Summary
Background
Symptomatic severe osteoarthritis and hip osteoporotic fractures are the main conditions requiring total hip arthroplasty (THA), whereas total knee arthroplasty (TKA) is mainly performed for pain, disability or deformity due to osteoarthritis. After surgery, some patients suffer from “painful prosthesis”, which currently represents a clinical problem.
Methods
A systematic review of scientific literature has been performed. A panel of experts has examined the issue of persistent pain following total hip or knee arthroplasty, in order to characterize etiopathological mechanisms and define how to cope with this condition.
Results
Four major categories (non infective, septic, other and idiopathic causes) have been identified as possible origin of persistent pain after total joint arthroplasty (TJA). Time to surgery, pain level and function impairment before surgical intervention, mechanical stress following prosthesis implant, osseointegration deficiency, and post-traumatic or allergic inflammatory response are all factors playing an important role in causing persistent pain after joint arthroplasty. Diagnosis of persistent pain should be made in case of post-operative pain (self-reported as VAS ≥3) persisting for at least 4 months after surgery, or new onset of pain (VAS ≥3) after the first 4 months, lasting ≥2 months. Acute pain reported as VAS score ≥7 in patients who underwent TJA should be always immediately investigated.
Conclusions
The cause of pain needs always to be indentified and removed whenever possible. Implant revision is indicated only when septic or aseptic loosening is diagnosed. Current evidence has shown that peri-and/or post-operative administration of bisphosphonates may have a role in pain management and periprosthetic bone loss prevention.
Keywords: hip arthroplasty, knee arthroplasty, persistent pain, painful prosthesis
Introduction: prosthesis and pain
Symptomatic severe osteoarthritis (OA), usually affecting hip and/or knee joints, is a leading cause of disability in elderly people and represents the main condition requiring surgical treatment with total joint arthroplasty (TJA) (1–3). The second major cause requiring TJA is hip fragility fracture due to osteoporosis, which currently represents about 30% of total hip replacements (4–6). Considering both severe osteoarthritis and osteoporosis, women are affected more frequently than men (7, 8). Joint arthroplasty is one of the most successful orthopedic interventions (9), and it is performed to reduce pain or functional disability (10), but persistent post-operative pain represents a problem that in some patients may negatively influence clinical outcomes (11). The International Association for the Study of Pain (IASP) has specifically defined persistent post-surgical pain as pain that developed after surgery which has been present for at least 3 months, an interval which is considered to be beyond the time for normal healing (12). The prevalence of persistent post-surgical pain is not clearly defined, being estimated between 10% and 50% of surgical patients (13), but surgery is known to be the second most common cause of persistent pain after degenerative conditions (14). According to the findings of Wylde et al., 44% of patients undergoing total knee arthroplasty (TKA) and 27% of those undergoing total hip arthroplasty (THA) suffer from persistent post-surgical pain of any severity, with severe or extremely severe pain being reported by 15% and 6% of operated patients, respectively (15).
It is unclear whether the persistence of pain after joint arthroplasty is a consequence of previous patient-related clinical factors, underlying vulnerability to pain, or if it should be simply regarded as a surgical complication due to aseptic (i.e. mechanical) or septic causes (15, 11). In some patients undergoing TJA it is very difficult to identify the origin (idiopathic) of painful symptoms. This latter condition has been defined as “painful prosthesis” (16, 17), which causes further sufferance, impaired function, and reduced quality of life to affected patients (17). In these patients, a re-intervention for prosthesis revision may be required, with uncertain clinical outcomes in relation to pain relief (16). Our study is aimed at providing an experts’ statement addressing the issue of definition (including possible causes), diagnosis, prevention, and treatment of persistent pain after total hip or knee arthroplasty. We have preliminary performed a systematic review both on Pumbed and Embase databases up to December 2012. There were 12 articles explicitly defining the condition of persistent pain after total hip or knee arthroplasty as “painful prosthesis” from 1975 to 2012 but 4 of these articles were published in this latter year. Total article generically addressing this issue were 1,559 (82 of which being review articles). By limiting the search to the articles specifically addressing the problem of pain following the implant of hip or knee prosthesis, we found 301 articles including 10 reviews on Pubmed – searching for “Prostheses and Implants”[Majr] AND “Pain”[Majr] AND (knee OR hip) – and 210 on Embase database – searching for ‘prosthesis’/exp AND ‘pain’/exp AND (knee OR hip) AND [embase]/lim. The experts have analyzed the available literature and the major topics concerning persistent pain after hip or knee arthroplasty.
Analysis of predictors in patients with severe osteoarthritis
Recent medical literature has identified some clinical factors as predictors of final outcome following TJA and possible onset of persistent post-surgical pain. Assessing predictors of clinical outcomes after TJA is becoming a critical issue, as a recent study by Judge et al. (the Eurohip study), carried out on 1,327 patients receiving primary THA for osteoarthritis (OA) across 20 European orthopedic centers, has estimated that such a relevant percentage of patients, that is between 14% and 36%, do not improve at 12 months after total hip arthroplasty (18). Similar evidence is available for a cohort of more than 8,000 OA patients one year after TKA (19). About 18% of operated patients declared they were not satisfied with the outcome of TKA at 12 months, with patients who reported higher scores concerning their pain assessment and functional evaluation being associated with worse post-operative satisfaction (19).
Age, musculoskeletal comorbidities, and preoperative pain
A specific study carried out by Nilsdotter et al. in 2003 (20) has assessed physical function of 339 patients undergoing THA both pre-operatively and post-operatively, by using the 36-Item Short-Form Health Survey (SF-36) and Western Ontario and McMaster Universities (WOMAC) questionnaires. In this study, age, sex, body mass index (BMI), presence of comorbidities (i.e. heart, peripheral arteries or lung, diseases, hypertension, diabetes, neurological problems, cancer, ulcer, kidney diseases, vision impairment, low back pain, and psychiatric disorders), widespread pain or pain at controlateral hip, the need of walking assistance, walking distance, and living alone, were evaluated both pre- and post-operatively and tested as potential predictors of post-operative outcomes. As a result, older age and higher preoperative higher scores in pain evaluation were shown to be predictors of a poor clinical outcome following THA. Moreover, patients with musculoskeletal comorbidities, such as low back pain and OA affecting the non-operated hip joint, were found to have less long term functional improvement after THA. Both low back pain and post-operative complications were associated with worse outcome. Notably, post-operative presence of low back pain was the only finding significantly associated with a non successful result in a multivariate analysis (20). The number of comorbidities preoperatively reported did not predict a worse post-operative outcome when assessed both by the WOMAC function and the by SF-36 PF (physical function) questionnaires (20). However, a better gradient with a lower number of comorbidities was reasonably presented. Low back pain and pain in the hip not operated on were characteristic of patients who did not reach the same level of function post-operatively as the age matched control group (20). According to a Canadian study carried out on 454 patients undergoing THA primary total hip arthroplasty (n = 197) or TKA (n = 257) who were evaluated within a month prior to surgery and 6 months post-operatively, age alone should not be considered a factor that affects the outcome of joint arthroplasty and should not be a limiting criterion when considering who should undergo TJA (21). Similar findings were provided by a study performed on 174 patients (mean age 75 years old), concluding that elderly patients undergoing THA or TKA for severe OA experienced excellent long-term outcomes (22). It must be pointed out that the latter evidence was provided by using the same evaluation tools for pain, function, and health-related quality of life (i.e. WOMAC, SF-36) (21, 22).
Time to surgery and preoperative disability
An emerging body of evidence has suggested that patients affected by symptomatic severe osteoarthritis may experience higher pain level and worse function the longer they wait for joint arthroplasty (23–26). Specifically, it seems that a wait time exceeding 1 year between first indication to surgery and surgical intervention is associated with worse clinical outcomes after TJA (23–26). It remains controversial whether long waiting lists may cause a worsening in pain or function of patients eligible for surgery. In fact, a recent metanalysis reported no deterioration concerning pain or self-reported functional status in OA patients waiting <180 days before receiving TJA (27), while other evidence suggested that waiting lists worsen both pain and function as measured by visual analogic scale (VAS), SF-36 and WOMAC, respectively (28).
According to a recent study carried out by Vergara et al. (29), long wait times are not free from adverse effects and might have irreversible consequences on clinical outcomes of surgery. Longer waiting time to surgery is possibly due to patient hesitation or suboptimal management of waiting lists for joint arthroplasty. In the latter case, the adoption of efficient procedures in the management of waiting lists – based only on pain and function level as selection criteria for prioritizing patients to surgery – has been shown to improve clinical outcomes after TJA (29). Time to surgery in OA patients undergoing joint arthroplasty is also a valuable marker of quality of care and system equity in terms of citizens’ access to health care services (30). Some authors have reported a mean waiting time to surgery of 6 months in European countries, although considerable differences between different nations and within the same country have been described (31). To explain the observed variations in wait times for elective surgery in OA patients, several major (demand, quality of life, pain, and disability) and minor (age, comorbidities, and other social variables such as the presence of caregivers) factors have been considered (26, 32, 33). However, opain and impaired function represent the most relevant criteria in the prioritization process for joint arthroplasty (23–29). All the variations in the waiting times observed between different hospitals are due to different management procedures of waiting lists or a complete absence of prioritization protocols, so that surgeons are allowed to arbitrarily use discretional selection criteria in patients eligible for TJA (33). A study performed by Quintana et al. (34) has pointed out the risks of not having clear and explicit criteria to prioritize patients eligible to surgery, which also determine inequality in the access to healthcare services.
