Summary
The morbidity and socioeconomic costs associated with bone healing are considerable. A number of fractures are complicated by impaired healing. This is prevalent in certain risk groups such as elderly, osteoporotics, post-menopausal women, and in people with malnutrition.
The biologic process of fracture healing is complex and impacted by multiple factors. Some of them, such as the nutritional and health conditions, are patient-dependent, while others depend on the trauma experienced and stability of the fracture. Fracture healing disorders negatively affect the patient’s quality of life and result in high health-care costs, as a second surgery is required to stabilize the fracture and stimulate bone biology. Future biotechnologies that accelerate fracture healing may be useful tools, which might also prevent the onset of these disorders.
We list the characteristics of the drugs used for osteoporosis, but we point out in particular the use of strontium ranelate and teriparatide in our clinical practice in elderly patients, especially females, who reported fractures with risk of nonunion.
This medical treatment could impaired fracture healing however, most of the evidence is obtained in animal studies and very few studies have been done in humans. Thus one could hypothesize the possibility of a medical treatment both as a preventive and as support to the synthesis. However, no clinical studies are available so far, and such studies are warranted before any conclusions can be drawn.
A positive effect of osteoporosis treatments on bone healing is an interesting possibility and merits further clinical research.
Keywords: fracture healing, strontium ranelate, teriparatide
Introduction
The bones fragility, typical of osteoporosis, is a main risk factor for the occurrence of first episodes of fractures and re-fractures (1–3). An accurate diagnosis of osteoporosis (4) and a proper treatment are today recognized to be the most important facts for prevention and for a correct arrangement and treatment of fragility fractures. In spite of the high incidence of osteoporosis, mainly due to the increase in life expectancy, especially in females, and to the high socio-economic impact of fragility fractures, we are currently often in front of cases of fractures, that repeat in the same patient who, to the detriment of the clinical picture, was never framed in terms of bone metabolism (5–9).
Osteoporosis drugs are prescribed to prevent this fragility fractures, which is the principal aim of the management of osteoporosis. However, if fracture does occur, then it is also important to promote a fast and uneventful healing process. Despite this, little is known about the effect of osteoporosis drugs on bone healing in humans (10).
The morbidity and socioeconomic costs associated with bone healing are considerable.
A number of fractures are complicated by impaired healing. This is prevalent in certain risk groups such as elderly, osteoporotics, postmenopausal women, and in people with malnutrition.
We define nonunion as the non consolidation at the fracture site within 6 months. In these cases only a change in treatment can lead to bone union. Sometimes there is no necessity to wait 6 months. This occurs when we have no progress in callus formation at fracture site at 4 weeks intervals follow up. On the other hand when there are indications of progress in callus formation, it is wise to wait more than 6 months (11).
Nonunion is permanent failure of healing process and we think that in most of cases a second intervention will necessary; it’s a severe complication for the patient which has a negative impact on his quality of life; undoubtedly, which is not exempt of risks and potential complications and increases healthcare costs.
Before coming to talk about non-union but we have a phase in which we note to delays in consolidation.
It is in these cases that we think need to give an additional stimulus to the repair process.
The biologic process of fracture healing is complex and impacted by multiple factors. Some of them, such as the nutritional and health conditions, are patient-dependent, while others depend on the trauma experienced and stability of the fracture. Fracture healing disorders negatively affect the patient’s quality of life and result in high healthcare costs, as a second surgery is required to stabilize the fracture and stimulate bone biology. Future biotechnologies that accelerate fracture healing may be useful tools, which might also prevent the onset of these disorders (12).
Fractures are a common occurrence and have a significant impact on the quality of life for patients and considerable economic consequences for our society. Healing occurs via rapid bone regeneration followed by osteoclast-mediated remodeling resulting in new bone with structural integrity and a geometrical configuration similar to that prior to the injury. In the majority of cases, healing is uneventful, but in 5–10% of cases delayed or impaired healing occurs (13). Nonunion of a fracture represents the most dramatic example of poor healing as complete restoration fails in the absence of some form of treatment.
Thus, the normal biologic healing process is deficient in achieving complete repair.
At present, no pharmacologic treatments are available. Thus, there is an unmet need for medications that can stimulate bone healing.
The main drugs that may support to bone healing are those discussed below.
