Summary
Osteoporosis is a multifactorial skeletal disorder characterized by the decrease of bone mass and the alteration of bone microarchitecture that leads to the increase of fracture risks.
Traditionally, osteoporosis has been classified into primary and secondary osteoporosis. Primary osteoporosis refers to osteoporotic conditions which are not related to other chronic illnesses and is usually associated with aging and decreased gonadal function, such as decreased level of estrogen, whereas secondary osteoporosis is the type of osteoporosis caused by other health problems. Disuse is one of the many reasons inducing bone loss and resulting in secondary osteoporosis.
The disuse osteoporosis appeared for the first time in the literature in 1974 when Minaire reported some histomorphometric analysis of iliac crest bone biopsies performed after a spinal cord injury. The most common skeleton sites in which disuse osteoporosis can be observed are knees and ankles.
There are three clinical situation in which this disease can be observed: neurological or muscular disease that causes a pathological and prolonged immobilization. The most frequent is caused by a spinal cord injury, long term bed rest or space flight that causes the immobilization linked to changes in mechanical environment and experimental immobilizations in healthy subjects. Physical exercise is essential for increasing or maintaining bone mass and strength.
In our study we wondered if the disuse of the upper limbs of a certain entity, lasting for a long time, can cause a decrease in BMD quantifiable with a densitometric evaluation of the distal radius and with an evaluation of the humeral cortical index such as to define a real osteoporosis from disuse.
We analyzed 30 female patients without secondary osteoporosis older than 60 years: everyone underwent to vit D evaluation, densitometric exams of spine, hip and distal radius, Constant score and femoral and humeral cortical index evaluation. We observed that the distal radius BMD and humeral cortical index were worse in patients with low upper limb functionality than in patients with normal shoulder function. The results of this study suggest that humeral cortical index and radial BMD can be useful methods of upper limb bone density evaluation and that they can be useful to select a correct surgical treatment in orthopaedic and traumatologic diseases.
Keywords: disuse osteoporosis, upper limb, demineralization, humeral bone quality
Introduction
Osteoporosis is a multifactorial skeletal disorder characterized by the decrease of bone mass and the alteration of bone microarchitecture that leads to the increase of fracture risks (1, 2).
The localized demineralization, as told by Alexander in 2011, is part of the orthopaedic culture since long time (3).
In fact, Wolff in 1986, after analyzing mathematical models of the loading forces acting on skeleton, told that the bone that loads is able to produce while the bone that doesn’t load is able to reabsorbe (4).
On the other side, the disuse osteoporosis (DO), or localized demineralization, is the bone loss due to skeletal mechanical unloading (5).
The disuse osteoporosis appeared for the first time in the literature in 1974 when Minaire P. reported some histomorphometric analysis of iliac crest bone biopsies performed after a spinal cord injury (6).
There are three clinical situation in which this disease can be observed (5): neurological or muscular disease that causes a pathological and prolonged immobilization. The most frequent is caused by a spinal cord injury, long term bed rest or space flight that causes the immobilization linked to changes in mechanical environment and experimental immobilizations in healthy subjects.
The etiology of this pathology is not well described but it appears to be related to a loss of mechanical stresses that causes subsequent metabolic changes in the osteoblasts and osteoclasts activity (7, 8).
Disuse osteoporosis has been shown to be a regional phenomenon in the areas with tremendous decrease in weight bearing like lower limbs.
There are three main forces groups that determinate bone density preservation (1): static-gravity-related wear-bearing, ground reaction forces and dynamic loading generated by muscle contractions during deambulation.
Physical exercise is essential for increasing or maintaining bone mass and strength (9).
However in the literature it cannot be found a lot about localized demineralization and what one can find is only about the lower limbs. In fact Minaire in 1974 described it as an exclusive condition of these (6).
Disuse osteoporosis was firstly described with the aim of radiography by Jones who identified four main patterns of demineralization: diffuse demineralization, a spotty configuration, band-like of translucency or linear band and cortical scalloping of the endosteum and subperiosteal layer (10, 11).