The role of mechanical stress following prosthesis implant
Mechanical factors, both those intrinsically related to prosthesis design or material, and those related to the implant in the bone, obviously play an important role in the onset of post-surgical pain. First generation hip prostheses presented a metal femoral implant articulating with a polyethylene acetabular cup that resulted in survival rates of only 34% at 10 years (35, 36). Failures were usually due to implant loosening secondary to osteolysis. This process was triggered by major volumetric wear of the polyethylene component articulating with the first generation femoral head (35–37). Relevant improvements have been achieved after the introduction of new bearing couples and thanks to the availability of new industrial machines for the production of better prostheses (38), with survival rates of last generation implants ranging from 80 to 95% at 15 years (39–41). Revision rates after TJA have been computed to be 6% at 5 years and 12% at 10 years both for hip and knee arthroplasties (42). Early revision surgery after total hip replacement is frequently associated with instability or loosening of the acetabular and femoral components (38, 43). Mechanical loosening of the prosthesis may also be associated with malalignment of the femoral implant, which is believed to increase shear forces at the bone-implant interface (35, 44, 45). Various physiopathological hypotheses (mechanical, vascular, biological) have been proposed to explain such phenomenon (46, 47). In general, the implantation of foreign materials in the human body results in several modifications and adaptations within the host tissue. Type and extent of these modifications depend on different factors: biocompatibility of the material, interference with the biomechanical characteristics of the host tissue, fragments of components from the implanted material, quality of the host tissue, local and general reactivity. Therefore the bone surrounding a prosthetic implant, both uncemented and cemented, normally experiences a progressive quantitative reduction (bone loss) as a result of two main factors: stress shielding and wear debris production (40).
Stress shielding is a physical phenomenon occurring when a hip-prosthesis is implanted into the bone tissue, so that loading axes (stresses) are bypassed by the prosthesis implant, thus discharging bone from weight bearing (40). The prosthesis shields bone from mechanical stresses that are necessary for maintenance of normal bone structure. When the bone tissue surrounding the implant is not subject to anabolic strain stimulus, bone is reabsorbed through an adaptive bone remodeling process mediated by the osteocytes (40). Under these conditions the implant will no longer hold and it slips out. Peri-prosthetic bone loss caused by stress shielding, which is more frequent in greater size, rigid and cemented implants, may be associated with aseptic loosening of femoral components (40, 48). The success of a total hip arthroplasty is strongly related to the initial stability of the femoral component and to the stress shielding effect. Inefficient primary stability is also a cause of thigh pain. In addition, bone adaptation after the surgery can lead to an excessive bone loss and, consequently, can compromise the success of the implant. However, prosthesis shape, design, material, and interface influence stress shielding and post-operative bone adaptation, so that optimization of implant performance and geometry may be useful in order to reduce the need for revisions and post-operative discomfort or pain (48–50). Poor quality of intertrochanteric cancellous bone does not seem as crucial as previously thought in influencing the risk of implant migration (51). It must also be taken into account that attrition of the prosthetic surfaces leads to the formation of wear debris, which may trigger osteolysis and finally result in the aseptic loosening of the implant. This debris is made of polyethylene particles originating from the acetabular cup of the prosthesis and causes a flogistic response leading to the production of inflammation mediators including cytokines (40). This activation enhances osteoclast recruitment and activity next to bone-implant interfaces, thus causing osteolysis and loosening of the implant. The presence of debris particles is not sufficient to trigger a foreign body reaction, which ultimately occurs when there is enough mobility of the prosthetic implant to increase the “effective articular space”, thus enabling the migration of the particles in the interface between bone and prosthesis. Therefore, periprosthetic osteolysis is determined by the combined action of an increase in bone resorption (directly induced by debris or through an inflammation process), and a reduced bone formation caused by a depression of osteoblastic activity as a result of debris direct toxicity (40).
Beyond direct mechanical causes, the role of biological factors and inflammation must be properly considered. In fact, the trauma resulting from the implant insertion into bone may trigger inflammatory response and lead to an activation of several cells, including osteoclasts, macrophages and angiogenic cells (46, 52). It has also been suggested that the apoptosis of osteocytes occurring in the area of the implant may foster the activation of osteoclasts, thus possibly resulting in alteration of the balance between bone resorption and formation (46). Once the resorption process preponderates, an impairment of early fixation might occur shortly after surgical intervention (46). On this basis, it has been suggested that post-operative pharmacological treatment with antiresorptive drugs may be useful in preventing periprosthetic bone loss, thus reducing the risk of implant migration (40). Several studies demonstrated that different antiresorptive drugs (i.e. ibandronate, alendronate, risedronate, zoledronic acid) can modulate periprosthetic bone loss related to osteoclastic activity enhanced by cytokines produced during flogistic response to wear debris (47, 52–59). Attempts to investigate the effect of weak antiresorptive – as strontium ranelate –periprosthetic bone loss are controversial (60). Dual-energy X-ray absorptiometry (DXA) can provide a surrogate measure of load redistribution of the prosthetic components after TJA and may be useful in monitoring the efficacy of pharmacological therapy to reduce the periprosthetic bone loss (40, 61). In this case, DXA is performed by using specific software algorithms for the evaluation of the bone around the implant. This technique provides information about BMD measured around the seven Gruen zones, with good reproducibility of the measurements (coefficients of variation range: 1.8–7.5%). It might be also useful to perform a pre-operative DXA analysis to support the choice of implant components (61).
Similar evidence is also available for the loosening of knee prostheses (55). The risk of late loosening of cemented knee prostheses is related to early fixation, which is defined as migration during the first and second year, as measured by radiostereometric analysis (RSA) (55, 62). Early fixation was considered as a purely mechanical phenomenon, but it is now clear that osteocytes next to the implant undergo post-operative death because of surgical trauma and circulatory disturbance (55). This leads to increased bone resorption at the interface, and reduced quality of the fixation (63). A fracture repair response to the trauma resulting in an increased bone formation has also been documented (55). Therefore, early knee implant fixation also depends on the balance between bone formation and resorption, and the first six months after surgical intervention seem to represent the most critical period for implant migration (64). In this perspective, the use of antiresorptive agents may be proposed in order to achieve a positive balance between bone formation and resorption, thus leading to a better early fixation of the knee prosthesis. In contrast with the normal bone remodeling process (where bone resorption and formation are coupled), in implant fixation bone resorption and formation are uncoupled processes, so that decreased resorption resulting from the action of antiresorptive agents do not hamper bone formation, thus allowing the achievement of a positive bone balance in case of pharmacological treatment (55). This net gain in bone balance has been shown in several animal models (65, 66), including those of early joint implant fixation. In these models, an anabolic effect has been recognized to be more important for strength of fixation than for preservation of pre-existing bone (67). Thus, it seems that patients at increased risk of implant loosening, such as young and/or very active people, could particularly benefit from possible improvements in implant fixation that can be achieved thanks to the administration of antiresorptive agents, and an increasing body of evidence seems to confirm the hypothesis that early implant migration involves osteoclasts activity (40, 55, 68, 69). Currently available data on bisphosphonates also show that post-operative oral treatment or peri-operative local application of antiresorptive agents in patients undergoing joint arthroplasty may have a measurable effect on long term mechanics of TJA, and can be useful in reducing implant migration (55).
Prosthesis osseointegration
Cementless or hybrid total joint prostheses currently represent the standard implants in many orthopedic centers. Press-fit insertion makes a cementless component stable immediately upon implantation, but secondary stability and long term survival of joint prostheses depend on osseointegration, which is defined as a direct structural and functional connection between ordered living bone and the surface of a load-carrying implant without intervening fibrous tissue (70). Osseointegration is achieved by the ingrowth of bone into the surface of the implant, and porous surfaces of prostheses could enhance this process. Preservation of intertrochanteric cancellous bone during surgical intervention seems not to significantly affect osseointegration of cementless stems (51). Titanium or titanium-alloy implants are the most biocompatible among the different materials investigated (71). Tissue integration requires the adherence and proliferation of cells on the surface of the implant, which can be further improved by coating it with calcium hydroxyapatite (HA), the most common constituent of natural bone mineral (71). Thanks to its osseoconductive properties, HA can support the ingrowth of capillaries, perivascular tissues and bone forming cells from the host into the structure of the implant (72). It has been shown that bone matrix and cells are damaged following the insertion of implants into bone, and that an inflammatory response is consequently triggered (73). This inflammatory response could foster bone resorption around the implant. Furthermore, it is known that disruption of the microcirculation and damage occur to the bone matrix resulting in osteocyte death around the trauma zone (73). Micro-cracks in bone matrix occurring upon surgical trauma lead to osteocyte apoptosis, which is supposed to start the resorptive process (74). As already observed, this remodeling process does not seem to involve the normal coupling between osteoclastic and osteoblastic activity, and there is a real risk for bone resorption around the implant (75). This may result in a weakening and potential early loss of fixation, and consequently in the loosening of the implant (76–79). An effective pharmacological strategy aimed to improve initial fixation and osseointegration of the implant would be particularly valuable. The modulation of the initial bone remodeling response towards increased net bone formation around the implant may represent a possible approach to accomplish this objective (75).
Many studies have addressed the issue of improving the osseointegration of joint implants thanks to different therapeutic approaches (55, 68, 69, 79–87). Because growth factors may potentially promote bony ingrowth (80), some experimental studies have been successfully carried out in order to determine whether TGF-h1 (human transforming growth factor-h1) and BMP-2 (human bone morphogenetic protein-2) are able increase osseointegration, thereby suggesting that early stability of joint implants can be improved with the use of these factors (80, 81). Also, antiresorptive agents may potentially enhance osseointegration of joint implants, because they are able to impair osteoclast-mediated bone resorption, with minimal inhibition of osteoblast activity (52, 82) and they also have some direct proliferative effects on osteoblasts (83). Bisphosphonates provide robust clinical efficacy in the management of osteopenia and osteoporosis by normalizing increased and negatively balanced bone turnover (84). As pre-clinical and clinical evidence suggests that osteoporosis may impair osseointegration (85, 86), antiresorptive agents may be worthy of further investigation for the enhancement of implant osseointegration in patients with low bone mass. This is of particular interest because the majority of patients undergoing total hip replacement are elderly people and, therefore, many of them may present or will develop osteopenia or osteoporosis (87). Also, mineralization defects (i.e. osteomalacia) may impair osseointegration, and induce prosthesis drift or loosening of the components. This has been confirmed by studies carried out both in animal models (88) and in patients with osteoarthritis undergoing total hip replacement (89), and therefore vitamin D deficiency should always be considered as a possible risk factor – which can be easily corrected – for a suboptimal outcome after TJA.