Strontium ranelate is an osteoporosis agent that increases bone formation and reduces bone resorption and may therefore be beneficial in fracture healing. Strontium ranelate is a strontium salt of ranelic acid that both increases deposition of new bone osteoblasts and reduces the resorption of bone by osteoclasts. It is therefore promoted as a “dual action bone agent”. Precaution is advised in patients at increased risk of venous thromboembolism (VTE), including patients with a history of VTE. Precaution is advised in patients with phenylketonuria, as strontium ranelate contains phenylalanine. Very little is known about the effect on fracture healing for the rest of the anti-osteoporotic agents currently in clinical use. However, recently interesting data have been published on strontium ranelate, which is supposed to have dual effects on bone turnover; in addition to its antiresorptive effects, some evidence also points toward an anabolic effect.
Therefore, it may have beneficial effects on bone healing, although no clinical trials have documented this effect but a few case reports (14) have indicated that there might be a clinical benefit of using strontium ranelate to induce fracture healing in humans. This is supported by a few in vivo studies. Interestingly, the effect only seems to be present under conditions of impaired fracture healing but not under normal physiologic conditions.
Thus, although the documentation of the effects of strontium ranelate on fracture healing is sparse some very intriguing studies have been published indicating that this compound may be useful in the induction of fracture healing, but that it only appears to be effective when used in states of impaired bone healing.
Parathyroid hormone (PTH) is the first bone anabolic drug approved for the treatment of osteoporosis and, intriguingly, a number of animal studies prove the ability of PTH to induce fracture healing. PTH may therefore be a potential novel treatment option in humans with impaired healing. However, more randomized clinical trials documenting the clinical efficacy of PTH as a promoter of fracture healing in the clinical setting are warranted (15).
Teriparatide is a synthetic polypeptide hormone that contains the 1–34 aminoacid fragment of recombinant human parathyroid hormone (PTH 1–34). It has been approved by the US Food and Drug Administration for the treatment of post-menopausal women with osteoporosis who are at high risk for sustaining a fragility fracture. Daily treatments with 20 μg of PTH (1–34) resulted in dose-dependent increases in bone mineral density in the lumbar spine and femoral neck in osteoporotic female and male patients.
Its anabolic effect is given by the stimulation of the osteoblast, which causes a net increase in both cancellous and cortical bone, thus improving bone architecture.
Teriparatide has different effects on trabecular and cortical bone. Because of the high degree of remodeling and apoptosis of trabecular bone osteoblasts, teriparatide has a more profound activity on trabecular as compared to cortical bone, which has a lower degree of osteoblastic apoptosis. It has been shown that teriparatide also accelerates fracture healing by improving the biomechanical properties of the fracture callus, increasing endochondral ossification and bone remodeling in animal models. This effect has been observed in several case reports.
Chintamaneni et al. (16) described a case of nonunion in the body of the sternum of a 67-year-old male after a motor vehicle accident who was treated with teriparatide; healing was achieved at 9 months. Rubery and Bukata (17) described a series with 3 cases of nonunions in type III odontoid fractures which, after conservative treatment with external immobilization, were successfully healed with teriparatide, with clinical improvement.
The existing basic science data suggest a role for PTH signaling in the regulation of chondrogenesis and osteogenesis. Investigations in humans have confirmed an anabolic role for PTH (1–34) in enhancing bone density and reducing fracture risk. Animal studies on fracture healing suggest that PTH signaling improves the biomechanical properties of fracture callus and accelerates callus formation, endochondral ossification, and bone remodeling.
Bisphosphonates – which are the most widely used drug for treating osteoporosis – delay the healing process slightly, although apparently not clinically relevant. Finally, a number of newer antiresorptive agents are available, but very few studies have addressed their effects on bone healing.
However, bisphosphonates inhibit bone remodeling through an initial inhibition of bone resorption and subsequently an inhibition of bone formation. In fracture healing, bone remodeling is important in the progression from callus formation through organization of the newly formed bone. Therefore, there is some controversy about the usefulness of bisphosphonates in the promotion of fracture healing (15).
Denosumab is another newly marketed antiresorptive drug that works through inhibiting osteoclast formation and function. In a mouse model, unilateral transverse femoral fractures were induced, and animals were treated with denosumab (10 mg/kg) or alendronate (0.1 mg/kg) biweekly for 42 days. Both groups showed increased callus and increased amounts of mineralized cartilage in the callus, but remodeling and organization of the callus was delayed.
