ingrowth and fibrous tissue formation in Co-Cr bead coated implants and these findings may be of concern as this may hinder the success of long-term fixation (1).
Titanium and its alloys
Ti alloys were first used in orthopaedics in the mid-1940s and have continued to gain attention because of their unique properties, including high specific strength, low weight, excellent corrosion resistance and biocompatibility. However, for bone replacement components, the strength of pure Ti is not sufficient and Ti alloys are preferred due to their superior mechanical properties (7).
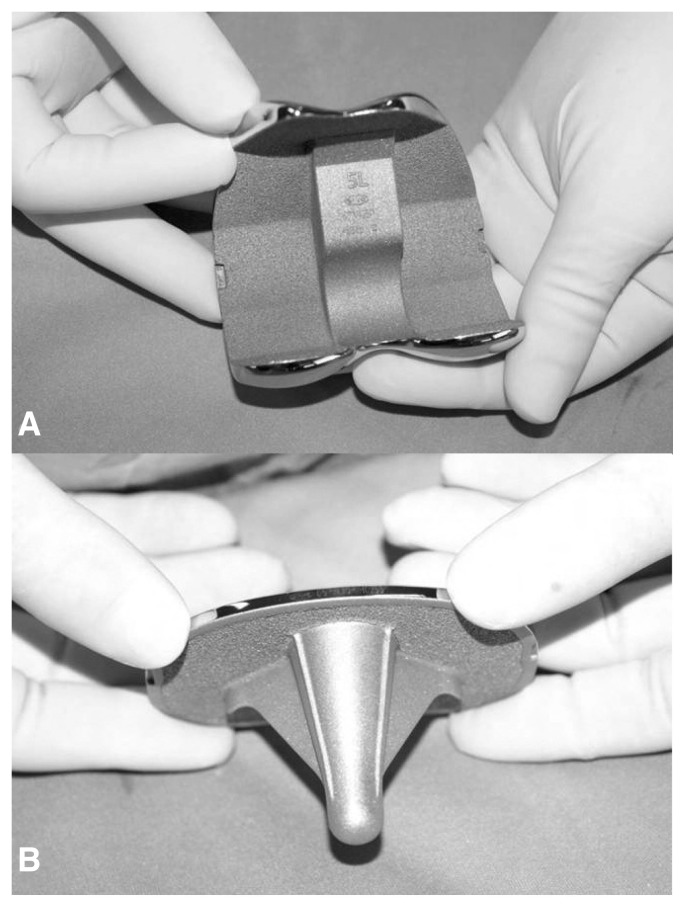
Titanium may be used for sintered beads and fiber metal mesh coatings. Plasma spray titanium coatings for total hip and knee arthroplasty have proven to be a safe, predictable material in long-term follow-up studies (1) (Figure 1). However coating possesses a low porosity ranging from 30–50% which limits the maximum interfacial strength that can form via bone ingrowth. Autopsy retrieval studies have demonstrated an average ingrowth of 15–30%. These traditional materials do not have the characteristics that would permit their use as bulk structural materials for bone implants or as bone graft substitutes. Similarly, these traditional implants require a significant proportion of host bone-implant contact for a successful outcome.
Figure 1.
Porous Plasma Spray (PPS) titanium coating in femural (A) and tibial component (B) for total knee replacement.
The metallic foam has an overall porosity ranging from 60 to 80% with pore sizes from 100–600 microns and may use in bulk form as well as a coating. These new porous metals show characteristics resembling those of cancellous bone with high surface friction properties, improved porosity levels, and relatively low modulus of elasticity; thus, potentially, providing a surface that will result in a long-lasting bond and substantial levels of bone ingrowth.
Titanium based foam are the extensively materials used to fabricate porous metal implant in primary and revision total hip, knee and shoulder arthroplasty (Figure 2). However this new materials have minimal peer-reviewed literature available. While the biomaterial properties of this foam are intriguing, it is important to maintain a tempered enthusiasm as we await intermediate and long-term follow-up with these implants.
Figure 2.