Different bisphosphonates have shown good results on prosthesis osseointegration both in animal models (52–57, 65–67, 90–92), and in vivo(55, 59, 68, 69, 93–98). Bisphosphonates act on osteoclasts and inhibit resorption, but the mechanism of action differs between bisphosphonates containing amino groups and those which do not (53–57). The amino-containing bisphosphonates interfere with the mevalonate pathway and thus prevent prenylation of down-stream enzymes, such as Ras and Rho, vital for cellular function. The non-amino-containing bisphosphonates are metabolized to non-hydrolyzable analogues of ATP and thus interfere with the cells’ ATP-dependent intracellular enzymes (90, 99). Both these mechanisms result in the impairment of function and finally in apoptosis of mature osteoclasts, in addition to a reduced recruitment of these cells (90, 94). There is additional evidence suggesting that some aminobisphosphonates may also inhibit osteocyte and osteoblast apoptosis (100), induce osteoblast proliferation and differentiation, and stimulate osteoprotegerin (OPG) production (101). Finally, bisphosphonates may also inhibit migration and promote apoptosis of inflammatory cells, primarily of the monocyte lineage (101, 102). Despite the fact that bisphosphonates are mainly used in clinical practice as anti-resorptive agents for the treatment of post-menopausal or secondary osteoporosis, they have been tested in clinical trials for several indications, and they are finding a role in many clinical areas ranging from rheumatology to oncology. Animal model (rats) evidence is available concerning the systemic use of ibandronate in modulating bone turn over at implantation sites of screws or pins used to stabilize fractures, resulting in the improvement of early fixation of implants (through dose-dependent effects on osseointegration) (52), although at dosages corresponding to those needed to treat patients with tumor disease. Lower doses equivalent to those for treatment of osteoporosis showed no beneficial effects in animals (53). Local applications of ibandronate (55) and alendronate (56), or systemic zolendronate at doses comparable to those used for the treatment of osteoporosis (57), showed a positive effect on osseointegration.
It has been shown that in implantation sites, apoptosis of osteocytes occurs around the inserted implant (50), and bone remodeling due to micro-cracks takes place in association with osteocytes apoptosis (103). Therefore, osteocytes death should lead to osteoclasts activation, together with resorption of the bone immediately adjacent to an inserted implant. Peri-operative and early post-operative factors influence the long-term survival of joint implants. Early migration has been shown to be a predicting factor for the survival of implants, and bisphosphonates have been proven to reduce prosthesis migration during the first post-operative year, thus confirming that early bone remodeling events play a crucial role in subsequent implant loosening (45, 58, 104–105). As bisphosphonates may strongly bind to bone in vivo and are generally safe, the major problem in their use for the prevention of implant loosening consists in the poor bioavailability of these drugs. Thus, the means of administration (oral, s.c., intra-operatory) seems to play a crucial role. This problem seems to be overcome by the recent introduction of bisphosphonates local administration in surgical practice (55, 105).
A recent double-blind randomized trial has investigated potential benefits of peri-operative application (1 minute before implant cementation) of 1 mg ibandronate directly to the tibial bone surface vs placebo (saline), finding a reduction in implant migration rate (measured by RSA) after 6, 12 and 24 months (55). Positive histological effects of local treatment with alendronate, with an observed increase in bone formation, have been reported (105). Post-operative oral administration of alendronate was proven to be active in reducing periprosthetic bone loss with persistence of the effect for two years (106). In fact, Arabmotlagh et al. have demonstrated (by using DXA measures) that patients undergoing total hip replacement experience a beneficial effect persisting at six years after surgery (with no significant changes in periprosthetic femoral BMD) when oral alendronate (10 mg/day for 10 weeks or 20 mg/day for 5 weeks) is administered (107). An improvement in the fixation of the tibial component of a total knee prosthesis has also been reported in a study with oral daily administration of clodronate for the first 6 months after TJA, although these data are quite controversial (68). In this latter study, authors documented a 25% reduction in implant migration rate (measured by RSA) at 6 months, with significant differences between treatment vs placebo groups persisting up to 4 years of follow-up (68). Also, data concerning the effect of strontium ranelate on bone-implant interface are controversial (60). Yamasaki et al. have evaluated the effects of risedronate on periprosthetic bone loss after cementless hip arthroplasty, finding that post-operative BMD reduction in the risedronate group was significantly lower than that of the placebo group at 6 months (93). These results suggest that post-operative treatment with bisphosphonate results in a long-standing beneficial effect for the prevention of femoral and knee periprosthetic bone loss following TJA. Although limited data are currently available on this topic, an alternative prophylactic approach to reduce periprosthetic bone loss might be the use of anabolic agents, which could enhance osseointegration by increasing bone formation around the implant (including teriparatide, parathyroid hormone, and strontium ranelate) (40).
Potential role of bisphosphonates in pain relief
In recent decades, bisphosphonates have been widely used in the management of pain for patients affected by metastatic cancer or severe osteoporosis (108). Before their use in clinical settings, several studies had shown that first and second-generation bisphosphonates, such as alendronate, clodronate and pamidronate, have analgesic effects in experimental animal models (109–110). Some data are also available on anti-nociceptive properties of the third-generation bisphosphonate zoledronic acid in rodents (111). However, very few studies are available on bisphosphonates’ mechanisms of action in pain relief. The mechanism by which bisphosphonates reduce pain is largely unknown. In fact, their analgesic efficacy is not fully explained by the main effect of all the drugs belonging to this pharmacological family, which consists in decreasing bone resorption by inhibiting osteoclast function (111–116). As local acidosis is a well-known cause of pain (117), a recent study performed by Yoneda et al. (118), has investigated the relationship between bone pain and acidic conditions due to proton release following local inflammation processes. In contrast with a previous theory according to which nociceptive sensory neurons would not innervate bone, this study also showed that specific sensory nociceptive neurons innervate mineralized bone and bone marrow, and can be directly involved in causing bone pain (107–119). These neurons express acid-sensing nociceptors (ASNs) such as the acid-sensing ion channels and transient receptor potential channel-vanilloid subfamily members (117, 118). Local acidosis is caused by bacterial activity, but also by bone-resorbing osteoclasts and inflammatory cells through the release of protons, responsible for the acidic bone microenvironment (120–122). Thus, acid signals received by ASNs subsequently activate intracellular signaling pathways and transcription factors in sensory neurons. Therefore, inflammation following prosthesis implant may have an important role in explaining both persistent post-operative bone pain, and the rationale of antiresorptive agent use.
A specific study (123) has recently been conducted in order to investigate the analgesic effect of amino-bisphosphonates, which are known to inhibit osteoclast-mediated bone resorption in women with postmenopausal osteoporosis, and reduce pain in patients with metastatic bone disease (116, 123–128). Some authors have documented a rapid appearance of pain relief after the administration of bisphosphonates in patients with bone disease from breast cancer, thus suggesting a possible dissociation between the analgesic and the metabolic effects of the drug (129). Although a potential anti-inflammatory action of bisphosphonates remains controversial (130), some anti-inflammatory effects of different molecules belonging to this class have been investigated in several studies (131–133). A fundamental study carried out by Bianchi et al. has shown that bisphosphonates are able to persistently reduce inflammatory edema and hyperalgesia in animal models of persistent pain with long lasting effects (still evident one week later) emerging three days after the administration of a single dose of the drug (ibandronate1 mg/kg) in rats (116). It is important to point out that the dosage used in the study was not able to cause clinically relevant nephrotoxicity after i.v. administration in the rat (134).
In addition to the potential role of local acidosis due to osteoclast activity and inflammation (118), which represents well known conditions occurring after TJA, experimental evidence suggests an important involvement of neuropeptide SP in the analgesic (namely anti-hyperalgesic) effect of bisphosphonates (116). In fact, peripheral inflammatory pain is associated with a complex pattern of local changes, as many pro-nociceptive and pro-inflammatory mediators are activated following tissue injury (116). These mediators lower nociceptive thresholds and increase neuronal membrane excitability, leading to hypernociception (135). Among these, the neuropeptide SP – synthesized in Dorsal Root Ganglia (DRG) –seems to have an important role (109). In the spinal cord, neuropeptide SP exerts excitatory action on dorsal horn neurons, thus determining an increased nociception sensation (135). Moreover, neuropeptide SP is released antidromically in the inflamed tissue, where it contributes to sustain so-called neurogenic inflammation (135, 136). In the peripheral nervous system, neuropeptide SP is able to increase the susceptibility of afferent fibers to nociceptive stimuli (136). Bianchi et al. have proven that administration of amino-bisphosphonates in animal models is able to completely abolish the increase in SP synthesis and release (116). Findings in animals also suggest that the decrease in neuropeptide SP release may have an impact on the production of IL-1 and TNF (116), which can either directly sensitize nociceptors, or induce the release of other pro-inflammatory and pro-nociceptive mediators (137–144). Interestingly, IL-1 has been reported to regulate neuropeptide SP production in DRG neurons, thus confirming that a positive loop between neuropeptide SP and cytokines is active in the maintenance and perpetration of inflammatory hyperalgesia (138).
This observation is important as it has been suggested that some bisphosphonates may directly modulate the production of cytokines from monocyte/macrophages, either increasing (145–148) or decreasing them (149, 150). Although a direct effect of bisphosphonates on cytokine production cannot be excluded, it is important to underline that no effect on cytokine production has been observed in absence of an inflammatory state (116). In experimental models, administration of bisphosphonates did not affect PGE-2 concentrations (116), thus confirming that these molecules do not interact with cyclooxygenase (both COX-1 and COX-2) enzyme activity (149). The mechanisms by which bisphosphonates might induce a decrease in neuropeptide SP production is still unknown. It has been hypothesized that the activation of osteoclasts in the frame of the inflammation process plays a role in nociceptor sensitization (150). This theory might coexists with the above discussed hypothesis based on the ability of osteoclasts localized in inflamed tissue to secrete protons (H+), so that cellular microenvironment may become acidic (118). In fact, it is well known that two classes of acid-sensing nociceptors are present in sensory neurons: the acid-sensing ionic channels (ASICS) and the transient receptor potential channel vanilloid member (TRPV1) (151). This latter can be activated directly by hydrogen ions (H+), and as a consequence its activation promotes inflammation mediated by neuropeptide SP in animal models (152). It has therefore been hypothesized that the inhibition of osteoclast activity induced by bisphosphonate administration might prevent them from secreting protons, and consequently reduce the activation of specific ionic channels, ultimately resulting in a reduced production of neuropeptide SP by primary afferents (116).