However, the mechanical strength at the fracture site was increased compared with the controls. Thus, despite the delay in healing, the strength at the fracture site was still improved compared with controls (18). This study indicates that the effects of the antiresorptive drug denosumab are similar to the bisphosphonates. However, more studies are necessary to further elucidate its effects on bone healing.
Our clinical use
All cases handled by us are elderly patients, especially females, who reported fractures with clinical and X ray signs of delay in consolidation. We never treated patients with secondary osteoporosis, referring to the various specialists treating these forms.
In our clinical practice we use since 2010 only strontium ranelate or teriparatide always associated with the supplement of Vitamin D and the use of electromagnetic fields. The supplementation of Vitamin D is made according to the initial values following this scheme. We start with 100.000 UI weekly for 4 weeks and then 25.000 UI every 2 weeks in maintaining.
The electromagnetic fields enhances osteogenesis having an action at the membrane of osteoblasts and bone with a piezoelectric effect with increased collagen production, structural order and orientation. Always recommend to perform it for about 6 hours per day, every day for about 2 months.
We prefer not to use strontium ranelate in patients with renal insufficiency and in patients bedridden because in the literature there are no data on bone safety in patients with severe renal impairment treated with strontium ranelate. It is not recommended in patients with creatinine clearance less than 30 ml/min. In accordance with good clinical practice is recommended periodic monitoring of renal function in patients with chronic renal failure. Furthermore, in a phase III placebo-controlled treatment with strontium ranelate was associated with an increase in the annual incidence of venous thromboembolism (VTE), including pulmonary embolism. Ranelate therefore should be used with caution in patients at increased risk of VTE, including patients with a past history of VTE and patients subjected to prolonged bed rest. With regard to the use of teriparatide, we consider a contraindication hypercalcemia, severe renal failure, metabolic bone diseases, including hyperparathyroidism and Paget’s disease of the bone, other than primary osteoporosis and glucocorticoid-induced osteoporosis. Should be excluded from treatment with teriparatide patients with unexplained elevations of alkaline phosphatase, which have made previous radiation therapy to the skeleton external or internal source (facility) and patients with skeletal malignancies or bone metastases. Caution should be exercised in patients with moderate renal impairment.
Bring into question 2 cases treated one with teriparatide and the other with strontium ranelate that we consider interesting to match this discussion.
One case is a caucasian 80-year-old white woman that had an accident in October 2011. She was diagnosed with right distal metaphysal femoral fracture on total knee artrhoplasty. She underwent surgery at our center consisting of ORIF with lateral femoral locking plate in October 2011.
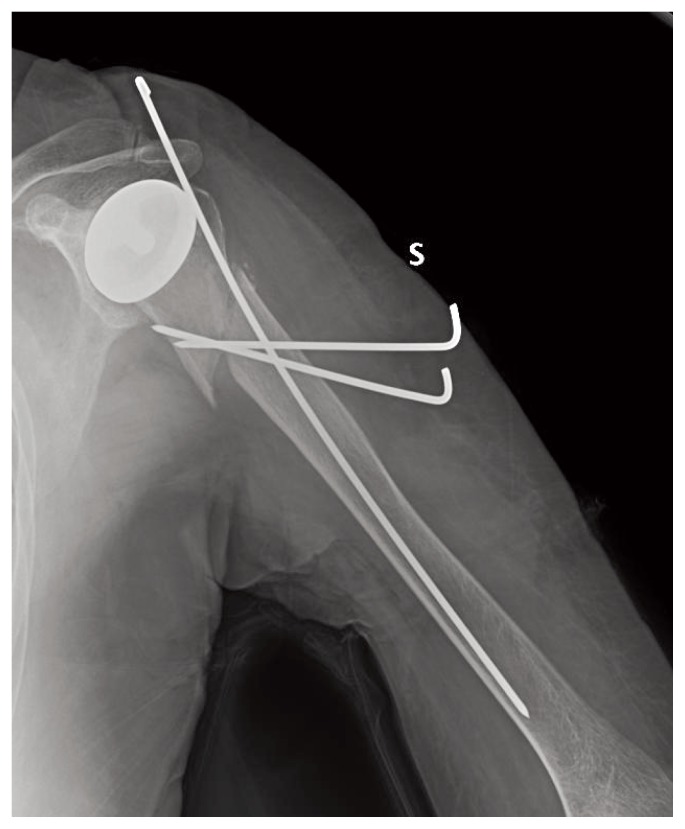
Radiological controls at 5 and 7 months did not show any signs of healing (Figure 1). The patient was treated also with pulsing electromagnetic fields. This was consistent with the clinical manifestations of pain and right leg weakness. The physical examination and laboratory tests, including white blood cell counts, C-reactive protein, and erythrocyte sedimentation rate, were normal, which permitted to rule out underlying infection. The patient denied smoking, alcohol abuse, and had no history of metabolic disease or glucocorticoid intake.