This findings generally appear after approximately 8 weeks of immobilization even if in younger patients we can find them sooner (10).
The RMN imaging typical findings of disuse osteoporosis are: accentuation of vertical and horizontal marrow lines, presence of subchondral lobules of fat, prominent bone vascularization and the presence of dotti foci of high signal intensity on T2-weighted fat-suppressed sequences (12).
On the other side Ding describes, in his study based on results of microcomputer tomography, the decrease of the volume and the number of intraosseous blood vessels that may play an important role in the development of post-nerve injury osteoporosis (13).
This confirms that it isn’t yet clear the etiology of disuse osteoporosis and the pathophysiology and its treatment.
The most common skeleton sites in which disuse osteoporosis can be observed are knees and ankles (7).
Really Donald in the past stated that upper limb disuse osteoporosis didn’t exist because of the difference of the upper extremities (presumably genetically programmed in a different way); in fact he said that the upper limb didn’t develop X-ray-detectable disuse osteoporosis typical demineralization except after several months of severe paralysis.
Rittweger et al. reported in their studies a reduction of bone mass in the cancellous bone-rich areas in subjects submitted to long-term bed rest of the lower limb instead of the upper limb. Bed rest is a recognized model for muscle atrophy and bone loss in space flight and in clinical medicine. They hypothesized that whole body vibration in combination with resistive exercise (RVE) would be an effective countermeasure. In this study the authors underline the importance of mechanical usage for the maintenance of the human skeleton (14).
Traditionally, osteoporosis has been classified into primary and secondary osteoporosis. Primary osteoporosis refers to osteoporotic conditions which are not related to other chronic illnesses and is usually associated with aging and decreased gonadal function, such as decreased level of estrogen, whereas secondary osteoporosis is the type of osteoporosis caused by other health problems. Disuse is one of the many reasons inducing bone loss and resulting in secondary osteoporosis (15).
Furthermore, muscular contraction seems to be the most important force out of the three categories of mechanical loading for keeping bone mass (1).
The disuse osteoporosis refers to bone mass decrements under conditions of decreased mechanical loading, including decreased ground force reaction, muscular contraction, and microgravity-related bone loss in astronauts after space flights. Although there are many effective treatments available for primary osteoporosis, there is a lack of effective treatments for disuse osteoporosis. This is because that the aetiology, pathophysiology, and resultant pathology of disuse osteoporosis differ from those of primary osteoporosis (1).
The degree of attenuation of trabecular bone loss and deterioration of its microarchitecture is closely dependent on the mechanical loading parameters within the regimen. Dynamic muscle stimulation as a preventive countermeasure for disuse osteopenia has been shown to be effective (16).
The movement of interstitial fluid in the lacunar-canalicular porosity of the bone plays an important role in bone preservation (17). The cyclic mechanical loading applied to the bone determines the regular interstitial fluid flows around the osteocytes in the lacunar and canalicular porosity (18).
Taking into account the fundamental role played by the muscle strength is likely to assume the possibility of disuse osteoporosis occurrence related to an important muscolar diminuited function especially in the upper limb that, in addition, is subject to a higher bone resorption because it is not under gravity load.
The close relationship between muscle and bone hypothesizes that a change in vascular perfusion within skeletal muscle can indeed cascade to a change in fluid pressure in bone (19).
Mechanical loading accentuates fluid shear stress on bone cells, thus releasing signaling molecules, e.g., nitric oxide, to further mediate bone remodeling (20, 21).
In orthopaedic culture is widely understood that humerus, that is not a static-gravity-related wear-bearing, presents a decreased bone density before the lower limb.
Rangan demonstrates that, in a population who have had a low Energy proximal humerus fractures, DEXA scans, taken at the hip and at the lumbar spine, misrepresent osteoporosis in the upper limb (22, 23).