The role of the nervous system in regulating bone biology has only recently been explored. However, an emerging number of anatomical and physiological evidences confirms the presence of sensory SP containing nerve in the bone (153–155). It has also been suggested that the rich innervation of periosteum by SP positive fibers may explain the role of this peptide in bone nociception. Several pro-inflammatory mediators, including TNF and IL-1, have been identified to exert a very important role in the development of hyperalgesia (156–157). Finally, Nagae et al. have shown that osteoclastic bone resorption is associated with an inflammatory state adjacent to bone (158), a finding which is particularly important after the recent acquisitions on bone sensory nociceptive innervations (ASNs) and the role of SP mediator (116, 118). Considering all these data on the involvement of osteoclasts and inflammation processes in determining bone pain, the ability of bisphosphonates to reduce inflammatory hyperalgesia, and to inhibit the mechanisms activated by mediators of inflammation, may contribute to explaining the reduction of pain observed during the treatment course with these drugs in patients affected by osteoporosis or in cancer pain associated with metastatic bone disease, and other conditions (116, 118). It is important to remember that bisphosphonates accumulate in the bone after repeated dosing, causing high drug concentrations in bone, and that part of the bone-bound drug is released during bone turnover (116, 159, 160). Bone concentration of bisphosphonates may therefore have a relevant impact on their anti-nociceptive action (116).
Expert guidance
Persistent post-surgical pain is an under-acknowledged condition, and can be severe in about 2–10% of all patients undergoing different types of major surgery (161). Since iatrogenic neuropathic pain is thought to be one of the most important causes of long-term postsurgical pain, surgical techniques that avoid nerve damage are applied whenever possible (161). However, only a minority of patients with intra-operative nerve damage develop chronic pain, thus suggesting the possible crucial role of individual genetic factors in developing persistent post-operative pain (161). In general, early administration of aggressive therapy for post-operative pain should be encouraged, since the intensity of acute post-operative pain correlates with the risk of developing a persistent pain state, probably by triggering nociceptive paths that remain permanently activated (161). Post-operative persistent pain is most commonly described as aching, tender, and tiring (15). Neuropathic origin is estimated to account for only about 1–6% of patients with painful prosthesis, while an even smaller proportion of subjects reporting severe persistent pain after TJA are more often affected by depressive symptoms (15). However, it has also been postulated that patients with persistent post-operative pain may have an underlying vulnerability to pain (15), as major depression and sufferance of different origins have been found to be significant, as well as independent post-operative determinants of persistent post-surgical pain (15). Some authors suggest that chronic pain following recovery from TKA is also influenced by psychosocial factors, including an individual’s pain-related illness cognitions, personal beliefs, and patient’s perception of his condition within the social context (162). Although the degree of pain is usually mild and an improvement in pain level vs pre-operative condition is achieved, patients undergoing TJA actually look forward to completely resolving their painful symptoms (15). This is the reason why long term persistence of pain after TJA represents a serious problem both for the patient and for the surgeon who has performed the arthroplasty.
Considering current clinical evidence and the IASP guidelines (12), authors of the present paper recommend that diagnosis of persistent pain should be made in case of post-operative pain following TJA – reported by the patient as VAS ≥3 – persisting for at least 4 months after surgery, or new onset of pain after the first 4 months after surgery (self-reported as VAS ≥3) lasting ≥2 months. Regardless of the duration of the painful symptoms, any episode of acute pain reported as VAS score ≥7 in patients who underwent TJA must be immediately investigated in order to find the origin of the pain. Early administration of drugs for post-operative pain is strongly recommended. Visual Analogic Scale (VAS) for pain evaluation should be systematically administered pre-operatively, in order to assess baseline pain of the patients, but also in all post-operative control points (both at hospital and in ambulatorial settings), at least after 3, 6, and 12 months. Once persistent post-operative pain has been diagnosed, we suggest approaching the patient according to the steps reported in the proposed algorithm (Table 1). We are aware that algorithms represent a reductionist approach, which are assumed to be important in current medical practice, and that greater attention should be paid to the totality of the patient, his environment, family and social milieu (163).
Table 1.
Proposed algorithm summarizing diagnostic and therapeutic approach to the patient with persistent pain after hip or knee arthroplasty.
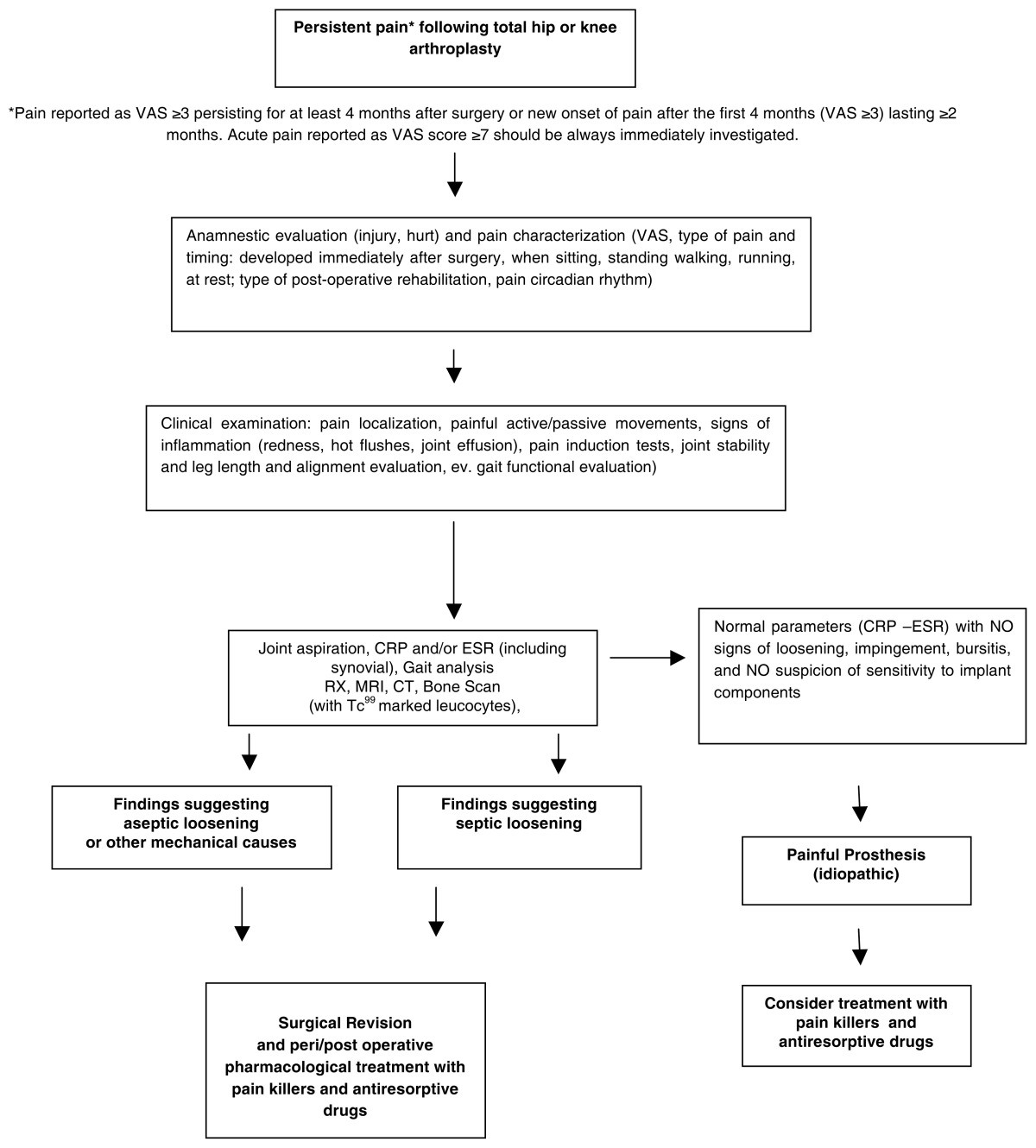
We have identified different categories of possible causes determining persistent pain after TJA (Table 2). The condition of “painful prosthesis” is defined when no specific causes for persistent post-operative pain can be identified (idiopathic origin). The cause of the pain should always be investigated to define the most appropriate surgical or pharmacological treatment. A complete anamnestic evaluation is aimed at excluding injury or harm. Quality and intensity of pain must be characterized. Type of pain and timing needs to be investigated. Patients must be asked if pain developed immediately after surgery, and if it occurs when sitting, standing, walking, running, or at rest. Type of post-operative rehabilitation followed by the patient and pain circadian rhythm should also be evaluated. Individual baropodometric assessment may be useful to investigate the role of pre-existing postural attitudes or changes if these measurements are available both before and after the intervention. Clinical exam and traditional radiology are usually able to confirm prosthesis instability requiring revision surgery. Newest MRI application, known as “patient specific instruments”, may be helpful in individual tailoring of prosthesis implant. Ultrasounds, computed tomography (CT), and magnetic resonance imaging (MRI) may disclose hidden hematomas. Specific neuro-electro-physiological exams may be useful for the detection of lesions to peripheral nerves following surgery or incorrect rehabilitative exercises. The cause of persistent pain should be removed whenever possible.
Table 2.