Figure 1.
Post operative X-ray after fracture fixation with lateral femoral locking plate at 7 months.
Other laboratory data, including serum alkaline phosphatase, PTH, and calcium, were normal part of a low blood level of Vitamin D.
The patient declined consented to an empiric, off-label therapy with teriparatide at approved doses for the treatment of osteoporosis (20 mg/day), as an attempt to treat the atrophic nonunion.
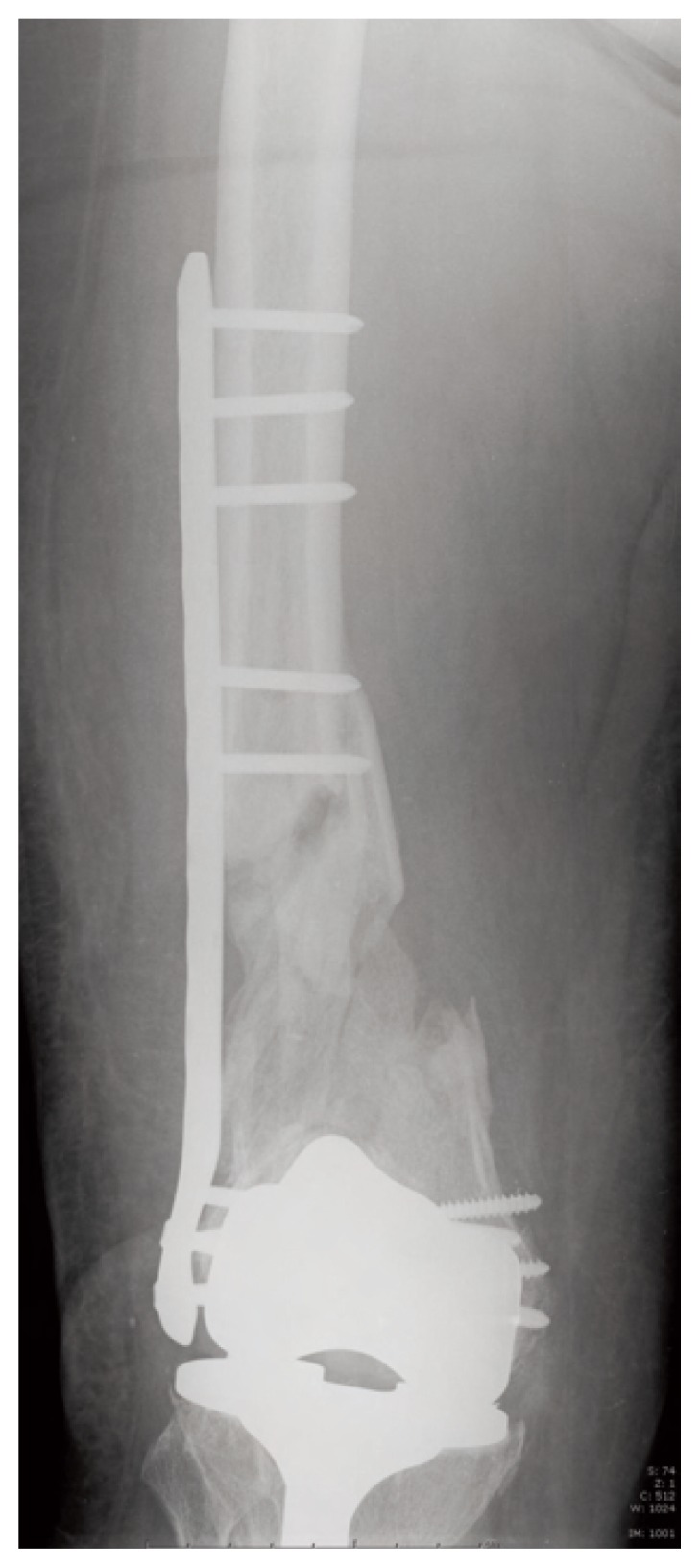
After 2 months of treatment with teriparatide, an x-ray showed the presence of bone bridges and a decreased gap between fragments. We also noticed the rx increased bone density in the site of fracture.