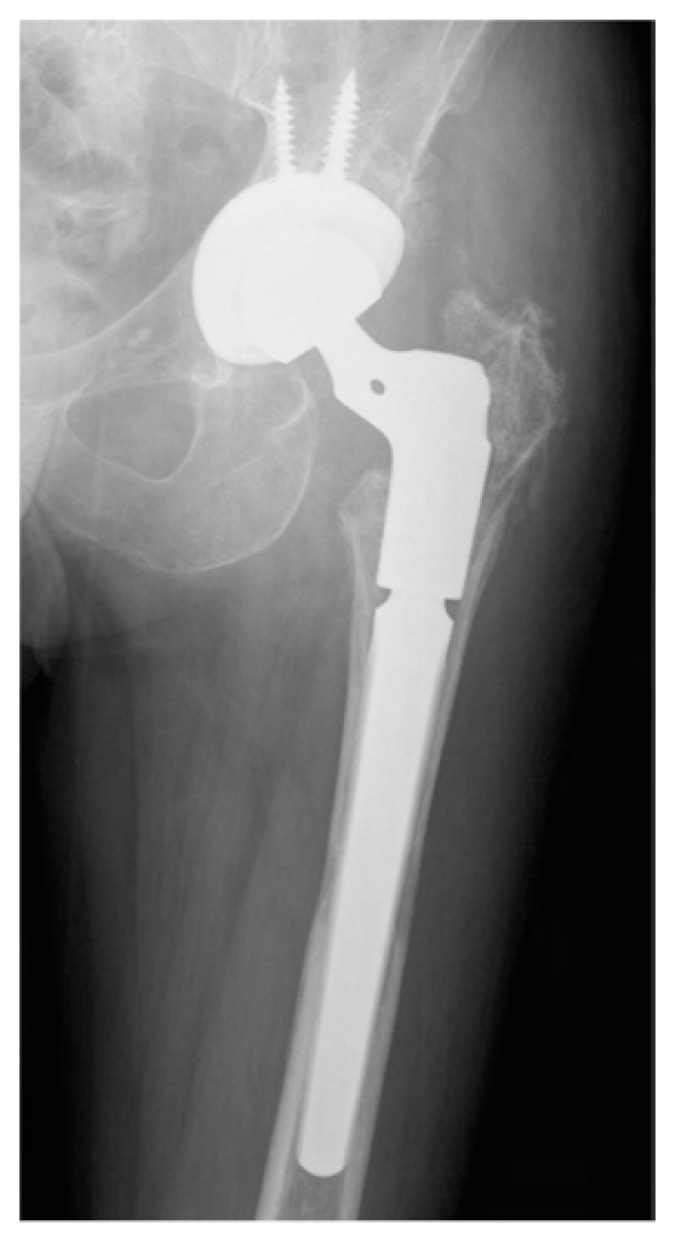
Titanium foam coating uncemented stem for revision hip arthroplasty. The X ray shows osteointegration of the implant to host bone.
Tantalum
Among metallic biomaterials, tantalum is gaining more attention as a new biomaterial. Extensive research has been performed on the mechanical properties of porous tantalum demonstrating that this material, despite a low modulus of elasticity and highly porous structure, can withstand physiologic load and support bone ingrowth under this stress. There is a theoretical benefit of decreased stress shielding, the potential for immediate postoperative weight bearing and a more normal pattern of bone remodeling adjacent to the component. In addition, tantalum is more resistant to corrosion than titanium but it presents high costs of production.
In vitro study showed excellent cellular adherence, growth and differentiation with abundant extracellular matrix formation on porous Ta structures compared to porous Ti controls. Balla et al. recently developed in a vitro biocompatibility study, using a human fetal osteoblast cell line (hFOB), on laser-deposited Ta coatings on Ti. Ta coating surface showed six times higher living cell density, excellent cellular adherence and growth with abundant extracellular matrix formation compared to the Ti surface (10). There have been a multitude of bone ingrowth studies completed on porous tantalum implants. Bobyn et al. revealed that at 1 year retrieval, new bone occupied up to 80% of the pores in acetabular components and haversian remodeling was demonstrated histologically (11).
Current clinical applications include acetabular components, femoral stems, tibia components, patellar components, spine implants, and humeral stems in total shoulder arthroplasty. Further, they are used in such stand-alone structures as acetabular augments, patellar augments, and osteonecrosis implants (Figures 3–5) (12–14).