Causes of persistent pain after joint arthroplasty.
| Non Infective Causes related to surgical intervention | Related to surgical technique and implant positioning | Surgical approach (anterior vs. lateral or postero-lateral) |
| Prosthesis components shape and size | ||
| Resurfacing arthroplasty | ||
| Primary instability | ||
| Revision arthroplasty | ||
| Painful scar | ||
| Periprosthetic fractures | ||
| Offset unbalance | ||
| Implant impingement | ||
| Leg length discrepancies | ||
| Implant breakage | ||
| Malalignment | ||
| Related to bone or immune reactions to the implant | Complex Pain Regional Syndrome (CPRS) | |
| Stress shielding determining bone resorption | ||
| Wear-induced osteolysis causing aseptic loosening | ||
| Sensitivity to implant components | ||
| Mineralization defect (i.e. osteomalacia) | ||
| Related to muscle conditions | Muscle spasms and contractures | |
| Muscle lesions (i.e. ileopsoas cist) | ||
| Impingement muscle-implant | ||
| Related to nerve conditions | Nerve lesions | |
| Nerve entrapment | ||
| Related to soft tissues conditions | Heterotopic ossification | |
| Bursitis | ||
| Pseudotumor | ||
| Septic causes | Early infections | Superficial or Deep soft tissue infections (including sinus and abscess) |
| Late Infections | Deep infections | |
| Other specific causes | Related to spine conditions | Radiculopathies |
| Neuropsichiatric conditions | Mood disorders | |
| Catastrophisizing patient | ||
| Idiopatic (“Painful Prosthesis”) | Undetermined origin | Unknown |
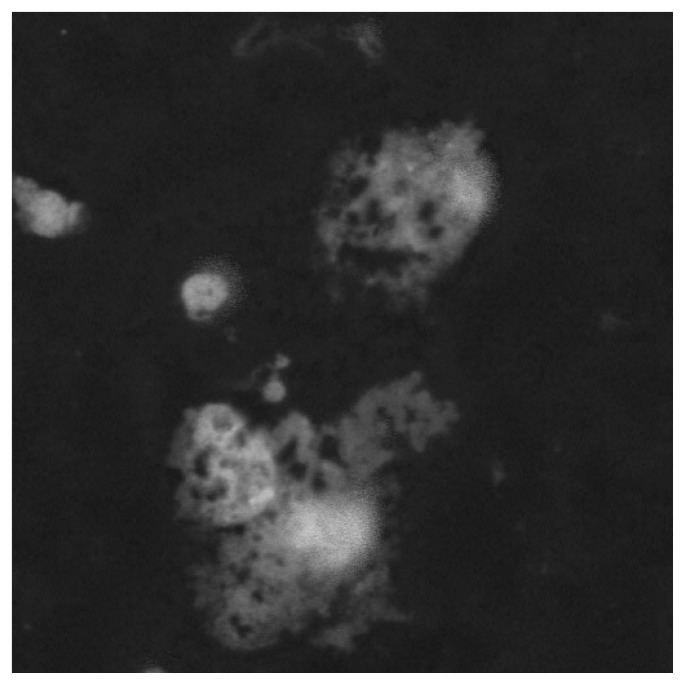
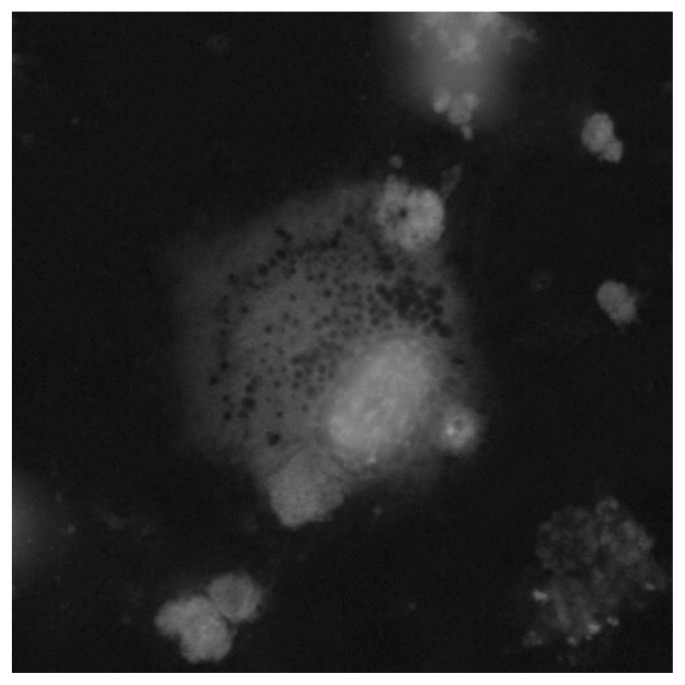
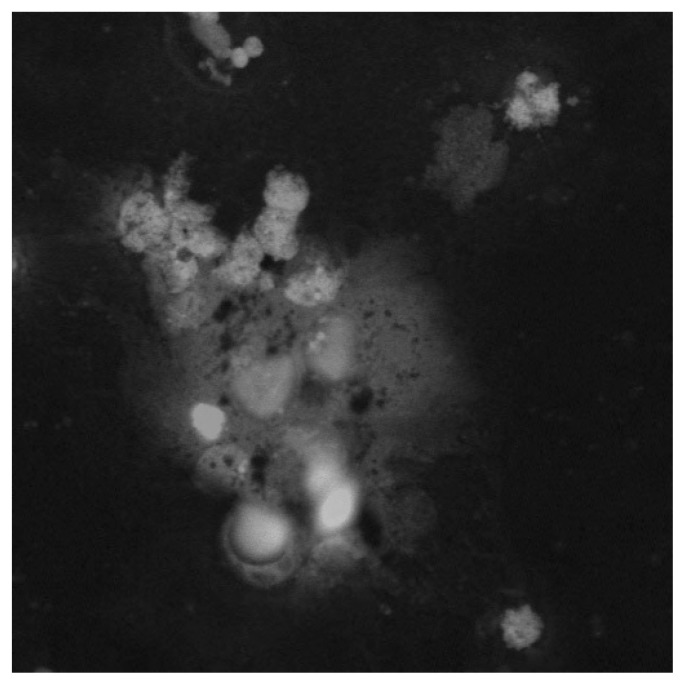
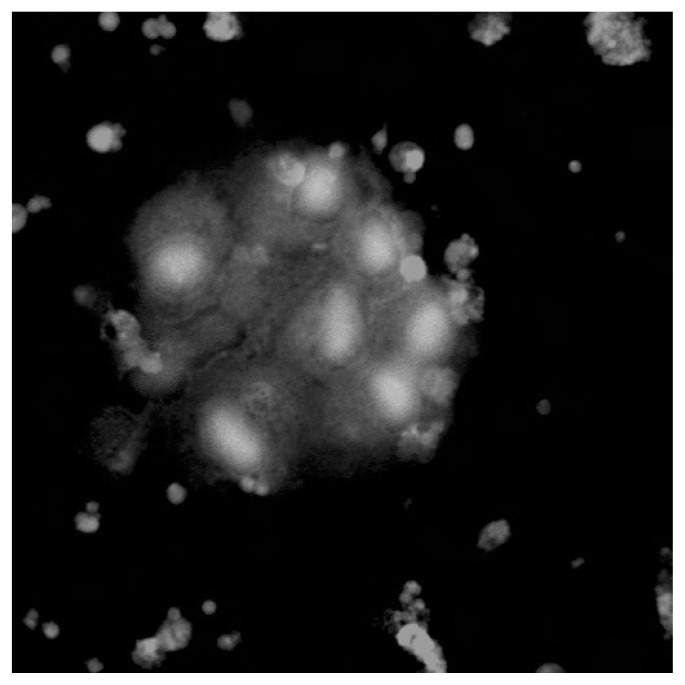
Infections or small infectious foci should be always suspected, although in some cases they may be too small for instrumental confirmation. Surgeons should be aware that specific categories of patients seem to be at higher risk of developing infections following total knee arthroplasty: diabetic and obese subjects, people suffering from rheumatoid arthritis, and those affected by hemophilic arthropaties (164). Patients whose clinical symptoms and history suggest the possibility of post-operative infection should undergo joint aspiration in case of abnormal values in ESR and/or PCR dosage. Differential diagnosis between post-operative infections (acute or delayed) and allergy should be always considered (165). Complementary Tc99 marked leucocytes bone scan has been shown to be more specific, accurate and sensitive (about 100%) than In111 marked scan alone in localizing infections (166, 167). Antibiotic therapy must be started only when the diagnosis of infection has been confirmed and possibly after the identification of the pathogen micro-organism on cultures. In this phase, strong cooperation between orthopedic surgeons, microbiologist and clinical laboratorists is very important. The duration of antibiotic treatment should be prolonged up to 6 weeks in case of early post-operative infections (less than 4 weeks from surgery) (168). A two stage revision intervention (i.e. prostheses of antibiotic loaded acrylic cement) after antibiotic therapy is recommended in case of systemic infections (blood dissemination) or delayed infections (>1 month from surgical intervention) (168–170). As allergic responses represent a relevant cause of inflammatory response, when possible, individual sensitivity to metal (especially to nickel and chrome) should be investigated (patch test, lymphocyte transformation test, dosage of cytokines produced by lymphocites following stimulation or incubation with metals). Figure 1 and Figure 2 show confocal microscopy images (a specific technique providing tridimensional image of 0.5 nm optical slides reconstructed by CT) of nickel- and chrome-stimulated mononucleate cells compared to normal situation. Titanium-stimulated cells usually show an appearance at confocal microscopy (Figure 3) which is more similar to the normal situation (Figure 4). Patch test positivity, high rate of cytokines production, metal inclusion in lymphocites, and cellular abnormalities at confocal microscopy are highly predictive of metal sensitivity.
Figure 1.
Confocal microscopy images of nickel stimulated mononucleate cells in peripheral blood showing disorganized cytoskeleton and peripheral nuclei which indicate cell damage.
Figure 2.
Confocal microscopy images of chrome-stimulated mononucleate cells in peripheral blood showing less abnormalities and cell damage compared to nickel- stimulated cells.
Figure 3.
Confocal microscopy images of titanium-stimulated cells in peripheral blood show a more normal appearance, with less disorganized cytoskeleton and peripheral nuclei.
Figure 4.
Normal situation: unstimulated cells in peripheral blood show a normal appearance.