After 3 months of treatment, healing was complete, coinciding with the disappearance of pain (Figure 2).
Figure 2.
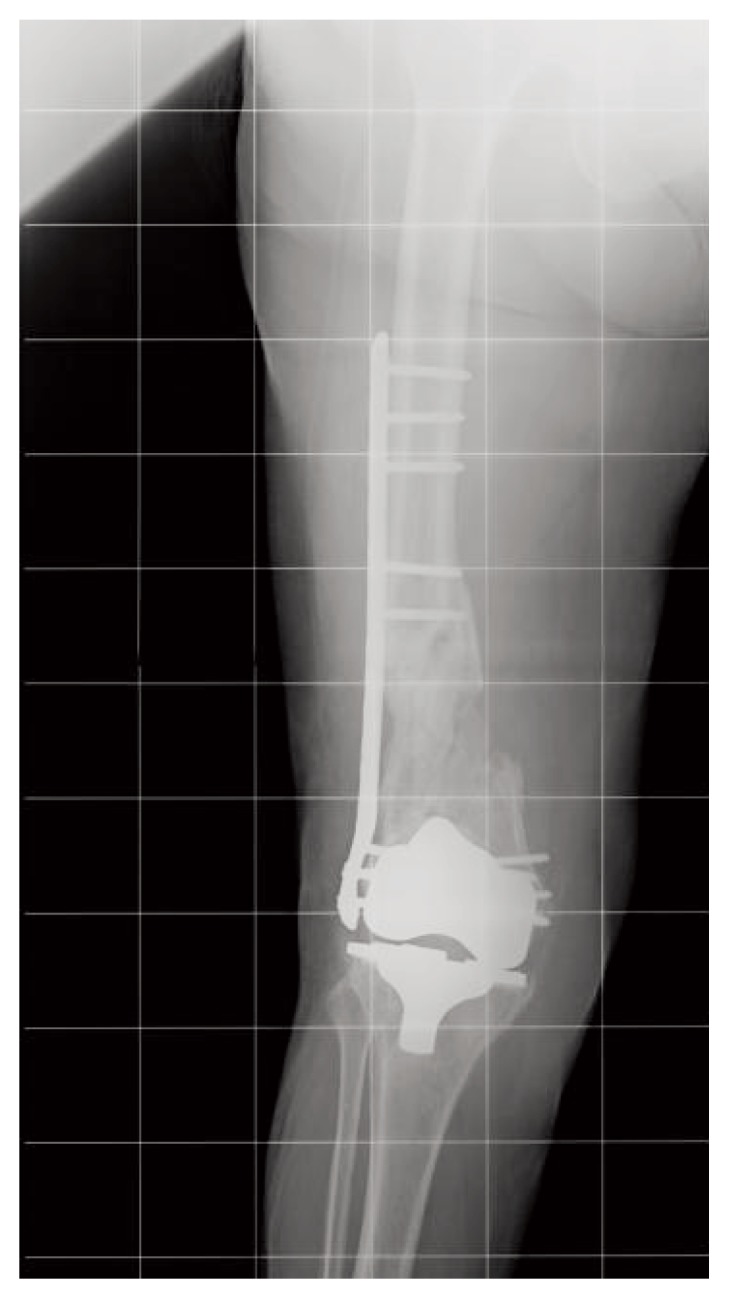
X-ray control at 10 months.