Moreover, humeral cortex index value in patients with humeral fragility fracture is worst than in patients who underwent a hip fracture surgery (24).
So it seems plausible to assume at least the possibility that even at the upper limb level an important reduction of the rotator cuff function is sufficient to determine a distrectual bone resorption.
Material and methods
In our study we wondered if the disuse of the upper limbs of a certain entity, lasting for a long time, can cause a decrease in BMD quantifiable with a densitometric evaluation of the distal radius and with an evaluation of the humeral cortical index such as to define a real osteoporosis from disuse.
Therefore the authors analyzed 30 female patients older than 60 years, in no case there was secondary osteoporosis, dividing them into two groups.
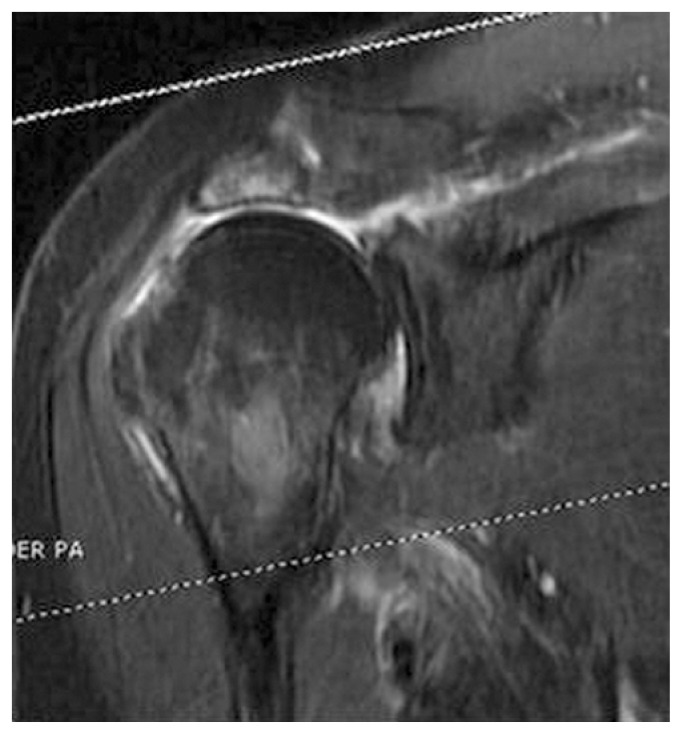
In the first group the authors considered patients with a significant decrease of upper limbs function, corresponding to a constant score less than or equal to 50 and present since at least 6 months before the evaluation (Figure 1).
Figure 1.
RMN in patient with a cuff arthropathy.
In the second group, they considered patients without upper limbs dysfunction.
The authors evaluated all the patients with humeral and femoral cortical index, ultradistal radio and femur densitometry. From the results it can be seen that, in the first group, the cortical index values and DEXA related to the upper limb, are worse (Figure 2), when compared to the age average values, then those of the lower limbs, and this demonstrates that it is a framework of the localized demineralization.
Figure 2.
DEXA ultradistal radius.
Instead, if we compare the measurements values of the upper limbs of the two groups, it can be noted that the values measured in the group with shoulder dysfunction are worst, demonstrating that we are in presence of a reabsorption due to disuse.
Moreover, knowing that physiologically, as shown by Rangan in 2009, the bone mineral density of the upper limbs are worse than that on the lower limbs in the same individual, we subjected the patients of the first group to a radial densitometric comparative assessment of the healthy upper limb compared to the limb affected by disuse.
The authors have thus observed, among the compromised limb and the healthy one, a clear difference of the average values of T-score of 1.5 points and of the average values of IC of 0.07 points.
Discussion
In the literature few information can be found about disuse osteoporosis and what you find is related to the lower limbs. To date it is established that it is a defined pathological entity, with causes and etiopathogenetic mechanisms different from those at the base of primary osteoporosis.
Consequently even the medical treatment proposed, although not yet codified, is different.
On the other hand, we face with an aging population in which the risk of fractures at the lower and upper limbs increases greatly.