The use of symptomatic drugs (i.e. FANS), analgesic treatments, antiresorptive or anabolic agents should be individually tailored on the basis of patient needs. Septic painful conditions would require proper antibiotic treatments, and in some cases specific microbiological exams should be required. Surgical revision of implants is indicated when aseptic or aseptic loosening is diagnosed. Idiopathic conditions (“painful prosthesis”) might benefit from pharmacological treatment with antiresorptive drugs. Bisphosponates resulted in significant post-intervention improvements in patients suffering from complex regional pain syndrome (CRPS) type I, also known as reflex sympathetic dystrophy, a condition that may occur after trauma or prolonged immobilization, characterized by focal pain and autonomic dysregulation, and trophic alterations (as those occurring in osteoporotic patients) (171–173). A lower amount of clinical data is available for anabolic therapies in patients undergoing TJA. As peri- and/or post-operative treatment with bisphosphonates are documented to have a role in pain management and prevention of periprosthetis bone loss after TJA (99–103), authors hyphotize the possible use of antiresorptive drugs in the treatment of idiopathic persistent pain (“painful prosthesis”), although specific double blind studies are needed. Peri-operative use of antiresorptive agents can also be taken into account when surgical revision of painful prosthesis is required, and in all patients eligible for joint arthroplasty who can be considered at higher risk of developing post-operative painful symptoms (i.e. OA patients with higher pain levels or function impairment who have waited too long before undergoing surgery; patients affected by rheumatoid arthritis). Post-operative treatment with antiresorptive or anabolic drugs should be continued for the first 6–12 months, as this is the time interval during which the risk of implant migration is highest. However, it must be pointed out once again that infections must be excluded before starting any therapy with bisphosphonates. In fact, the use of these drugs after primary THA was associated with an increased risk of revision due to deep infection in a recent study carried out by Thillemann et al. (174). In the same study, long-term use was associated with a reduced risk of revision of any type (174), thus underlining the efficacy of post-operative treatment with bisphosphonates when infective processes have been properly excluded.
In order to prevent post-operative pain related to mechanic factors or osseointegration, a DXA examination provided with specific software application for the evaluation of periprosthetic bone should be performed prior to surgery (to assess load redistribution after the implant and to support the choice of implant components) and after 12–18 months (for the evaluation of periprosthetic bone loss and stress shielding). Postoperative administration of antiresorptive drugs is also recommended in osteoporotic patients who are not being treated with any drugs. Surgical techniques, materials, and operative accesses must be carefully planned. The use of intra/peri-operative biologic modulators of osseointegration (i.e. platelet rich plasma, PRP and others) and antiresorptive agents can be useful, and therefore surgeons are encouraged to consider their use according to individual clinical needs. It must be kept in mind that surgical revision of painful prosthesis due to idiopathic persistent pain often leads to unpredictable results. Laskin et al. have reported a very high failure rate (about 80%) in painful knee revision case series when the cause of the pain was undetermined (16). On this basis, when approaching patients with painful prosthesis of undetermined origin, we recommend starting treatment with an effective antiresorptive agent (wait and see strategy), before considering the possibility of surgical revision if no improvement in pain symptoms (measured by VAS) are achieved after 12–18 months. In these cases, it may also be useful to carefully assess the rehabilitative exercises followed by the patient, as well as his/her postural characteristics, as they might influence load distribution. Finally, based on available evidence, in order to prevent persistent post-operative pain and poor functional outcomes, the authors recommend considering only pain level and disability in the decision-making process for prioritization of patients affected by symptomatic severe osteoarthritis requiring joint arthroplasty.
Acknowledgments
Authors are grateful to the staff of the Italian Society for Orthopedics and Medicine (OrtoMed) and the FIRMO foundation for research in the field of osteoporosis (Florence, Italy) for their support while carrying out this study.
Footnotes
Disclosures. This study has been funded by OrtoMed (Florence). Authors disclose the following conflicts of interest: Prisco Piscitelli has received consulting fees from Amgen-Dompè, Sanofi-Aventis, Eli-Lilly, Servier; Giovanni Iolascon has received consulting fees from Merck, Sharp and Dohme, Warner Chilcott; Massimo Innocenti has received consulting fees and research grants from Smith & Nephew, Biomet, Tornier; Roberto Civinini: no disclosures; Alessandro Rubinacci: no disclosures; Maurizio Muratore: no disclosures; Michele D’Arienzo: no disclosures; Paolo Tranquilli Leali: no disclosures; Annamaria Carossino: no disclosures; Maria Luisa Brandi has received consulting fees and research grants from Servier, Eli-Lilly, Novartis, Amgen, Merck, Sharp and Dohme.
References
- 1.D’Ambrosia RD. Epidemiology of osteoarthritis. Orthopedics. 2005;28(Suppl):s201–s205. doi: 10.3928/0147-7447-20050202-04. [DOI] [PubMed] [Google Scholar]
- 2.Woolf AD. The bone and joint decade 2000–2010. Annals of RheumaticDisease. 2000;59:81–2. doi: 10.1136/ard.59.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shane Anderson A, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010 Feb;24(1):15–26. doi: 10.1016/j.berh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iolascon G, Piscitelli P, Guida G, et al. Hip fractures in Italy, analysis of DRG data. Aging Clin Exp Res. 2007;19(Suppl. to No. 3):2–4. [PubMed] [Google Scholar]
- 5.Piscitelli P, Iolascon G, et al. Femoral fractures and orthopaedic surgery in Italy. J Orthop Traum. 2005;6:202–205. [Google Scholar]
- 6.Piscitelli P, Iolascon G, Brandi ML, et al. Hip Fractures in Italy: 2000–2005 extension study. Osteoporos Int. 2009 doi: 10.1007/s00198-009-1084-x. published online 7 October 2009. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor MI. Osteoarthritis of the hip and knee: sex and gender differences. Orthop Clin North Am. 2006;37:559–568. doi: 10.1016/j.ocl.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Adami S, Giannini S, Giorgino R, et al. The effect of age, weight, and lifestyle factors on calcaneal quantitative ultrasound: the ESOPO study. Osteoporos Int. 2003;14:198–207. doi: 10.1007/s00198-002-1352-5. [DOI] [PubMed] [Google Scholar]
- 9.Herberts P, Malchau H. Long-term registration has improved the quality of hip replacement: a review of the Swedish THR Register comparing 160,000 cases. Acta Orthop Scand. 2000;71:111–21. doi: 10.1080/000164700317413067. [DOI] [PubMed] [Google Scholar]
- 10.Bozic KJ, Saleh KJ, Rosenberg AG, Rubash HE. Economic evaluation in total hip arthroplasty: analysis and review of the literature. J Arthroplasty. 2004;19:180–189. doi: 10.1016/s0883-5403(03)00456-x. [DOI] [PubMed] [Google Scholar]
- 11.Nilsdotter AK, Lohmander LS. Age and waiting time as predictors of outcome after total hip replacement for osteoarthritis. Rheumatology (Oxford) 2002;41:1261–1267. doi: 10.1093/rheumatology/41.11.1261. [DOI] [PubMed] [Google Scholar]
- 12.IASP. Classification of chronic pain. Pain. 1986:S1–226. [PubMed] [Google Scholar]
- 13.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 14.Macrae WA. Chronic postsurgical pain: 10 years on. Br J Anaesth. 2008;101:77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 15.Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: Prevalence, sensory qualities, and post-operative determinants. Pain. 2011;152:566–572. doi: 10.1016/j.pain.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Laskin, et al. The painful knee. Orthopedics. 1999 Sep;22(9):869–870. doi: 10.3928/0147-7447-19990901-27. [DOI] [PubMed] [Google Scholar]
- 17.Martucci E, Savini R, Galletti S, Borghi A, Salvati EA. Painful prosthesis of the hip joint: diagnostic criteria. Chir Organi Mov. 1987 Jul-Sep;72(3):231–40. [PubMed] [Google Scholar]
- 18.Judge A, Cooper C, Williams S, Dreinhoefer K, Dieppe P. Patient-reported outcomes one year after primary hip replacement in a European Collaborative Cohort. Arthritis Care Res. 2010 Apr;62(4):480–8. doi: 10.1002/acr.20038. [DOI] [PubMed] [Google Scholar]
- 19.Baker PN, van der Meulen JH, Lewsey J, Gregg PJ. The role of pain and function in determining patient satisfaction after total knee replacement: data from the National Joint Registry for England and Wales. J Bone Joint Surg Br. 2007;89B:893–900. doi: 10.1302/0301-620X.89B7.19091. [DOI] [PubMed] [Google Scholar]
- 20.Nilsdotter AK, Petersson IF, Roos EM, Lohmander LS. Predictors of patient relevant outcome after total hip replacement for osteoarthritis: a prospective study. Ann Rheum Dis. 2003 Oct;62(10):923–30. doi: 10.1136/ard.62.10.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. The effect of age on pain, function, and quality of life after total hip and kneearthroplasty. Arch Intern Med. 2001;161:454–60. doi: 10.1001/archinte.161.3.454. [DOI] [PubMed] [Google Scholar]