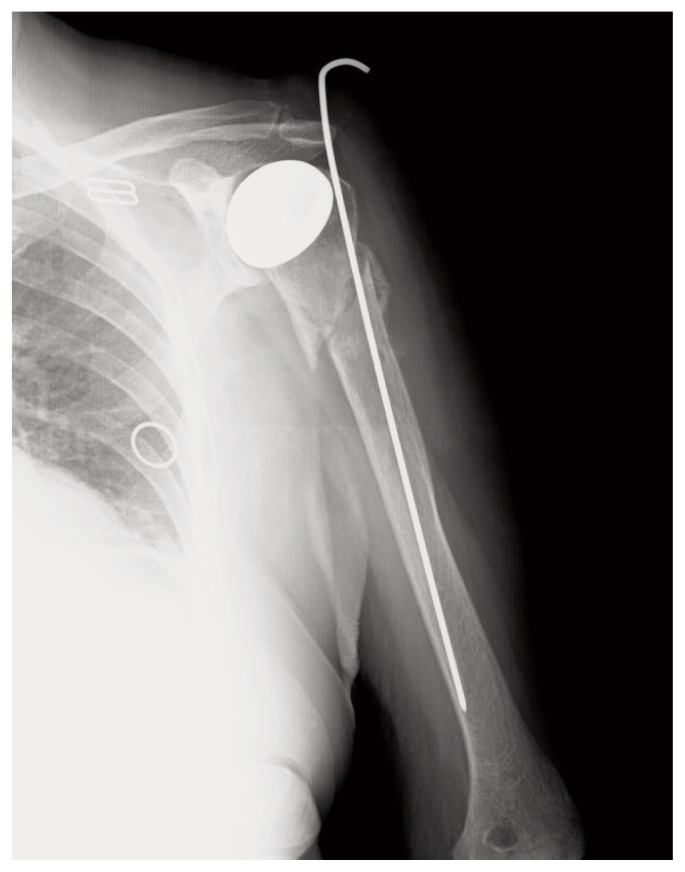
The other case is a female, 78 years old, right-handed, former smoker, with a history of high blood pressure in 2007 and 2009 was subjected to surgical positioning bilateral shoulder resurfacing. The patient reported good function of both shoulder. In March 2012, following a fall, the patient reported humeral periprosthetic fracture and femoral neck fracture on the left side. The femoral fracture was treated by reduction and fixation with intramedullary nail and humeral fracture reduction and fixation by K-wires.
The reduction performed in humerus did not give absolute stability, but we chose this treatment by assessing the risks and benefits of the patient looking for a less invasive surgery (Figure 3).
Figure 3.
Post operative X-ray after fracture fixation with K-wires.
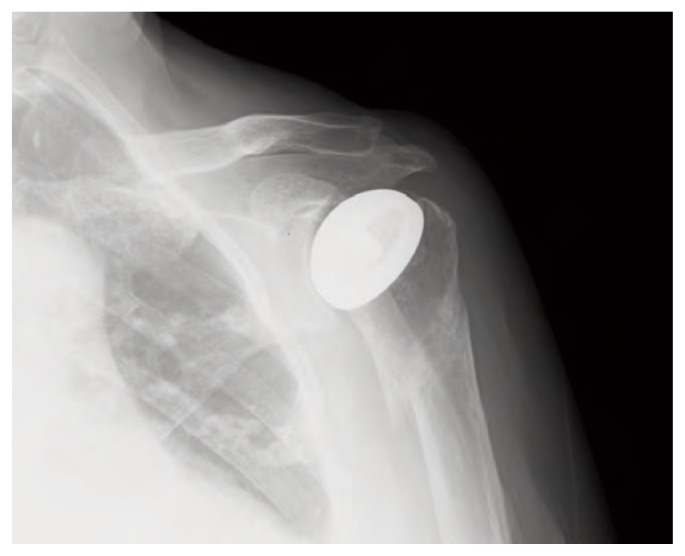
In June 2012 the radiographs showed delay in consolidation of the humerus (Figure 4). Blood tests revealed that values in the media but the lower limits of the reference parameters: Vit A 23.4 g/l, PTH 14.00 pg/ml and alkaline phosphatase 115 U/l, urinary calcium 103, 7 mg/24 h.
Figure 4.
X-ray that shows delay in consolidation.
Since June 2012, the patient began therapy with strontium ranelate 2 gr daily and the control after 2 months treatment showed complete bone healing (Figure 5). According to the Italian law, Ethical approval for this study was not required because it involved only routine clinical follow-up and radiographic examination. Written informed consent has been obtained from these patients. With these consent the patients authorize the surgical treatment and also the collection and publication of clinical data about their cases for scientific and educational purposes even outside the institution.
Figure 5.
X-ray control that shows consolidation after 2 months therapy of strontium ranelate.
Discussion
Fractures are the most feared osteoporosis complications and often result in disability and loss of autonomy of patients, with socio-economic consequences. Moreover many studies suggest a negative correlation between age and fracture healing resulting in further difficulties in achieving clinical-radiographic healing of fractures in elderly patients.