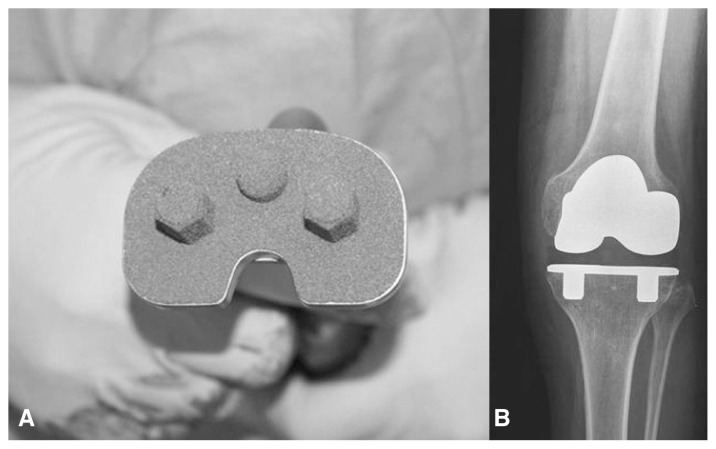
Figure 3.
(A) Tantalum trabecular metal tibial baseplate for uncemented total knee replacement. (B) X ray shows implant osteointegration at 1 year of follow-up.
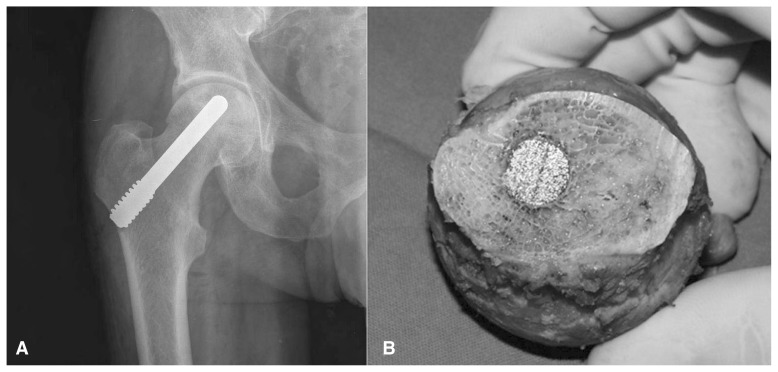
Figure 5.
(A) Tantalum rods implanted for avascular necrosis of the hip to sustain the subcondral bone and avoid collapse of the femoral head. (B) Osteointegration of the rod into the bone in one case failed treated with total hip replacement.
Several clinical studies reported that tantalum implants or augmentations can provide a good substrate for attachment, formation and ingrowth of bone tissue in vivo even under difficult conditions. Nevertheless, some fundamental conditions must be met such the largest possible interface between the tantalum implant and the host bone.
Conclusion
The development of porous metals and coatings for osseointegration has revolutionized the field of orthopaedics, particularly total joint reconstruction. However, classic implants are fabricated using traditional materials (i.e. sintered beads, fiber metal, plasma spray) which have several inherent biomaterial limitations.
Several new highly porous metals have been recently introduced to improve the biomaterial properties of these traditional metals, namely porosity, surface coefficient, and modulus of elasticity. These new biomaterials all share a microscopic characteristic appearance similar to cancellous bone. The open-cell structure of these materials affords several intriguing properties, including high volumetric porosity (60–80%) and low modulus of elasticity.
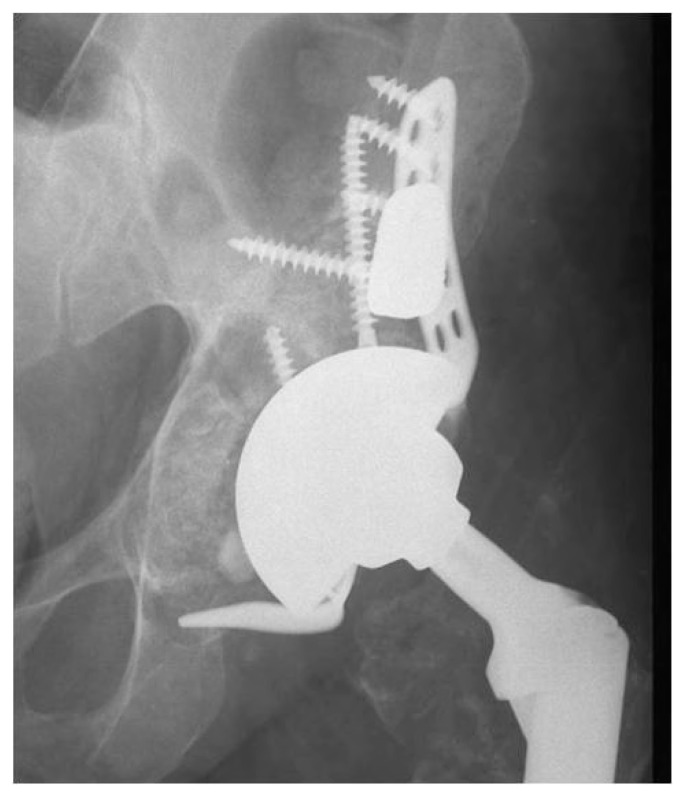
Figure 4.