- 22.Hamel MB, Toth M, Legedza A, Rosen MP. Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, post-operative recovery, and clinical outcomes. Arch Intern Med. 2008 Jul 14;168(13):1430–40. doi: 10.1001/archinte.168.13.1430. [DOI] [PubMed] [Google Scholar]
- 23.Fortin PR, Penrod JR, Clarke AE, St-Pierre Y, Joseph L, Belisle P, Liang MH, Ferland D, Phillips CB, Mahomed N, Tanzer M, Sledge C, Fossel AH, Katz JN. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46:3327–3330. doi: 10.1002/art.10631. [DOI] [PubMed] [Google Scholar]
- 24.Hajat S, Fitzpatrick R, Morris R, Reeves B, Rigge M, Williams O, Murray D, Gregg P. Does waiting for total hip replacement matter? Prospective cohort study. J Health Serv Res Policy. 2002;7:19–25. doi: 10.1258/1355819021927638. [DOI] [PubMed] [Google Scholar]
- 25.Ostendorf M, Buskens E, van Stel H, Schrijvers A, Marting L, Dhert W, Verbout A. Waiting for total hip arthroplasty: avoidable loss in quality time and preventable deterioration. J Arthroplasty. 2004;19:302–309. doi: 10.1016/j.arth.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Quintana JM, Escobar A, Arostegui I, Bilbao A, Azkarate J, Goenaga I, Arenaza JC. Health-related quality of life and appropriateness of knee or hip joint replacement. Arch Intern Med. 2006;166:200–226. doi: 10.1001/archinte.166.2.220. [DOI] [PubMed] [Google Scholar]
- 27.Hoogeboom TJ, van den Ende CH, van der Sluis G, Elings J, Dronkers JJ, Aiken AB, van Meeteren NL. The impact of waiting for total joint replacement on pain and functional status: a systematic review. Osteoarthritis Cartilage. 2009 Nov;17(11):1420–7. doi: 10.1016/j.joca.2009.05.008. Epub 2009 May 20. [DOI] [PubMed] [Google Scholar]
- 28.McHugh GA, Luker KA, Campbell M, Kay PR, Silman AJ. Pain, physical functioning and quality of life of individuals awaiting total joint replacement: a longitudinal study. J Eval Clin Pract. 2008 Feb;14(1):19–26. doi: 10.1111/j.1365-2753.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 29.Vergara I, Bilbao A, Gonzalez N, Escobar A, Quintana JM. Factors and Consequences of Waiting Times for Total Hip Arthroplasty. Clin Orthop Relat Res. 2011;469:1413–1420. doi: 10.1007/s11999-010-1753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Judge A, Welton NJ, Sandhu J, Ben-Shlomo Y. Equity in access to total joint replacement of the hip and knee in England: cross sectional study. BMJ. 2010 Aug 11;341:c4092. doi: 10.1136/bmj.c4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siciliani L, Hurst J. Tackling excessive waiting times for elective surgery: a comparative analysis of policies in 12 OECD countries. Health Policy. 2005;72:201–215. doi: 10.1016/j.healthpol.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Birrell F, Johnell O, Silman A. Projecting the need for hip replacement over the next three decades: influence of changing demography and threshold for surgery. Ann Rheum Dis. 1999;58:569–572. doi: 10.1136/ard.58.9.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright JG, Coyte P, Hawker G, Bombardier C, Cooke D, Heck D, Dittus R, Freund D. Variation in orthopedic surgeons’ perceptions of the indications for and outcomes of knee replacement. CMAJ. 1995;152:687–697. [PMC free article] [PubMed] [Google Scholar]
- 34.Quintana JM, Aróstegui I, Azkárate J, Goenaga JI, Elexpe X, Letona J, Arcelay A. Evaluation of explicit criteria for total hip joint replacement. J Clin Epidemiol. 2000;53:1200–1208. doi: 10.1016/s0895-4356(00)00244-4. [DOI] [PubMed] [Google Scholar]
- 35.Laffossea JM, Aubinb K, Lavigneb M, Royb A, Vendittoli PA. Radiographic changes of the femoral neck after total hip resurfacing. Orthopaedics & Traumatology: Surgery & Research. 2011;97:229–240. doi: 10.1016/j.otsr.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Yue EJ, Cabanela ME, Duffy GP, Heckman MG, O’Connor MI. Hip resurfacing arthroplasty: risk factors for failure over 25 years. Clin Orthop Relat Res. 2009;467:992–9. doi: 10.1007/s11999-008-0506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritter MA, Lutgring JD, Berend ME, Pierson JL. Failure mechanisms of total hip resurfacing: implications for the present. Clin Orthop Relat Res. 2006;453:110–4. doi: 10.1097/01.blo.0000238849.23744.8e. [DOI] [PubMed] [Google Scholar]
- 38.Amstutz HC, Ball ST, Le Duff MJ, Dorey FJ. Resurfacing THA for patients younger than 50 years: results of 2- to 9-year followup. Clin Orthop Relat Res. 2007;460:159–64. doi: 10.1097/BLO.0b013e318041f0e7. [DOI] [PubMed] [Google Scholar]
- 39.Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg (Br) 2004;86:177–84. doi: 10.1302/0301-620x.86b2.14600. [DOI] [PubMed] [Google Scholar]
- 40.Iolascon G, Di Pietro G, Capaldo A, Gioia C, Gatto S, Gimigliano F. Periprosthetic bone density as outcome of therapeutic response. Clin Cases Miner Bone Metab. 2010 Jan-Apr;7(1):27–31. [PMC free article] [PubMed] [Google Scholar]
- 41.Malchau H, Herberts P, Eisler T, et al. The Swedish Total Hip Replacement Register. J Bone Joint Surg Am. 2002;84-A(Suppl 2):2–20. doi: 10.2106/00004623-200200002-00002. [DOI] [PubMed] [Google Scholar]
- 42.Labek G, Thaler M, Janda W, Agreiter M, Stöckl B. Revision rates after total joint replacement: cumulative results from worldwide joint register datasets. J Bone Joint Surg Br. 2011 Mar;93(3):293–7. doi: 10.1302/0301-620X.93B3.25467. [DOI] [PubMed] [Google Scholar]
- 43.Falez F, Favetti F, Casella F, Panegrossi G. Hip resurfacing: why does it fail? Early results and critical analysis of our first 60 cases. Int Orthop. 2008;32:209–16. doi: 10.1007/s00264-006-0313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beaulé P, Dorey F, Leduff M, Gruen T, Amstutz H. Risk factors affecting outcome of metal-on-metal surface arthroplasty of the hip. Clin Orthop Relat Res. 2004;418:87–93. [PubMed] [Google Scholar]
- 45.Amstutz H, Beaulé P, Dorey F, Le Duff M, Campbell P, Gruen T. Metal-on-metal hybrid surface arthroplasty: 2 to 6-year followup study. J Bone Joint Surg Am. 2004;86:28–39. [PubMed] [Google Scholar]
- 46.Hing CB, Young DA, Dalziel RE, Bailey M, Back DL, Shimmin AJ. Narrowing of the neck in resurfacing arthroplasty of the hip: a radiological study. J Bone Joint Surg (Br) 2007;89:1019–24. doi: 10.1302/0301-620X.89B8.18830. [DOI] [PubMed] [Google Scholar]
- 47.Venesmaa PK, Kröger HP, Miettinen HJ, et al. Alendronate reduces periprosthetic bone loss after uncemented primary total hip arthroplasty: a prospective randomized study. J Bone Miner Res. 2001;16:2126–131. doi: 10.1359/jbmr.2001.16.11.2126. [DOI] [PubMed] [Google Scholar]
- 48.Leichtle UG, Leichtle CI, Schmidt B, Martini F. Peri-prosthetic bone density after implantation of a custom-made femoral component. A five-year follow-up. J Bone Joint Surg Br. 2006 Apr;88(4):467–71. doi: 10.1302/0301-620X.88B4.16613. [DOI] [PubMed] [Google Scholar]
- 49.Heller MO, Mehta M, Taylor WR, Kim DY, Speirs A, Duda GN, Perka C. Influence of prosthesis design and implantation technique on implant stresses after cementless revision THR. J Orthop Surg Res. 2011 May 13;6:20. doi: 10.1186/1749-799X-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyle C, Kim IY. Comparison of different hip prosthesis shapes considering micro-level bone remodeling and stress-shielding criteria using three-dimensional design space topology optimization. J Biomech. 2011 Jun 3;44(9):1722–8. doi: 10.1016/j.jbiomech.2011.03.038. Epub 2011 Apr 16. [DOI] [PubMed] [Google Scholar]
- 51.Moritz N, Alm JJ, Lankinen P, Mäkinen TJ, Mattila K, Aro HT. Quality of intertrochanteric cancellous bone as predictor of femoral stem RSA migration in cementless total hip arthroplasty. J Biomech. 2011 Jan 11;44(2):221–7. doi: 10.1016/j.jbiomech.2010.10.012. Epub 2010 Nov 11. [DOI] [PubMed] [Google Scholar]
- 52.Skoglund B, Holmertz J, Aspenberg P. Systemic and local ibandronate enhance screw fixation. Journal of Orthopaedic Research. 2004;22:1108–1113. doi: 10.1016/j.orthres.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 53.Eberhardt C, Schwarz M, Kurth AH. High dosage treatment of nitrogen-containing bisphosphonate ibandronate is required for osseointegration of cementless metal implants. J Orthop Sci. 2005 Nov;10(6):622–6. doi: 10.1007/s00776-005-0955-z. [DOI] [PubMed] [Google Scholar]
- 54.Arlot M, Meunier PJ, Boivin G, Haddock L, Tamayo J, Correa-Rotter R, Jasqui S, Donley DW, Dalsky GP, Martin JS, Eriksen EF. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res. 2005 Jul;20(7):1244–53. doi: 10.1359/JBMR.050309. Epub 2005 Mar 14. [DOI] [PubMed] [Google Scholar]
- 55.Hilding M, Aspenberg P. Local peroperative treatment with a bisphosphonates improves the fixation of total knee prostheses: a randomized, double-blind radiostereometric study of 50 patients. Acta Orthopaedica. 2007;78(6):795–799. doi: 10.1080/17453670710014572. [DOI] [PubMed] [Google Scholar]
- 56.Jakobsen T, Kold S, Bechtold JE, Elmengaard B, Søballe K. Local alendronate increases fixation of implants inserted with bone compaction: 12-week canine study. J Orthop Res. 2007 Apr;25(4):432–41. doi: 10.1002/jor.20276. [DOI] [PubMed] [Google Scholar]
- 57.Carvas JS, Pereira RM, Caparbo VF, Fuller P, Silveira CA, Lima LA, Bonfa E, Mello SB. A single dose of zoledronic acid reverses the deleterious effects of glucocorticoids on titanium implant osseointegration. Osteoporos Int. 2010 Oct;21(10):1723–9. doi: 10.1007/s00198-009-1125-5. Epub 2009 Dec 9. [DOI] [PubMed] [Google Scholar]