There is no doubt that PTH given intermittently can potentially be used in the treatment of impaired fracture healing. Strontium ranelate is the only osteoporosis treatment that dissociates the processes of bone resorption and bone formation (19). Our cases seem to confirm the effect of these medications as bone inductor through a more rapid healing of fractures and moreover, a case was an upper limb.
Additionally we would like to underline that the case of the upper limb, subjected to percutaneus surgery (Kirschner wires), had as a disadvantage on healing because the reduction could not be considered very stable. This increases the chances of nonunion. Hence the fact that strontium ranelate has still led to a fracture healing less helped by surgery greatly strengthens its contribution as a determinant agent in bone healing.
They could have a potentially important role in treating some forms of nonunion especially where bone metabolism is compromised. According to their mode of action, the osteoporosis treatments may affect the proliferation of early callus tissue, the differentiation of chondrocytes or osteoblasts, capillary formation, and/or sensitivity to mechanical input (20, 21). All of these effects could influence the healing time or the rate of fracture complication.
There are a number of limitations to make conclusions from clinical cases. We know that in our study we had to remove some of the factors that may have affected on the resolution of these cases, such as variations in loading, alignment, and individual physiology.
Despite the limitations above, these cases highlight a potential application and are supported by preclinical studies in experimental animals.
In our opinion is crucial that the treatment used by orthopaedic surgeons is not related only to the surgical treatment but take into consideration both the underlying disease and the possibility of positively affect bone healing with specific drug therapy (22–25).
Thus one could hypothesize the possibility of a medical treatment both as a preventive and as support to the synthesis. A positive effect of osteoporosis treatments on bone healing is an interesting possibility and merits further clinical research.
Footnotes
Conflict of interest
No benefits or funds were received in support of this study. None of the authors has any conflict of interests to disclose.
References
- 1.Leali PT, Muresu F, Melis A, Ruggiu A, Zachos A, Doria C. Skeletal fragility definition. Clin Cases Miner Bone Metab. 2011 May;8(2):11–13. [PMC free article] [PubMed] [Google Scholar]
- 2.Della Carbonare L, Giannini S. Bone microarchitecture as an important determinant of bone strength. J Endocrinol Invest. 2004 Jan;27(1):99–105. doi: 10.1007/BF03350919. [DOI] [PubMed] [Google Scholar]
- 3.Piscitelli P, Brandi ML, Chitano G, Argentiero A, Neglia C, Distante A, Saturnino L, Tarantino U. Epidemiology of fragility fractures in Italy. Clin Cases Miner Bone Metab. 2011 May;8(2):29–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett E, Nordin BEC. The radiological diagnosis of osteoporosis a new approach. Clin Radiol. 1960;11:166–9. doi: 10.1016/s0009-9260(60)80012-8. [DOI] [PubMed] [Google Scholar]
- 5.Piscitelli P, Tarantino U, Chitano G, Argentiero A, Neglia C, Agnello N, Saturnino L, Feola M, Celi M, Raho C, Distante A, Brandi ML. Updated incidence rates of fragility fractures in Italy: extension study 2002–2008. Clin Cases Miner Bone Metab. 2011 Sep;8(3):54–61. [PMC free article] [PubMed] [Google Scholar]
- 6.Guido G, Giannotti S, Bottai V, Ghilardi M, Bianchi MG, Ceglia MJ. Femoral fractures in the extremely elderly. Clin Cases Miner Bone Metab. 2011 May;8(2):35–37. [PMC free article] [PubMed] [Google Scholar]
- 7.Giannotti S, Bottai V, Ghilardi M, Dell’Osso G, Guido G. Shoulder resurfacing with Durom Cup: clinical and radiological re-assessment. J Orthop Sci. 2012 Sep;17(5):545–50. doi: 10.1007/s00776-012-0256-2. [DOI] [PubMed] [Google Scholar]