The upper limbs are particularly sensitive to a reduction in bone density due to a reduction of muscular activity as occurs in older age, because the upper limbs are segments where the only load forces that act are those of muscle tendon (25). There is a relationship of proportionality between the cortical index values and the ultradistal radio densitometry and both can be considered as valid methods to define the bone resorption and the risk of fractures of the upper limb.
Regarding the surgical treatment, where it is indicated, for proximal humerus fractures, the treatment choice and consequently its success are strongly linked to the state of the humeral bone quality. However, until today there aren’t yet “standardization” in the measurement of bone mineral density humeral able to identify the values below which it is conceivable that there is a poor seal of the means of synthesis.
The disuse osteoporosis can be defined as the reduction of bone mass in the upper limb that occurs for an important reduction of the normal joint function of the shoulder with insufficiency of the rotator cuff protracted for at least 4–6 months.
The assessment of this framework is important both in the prevention of osteoporotic fractures in these kind of patients and in the choice of surgical treatment where it is necessary.
Finally, perhaps, disuse osteoporosis of the upper limb has to be considered a real disease on its own with all the medical (26–29) and surgical implications (both for the election pathology and post-traumatic one) that follow.
References
- 1.Lau RY, Guo X. A review of current osteoporosis research: with special focus on disuse bone loss. J Osteoporosis. 2011 doi: 10.4061/2011/293808. articli ID 293808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guido G, Giannotti S, Bottai V, Ghilardi M, Bianchi MG, Ceglia MJ. Femoral fractures in the extremely elderly. Clin Cases Miner Bone Metab. 2011 May;8(2):35–37. [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandre C, et al. Pathophysiology of bone loss in disuse osteoporosis. Joint Bone Spine. 2011 Dec;78(6):572–6. doi: 10.1016/j.jbspin.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Wolff J. The Law of Bone Remodeling. Berlin Heidelberg New York: Springer; 1986. [Google Scholar]
- 5.Alexandre C, Laurence V. Pathophisiology of bone loss in disuse osteoporosis. Jone Bone Spine. 2011;78:572–576. doi: 10.1016/j.jbspin.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Minaire P, Meunier P, Edouard C, et al. Quantitative histological data on disuse osteoporosis: comparison with biological data. Calcif tissue res. 1974;17:57–73. doi: 10.1007/BF02547214. [DOI] [PubMed] [Google Scholar]
- 7.Resnick D. Diagnosis of bone and joint disorders. 4th edition. Philadelphia: Saunders; 2002. Regional osteoporosis; pp. 1795–98. [Google Scholar]
- 8.Takata S, Yasui N. Disuse Osteoporosis. J Med Investig. 2001;48(3–4):147–56. [PubMed] [Google Scholar]
- 9.Rutherford O. The role of exercise in prevention of osteoporosis. Physiotherapy. 1990;76:522–526. [Google Scholar]
- 10.Jones G. Radiological appearances of disuse osteoporosis. Clin Radiol. 1969;20:345. doi: 10.1016/s0009-9260(69)80082-6. [DOI] [PubMed] [Google Scholar]
- 11.Joyce JM, Keats T. Disuse osteoporosis: mimico of neoplastic disease. Skeletal radiol. 1986;15(2):129–32. doi: 10.1007/BF00350206. [DOI] [PubMed] [Google Scholar]
- 12.de Abreu Marcelo R, et al. Bone marrow MR imaging findings in disuse osteoporosis. Skeletal radiol. 2011;40:571–575. doi: 10.1007/s00256-010-1042-x. [DOI] [PubMed] [Google Scholar]