Trabecular metal acetabular revision system with cage and augments employed to fill bone defects in complex revision case.
References
- 1.Fabi DW, Levine BR. Porous Coatings on Metallic Implant Materials. ASM Handbook, Volume 23: Materials for Medical Devices. 2012:307–319. [Google Scholar]
- 2.Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012 Oct;30(10):546–54. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matassi F, Nistri L, Chicon Paez D, Innocenti M. New biomaterials for bone regeneration. Clin Cases Miner Bone Metab. 2011 Jan;8(1):21–4. [PMC free article] [PubMed] [Google Scholar]
- 4.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg Am. 2008 Feb;90(Suppl 1):36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 5.Woodard JR, Hilldore AJ, Lan SK, Park CJ, Morgan AW, Eurell JA, Clark SG, Wheeler MB, Jamison RD, Wagoner Johnson AJ. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials. 2007 Jan;28(1):45–54. doi: 10.1016/j.biomaterials.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Dabrowski B, Swieszkowski W, Godlinski D, Kurzydlowski KJ. Highly porous titanium scaffolds for orthopaedic applications. J Biomed Mater Res B Appl Biomater. 2010 Oct;95(1):53–61. doi: 10.1002/jbm.b.31682. [DOI] [PubMed] [Google Scholar]
- 7.Nouri Alireza, Peter D. Hodgson, Cui’e Wen Biomimetic Porous Titanium Scaffolds for Orthopedic and Dental Applications. Biomimetics Learning from Nature. 2010;21:415–450. [Google Scholar]
- 8.Ryan G, Pandit A, Apatsidis DP. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials. 2006 May;27(13):2651–70. doi: 10.1016/j.biomaterials.2005.12.002. Epub 2006 Jan 19. [DOI] [PubMed] [Google Scholar]
- 9.Balla VK, Bodhak S, Bose S, Bandyopadhyay A. Porous tantalum structures for bone implants: fabrication, mechanical and in vitrobiological properties. Acta Biomater. 2010 Aug;6(8):3349–59. doi: 10.1016/j.actbio.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balla, Bodhak Subhadip, Bose Susmita, Amit Bandyopadhyay Balla VK, Banerjee S, Bose S, Bandyopadhyay A. Direct laser processing of tantalum coating on titanium for bone replacement structures. Acta Biomater. 2010;6:2329–34. doi: 10.1016/j.actbio.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobyn JD, Toh KK, Hacking SA, Tanzer M, Krygier JJ. Tissue response to porous tantalum acetabular cups: a canine model. J Arthroplasty. 1999 Apr;14(3):347–54. doi: 10.1016/s0883-5403(99)90062-1. [DOI] [PubMed] [Google Scholar]
- 12.Civinini R, Carulli C, Matassi F, Nistri L, Innocenti M. A dual-mobility cup reduces risk of dislocation in isolated acetabular revisions. Clin Orthop Related Res. 2012 Dec;470(12):3542–8. doi: 10.1007/s11999-012-2428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Civinini R, De Biase P, Carulli C, Matassi F, Nistri L, Capanna R, Innocenti M. The use of an injectable calcium sulphate/calcium phosphate bioceramic in the treatment of osteonecrosis of the femoral head. Int Orthop. 2012 Aug;36(8):1583–8. doi: 10.1007/s00264-012-1525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carulli C, Matassi F, Civinini R, Innocenti M. Tissue engineering applications in the management of bone loss. Clin Cases Miner Bone Metab. 2013 Jan;10(1):22–5. doi: 10.11138/ccmbm/2013.10.1.022. [DOI] [PMC free article] [PubMed] [Google Scholar]