- 8.Giannotti S, Bottai V, Dell’Osso G, Donati D, Bugelli G, De Paola G, Guido G. Indices of risk assessment of fracture of the proximal humerus. Clin Cases Miner Bone Metab. 2012 Jan;9(1):37–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Giannotti S, Bottai V, Dell’Osso G, Donati D, Bugelli G, De Paola G, Guido G. Humeral bone fragility in patients with shoulder prosthesis: a case of humeral periprosthetic refracture. Clin Cases Miner Bone Metab. 2012 Jan;9(1):56–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Alegre DN, Ribeiro C, Sousa C, Correia J, Silva L, de Almeida L. Possible benefits of strontium ranelate in complicated long bone fractures. Rheumatol Int. 2012;32:439–443. doi: 10.1007/s00296-010-1687-8. [DOI] [PubMed] [Google Scholar]
- 11.Paraschou S, Bekir H, Anastasopoulos H, et al. Evaluation of interlocking intramedullary nailing in distal tibial fractures and nonunions. Acta Orthop Traumatol Turc. 2009;43(6):472–7. doi: 10.3944/AOTT.2009.472. [DOI] [PubMed] [Google Scholar]
- 12.Otero-Alvaro A, Moreno E. Atrophic humeral shaft nonunion treated with teriparatide (rh PTH 1–34): a case report. J Shoulder Elbow Surg. 2010;19(7):e22–8. doi: 10.1016/j.jse.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31(6):721–727. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alegre DN, Ribeiro C, Sousa C, Correia J, Silva L, de AL. Possible benefits of strontium ranelate in complicated long bone fractures. Rheumatol Int. 2012 Feb;32(2):439–43. doi: 10.1007/s00296-010-1687-8. [DOI] [PubMed] [Google Scholar]
- 15.Jørgensen NR, Schwarz P. Effects of Anti-osteoporosis Medications on Fracture Healing. Curr Osteoporos Rep. 2011;9:149–155. doi: 10.1007/s11914-011-0065-0. [DOI] [PubMed] [Google Scholar]
- 16.Chintamaneni S, Finzel K, Gruber BL. Successful treatment of sternal fracture nonunion with teriparatide. Osteoporos Int. 2010;21(6):1059–63. doi: 10.1007/s00198-009-1061-4. [DOI] [PubMed] [Google Scholar]
- 17.Rubery PT, Bukata SV. Teriparatide may accelerate healing in delayed unions of type III odontoid fractures: A report of 3 cases. J Spinal Disord Tech. 2010;23(2):151–5. doi: 10.1097/BSD.0b013e31819a8b7a. [DOI] [PubMed] [Google Scholar]
- 18.Gerstenfeld LC, Sacks DJ, Pelis M, Mason ZD, Graves DT, Barrero M, Ominsky MS, Kostenuik PJ, Morgan EF, Einhorn TA. Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res. 2009;24:196–208. doi: 10.1359/jbmr.081113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marie PJ, Ammann P, Boivin G, et al. Mechanisms of action and therapeutic potential of strontium in bone. Calcif Tissue Int. 2001;69:121–129. doi: 10.1007/s002230010055. [DOI] [PubMed] [Google Scholar]
- 20.Aspenberg P. Drugs and fracture repair. Acta Orthop. 2005;76:741–748. doi: 10.1080/17453670510045318. [DOI] [PubMed] [Google Scholar]
- 21.Matassi F, Nistri L, Chicon Paez D, Innocenti M. New biomaterials for bone regeneration. Clin Cases Miner Bone Metab. 2011 Jan-Apr;8(1):21–24. [PMC free article] [PubMed] [Google Scholar]
- 22.Giannotti S, Bottai V, Dell’Osso G, De Paola G, Pini E, Guido G. Atrophic femoral nonunion successfully treated with teriparatide. Eur J Orthop Surg Traumatol. 2012 doi: 10.1007/s00590-012-1143-4. [DOI] [PubMed] [Google Scholar]
- 23.Giannotti S, Bottai V, Pini E, Dell’Osso G, De Paola G, Guido G. Clinical and surgical approach of severe bone fragility fracture: clinical case of 4 fragility fracture in patient with heavy osteoporosis. Clin Cases Miner Bone Metab. 2013;10(1):52–55. doi: 10.11138/ccmbm/2013.10.1.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannotti S, et al. Treatment of pseudoarthrosis of the upper limb using expanded mesenchymal stem cells: a pilot study. Eur Rev Med Pharmacol Sci. 2013 Jan;17(2):224–7. [PubMed] [Google Scholar]
- 25.Giannotti S, Bottai V, Dell’Osso G, De Paola G, Ghilardi M, Guido G. Pseudoartrosis in atypical femoral fracture: case report. Osteoporos Int. 2013 doi: 10.1007/S00198-013-2397-3. [DOI] [PubMed] [Google Scholar]