- 13.Ding WG, Yan WH, Wei ZX, Liu JB. Difference in intraosseous blood vessel volume and number in osteoporotic model mice induced by spinal cord injury and sciatic nerve resection. J Bone Miner Metab. 2012 Jul;30(4):400–7. doi: 10.1007/s00774-011-0328-y. [DOI] [PubMed] [Google Scholar]
- 14.Rittweger J, et al. Muscle atrophy and bone loss after 90 day’s bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone. 2005;36(6):1019–1029. doi: 10.1016/j.bone.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Howard A. Coding for bone diseases. For The Record. 2011;23(9):27. [Google Scholar]
- 16.Hoyan L, et al. Bone. 2011 Feb;48(2):399–405. [Google Scholar]
- 17.Fritton SP, et al. Fluid and solute transport in bone: flow-induced machanotrasduction. Annu Rev Fluid Mech. 2009;41:347–74. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Divya Sharma, et al. Alterations in the osteocyte lacunar-canalicular microenviroment due to estrofen deficiency. Bone. 2012;51 doi: 10.1016/j.bone.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin YX, Lam H. Intramedullary pressure and matrix strain induced by oscillatory skeletal muscle stimulation and its potential in adaptation. J Biomech. 2009;42:140–5. doi: 10.1016/j.jbiomech.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAllister TN, et al. Fluid shear stress stimulates prostaglandin and nitric oxide release in bone marrow-derived preosteoclast-like cells. Biochem Biophys Res Commun. 2000;270:643–8. doi: 10.1006/bbrc.2000.2467. [DOI] [PubMed] [Google Scholar]
- 21.Mullender MG, et al. Release of nitric oxide, but not prostaglandin E2, by bone cells depends on fluid flow frequency. J Orthop Res. 2006;24:1170–7. doi: 10.1002/jor.20179. [DOI] [PubMed] [Google Scholar]
- 22.Giannotti S, Bottai V, Dell’Osso G, Donati D, Bugelli G, De Paola G, Guido G. Humeral bone fragility in patients with shoulder prosthesis: a case of humeral periprosthetic refracture. Clin Cases Miner Bone Metab. 2012 Jan;9(1):56–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson J, et al. Variation in bone mineral density by anatomical site in patients with proximal humeral fractures. J Bone Joint Surg Br. 2009 Jun;91(6):772–5. doi: 10.1302/0301-620X.91B6.22346. [DOI] [PubMed] [Google Scholar]
- 24.Giannotti S, Bottai V, Dell’Osso G, Donati D, Bugelli G, De Paola G, Guido G. Indices of risk assessment of fracture of the proximal humerus. Clin Cases Miner Bone Metab. 2012 Jan;9(1):37–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Giannotti S, Bottai V, Ghilardi M, Dell’Osso G, Guido G. Shoulder resurfacing with Durom Cuup: clinical and radiological reassessment. J Orthop Sci. 2012 Sep;17(5):545–50. doi: 10.1007/s00776-012-0256-2. [DOI] [PubMed] [Google Scholar]
- 26.Giannotti S, Bottai V, Dell’Osso G, De Paola G, Pini E, Guido G. Atrophic femoral nonunion successfully treated with teriparatide. Eur J Orthop Surg Traumatol. 2012 doi: 10.1007/s00590-012-1143-4. [DOI] [PubMed] [Google Scholar]
- 27.Giannotti S, Bottai V, Pini E, Dell’Osso G, De Paola G, Guido G. Clinical and surgical approach of severe bone fragility fracture: clinical case of 4 fragility fracture in patient with heavy osteoporosis. Clin Cases Miner Bone Metab. 2013;10(1):52–55. doi: 10.11138/ccmbm/2013.10.1.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giannotti S, et al. Treatment of pseudoarthrosis of the upper limb using expanded mesenchymal stem cells: a pilot study. Eur Rev Med Pharmacol Sci. 2013 Jan;17(2):224–7. [PubMed] [Google Scholar]
- 29.Giannotti S, Bottai V, Dell’Osso G, De Paola G, Ghilardi M, Guido G. Pseudoartrosis in atypical femoral fracture: case report. Osteoporos Int. 2013 doi: 10.1007/S00198-013-2397-3. [DOI] [PubMed] [Google Scholar]