- 58.McKenzie K, Dennis Bobyn J, Roberts J, Karabasz D, Tanzer M. Bisphosphonate remains highly localized after elution from porous implants. Clin Orthop Relat Res. 2011 Feb;469(2):514–22. doi: 10.1007/s11999-010-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jakobsen T, Kold S, Bechtold JE, Elmengaard B, Søballe K. Effect of topical alendronate treatment on fixation of implants inserted with bone compaction. Clin Orthop Relat Res. 2006 Mar;444:229–34. doi: 10.1097/01.blo.0000191273.34786.40. [DOI] [PubMed] [Google Scholar]
- 60.Vestermark MT. Strontium in the bone-implant interface. Dan Med Bull. 2011 May;58(5):B4286. [PubMed] [Google Scholar]
- 61.Hendrikus van der Wal BC, Rahmy A, Grimm B, et al. Preoperative bone quality as a factor in dual-energy X-rayabsorptiometry analysis comparing bone remodeling between two implant types. International Orthopaedics (SICOT) 2008;32:39–45. doi: 10.1007/s00264-006-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryd L, Albrektsson BE, Carlsson L, Dansgard F, Herberts P, Lindstrand A, Regner L, Toksvig-Larsen S. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg (Br) 1995;77(3):377–83. [PubMed] [Google Scholar]
- 63.Dhert WJ, Thomsen P, Blomgren AK, Esposito M, Ericson LE, Verbout AJ. Integration of press-fit implants in cortical bone: a study on interface kinetics. J Biomed Mater Res. 1998;41(4):574–83. doi: 10.1002/(sici)1097-4636(19980915)41:4<574::aid-jbm9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 64.Mjoberg B. The theory of early loosening of hip prostheses. Orthopedics. 1997;20(12):1169–75. doi: 10.3928/0147-7447-19971201-12. [DOI] [PubMed] [Google Scholar]
- 65.Bobyn JD, Hacking SA, Krygier JJ, Harvey EJ, Little DG, Tanzer M. Zoledronic acid causes enhancement of bone growth into porous implants. J Bone Joint Surg (Br) 2005;87(3):416–20. doi: 10.1302/0301-620x.87b3.14665. [DOI] [PubMed] [Google Scholar]
- 66.Little DG, Smith NC, Williams PR, Briody JN, Bilston LE, Smith EJ, Gardiner EM, Cowell CT. Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J Bone Miner Res. 2003;18(7):1300–7. doi: 10.1359/jbmr.2003.18.7.1300. [DOI] [PubMed] [Google Scholar]
- 67.Wermelin K, Tengvall P, Aspenberg P. Surface bound bisphosphonates enhance screw fixation in rats. Increasing effect up to 8 weeks after insertion. Acta Orthop. 2007;78(3):385–92. doi: 10.1080/17453670710013979. [DOI] [PubMed] [Google Scholar]
- 68.Hilding M, Ryd L, Toksvig-Larsen S, Aspenberg P. Clodronate prevents prosthetic migration: a randomized radiostereometric study of 50 total knee patients. Acta Orthop Scand. 2000;71(6):553–7. doi: 10.1080/000164700317362163. [DOI] [PubMed] [Google Scholar]
- 69.Hilding M, Aspenberg P. Post-operative clodronate decreases prosthetic migration: 4-year follow-up of a randomized radiostereometric study of 50 total knee patients. Acta Orthop. 2006;77(6):912–6. doi: 10.1080/17453670610013213. [DOI] [PubMed] [Google Scholar]
- 70.Kurth AHA, Eberhardt C, Muller S, Steinacker M, Schwarz M, Bauss F. The bisphosphonate ibandronate improves implant integration in osteopenic ovariectomized rats. Bone. 2005;37:204–210. doi: 10.1016/j.bone.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 71.Kienapfel H, Sprey C, Wilke A, Griss P. Implant fixation by bone ingrowth. J Arthroplasty. 1999;14:355–68. doi: 10.1016/s0883-5403(99)90063-3. [DOI] [PubMed] [Google Scholar]
- 72.Jaffe WL, Scott DF. Total hip arthroplasty with hydroxyapatite-coated prostheses. J Bone Joint Surg Am. 1996;78:1918–34. doi: 10.2106/00004623-199612000-00018. [DOI] [PubMed] [Google Scholar]
- 73.Qui S-J, Hoshaw S, Gibson G, Lundin-Cannon KMS. Osteocyte apoptosis after acute matrix injury in compact bone. Trans Orthop Res SOC; San Francisco: 1997. p. 22.p. 89. [Google Scholar]
- 74.Verborgt O, Tatton N, Majeska RMS. Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? J Bone Miner Res. 2002;17:907–14. doi: 10.1359/jbmr.2002.17.5.907. [DOI] [PubMed] [Google Scholar]
- 75.Glimcher MJ, Kenzora JE. The biology of osteonecrosis of the human femoral head and its clinical implications: discussion of the etiology and genesis of the pathological sequelae; comments on treatment. Clin Orthop. 1979:273–312. [PubMed] [Google Scholar]
- 76.Aspenberg P, Herbertsson P. Periprosthetic bone resorption. Particles versus movement. J Bone Joint Surg Br. 1996;78:641–6. [PubMed] [Google Scholar]
- 77.Karrholm J, Borssen B, Lowenhielm G, Snorrason F. Does early micromotion of femoral stem prostheses matter? &7-year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg Br. 1994;76:912–7. [PubMed] [Google Scholar]
- 78.Mjoberg B. The theory of early loosening of hip prostheses. Orthopedics. 1997;20(1):169–75. doi: 10.3928/0147-7447-19971201-12. [DOI] [PubMed] [Google Scholar]
- 79.Ryd L, Albrektsson BE, Carlsson L, Dansgard F, Herberts P, Lindstrand A, et al. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg Br. 1995;77:377–83. [PubMed] [Google Scholar]
- 80.Riley EH, Lane JM, Urist MR, Lyons KM, Lieberman JR. Bone morphogenetic protein-2. Biology and applications. Clin Orthop. 1996;324:39–46. [PubMed] [Google Scholar]
- 81.Lind M, Overgaard S, Ongpipattanakul B, Nguyen T, Bunger C, Søballe K. Transforming growth factor-h1 stimulates bone on growth to weight-loaded tri-calcium phosphate coated implants. J Bone Joint Surg (Br) 1996;78:377–82. [PubMed] [Google Scholar]
- 82.Fleisch H. The bisphosphonate ibandronate, given daily as well as discontinuously, decreases bone resorption and increases calcium retention as assessed by 45C kinetics in the intact rat. Osteoporos Int. 1996;6:166–70. doi: 10.1007/BF01623942. [DOI] [PubMed] [Google Scholar]
- 83.Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 84.Fromigue O, Body JJ. Bisphosphonates influence the proliferation and the maturation of normal human osteoblasts. J Endocrinol Invest. 2002;25:539–46. doi: 10.1007/BF03345497. [DOI] [PubMed] [Google Scholar]
- 85.Papapoulos SE. Bisphosphonates in the management of postmenopausal osteoporosis. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. New York’: Academic Press; 2001. pp. 631–49. [Google Scholar]
- 86.Motohashi M, Shirota T, Tokugawa Y, Ohno K, Michi K, Yamaguchi A. Bone reactions around hydroxyapatite-coated implants in ovariectomized rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endo. 1999;87:145–52. doi: 10.1016/s1079-2104(99)70264-7. [DOI] [PubMed] [Google Scholar]
- 87.Melton LJ., III How many women have osteoporosis now? J Bone Miner Res. 1995;10:175–7. doi: 10.1002/jbmr.5650100202. [DOI] [PubMed] [Google Scholar]
- 88.Nawabi DH, Chin KF, Keen RW, Haddad FS. Vitamin D deficiency in patients with osteoarthritis undergoing total hip replacement: a cause for concern? J Bone Joint Surg (Br) 2010 Apr;92(4):496–9. doi: 10.1302/0301-620X.92B3.23535. [DOI] [PubMed] [Google Scholar]
- 89.Kelly J, Lin A, Wang CJ, Park S, Nishimura I. Vitamin D and bone physiology: demonstration of vitamin D deficiency in an implant osseointegration rat model. J Prosthodont. 2009 Aug;18(6):473–8. doi: 10.1111/j.1532-849X.2009.00446.x. Epub 2009 Mar 26. [DOI] [PubMed] [Google Scholar]
- 90.Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–78. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 91.Eberhardt C, Habermann B, Müller S, Schwarz M, Bauss F, Kurth AH. The bisphosphonate ibandronate accelerates osseointegration of hydroxyapatite-coated cementless implants in an animal model. J Orthop Sci. 2007 Jan;12(1):61–6. doi: 10.1007/s00776-006-1081-2. Epub 2007 Jan 31. [DOI] [PubMed] [Google Scholar]
- 92.Eberhardt C, Müller S, Steinacker M, Schwarz M, Bauss F. The bisphosphonate ibandronate improves implant integration in osteopenic ovariectomized rats. Kurth AH. Bone. 2005 Aug;37(2):204–10. doi: 10.1016/j.bone.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 93.Yamasaki S, Masuhara K, Yamaguchi K, et al. Risedronate reduces postoperative bone resorption after cementless total hip arthroplasty. Osteoporos Int. 2007;18:1009–1015. doi: 10.1007/s00198-007-0339-7. [DOI] [PubMed] [Google Scholar]
- 94.Goodship AE, Blunn GW, Green J, Coathup MJ. Prevention of strain-related osteopenia in aseptic loosening of hip prostheses using perioperative bisphosphonate. J Orthop Res. 2008 May;26(5):693–703. doi: 10.1002/jor.20533. [DOI] [PubMed] [Google Scholar]
- 95.Kinov P, Tivchev P, Doukova P, Leithner A. Effect of risedronate on bone metabolism after total hip arthroplasty: a prospective randomised study. Acta Orthop Belg. 2006 Jan;72(1):44–50. [PubMed] [Google Scholar]
- 96.Sköldenberg OG, Salemyr MO, Bodén HS, Ahl TE, Adolphson PY. The effect of weekly risedronate on periprosthetic bone resorption following total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2011 Oct 19;93(20):1857–64. doi: 10.2106/JBJS.J.01646. [DOI] [PubMed] [Google Scholar]
- 97.Arabmotlagh M, Rittmeister M, Hennigs T. Alendronate prevents femoral periprosthetic bone loss following total hip arthroplasty: prospective randomized double-blind study. J Orthop Res. 2006 Jul;24(7):1336–41. doi: 10.1002/jor.20162. [DOI] [PubMed] [Google Scholar]
- 98.Trevisan C, Ortolani S, Romano P, Isaia G, Agnese L, Dallari D, Grappiolo G, Cherubini R, Massari L, Bianchi G. Decreased periprosthetic bone loss in patients treated with clodronate: a 1-year randomized controlled study. Calcif Tissue Int. 2010 Jun;86(6):436–46. doi: 10.1007/s00223-010-9356-1. [DOI] [PubMed] [Google Scholar]
- 99.Vasikaran SD. Bisphosphonates: an overview with special reference to alendronate. Ann Clin Biochem. 2001;38:608–23. doi: 10.1258/0004563011901037. [DOI] [PubMed] [Google Scholar]
- 100.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–74. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fromigue O, Body JJ. Bisphosphonates influence the proliferation and the maturation of normal human osteoblasts. J Endocrinol Invest. 2002;25:53946. doi: 10.1007/BF03345497. [DOI] [PubMed] [Google Scholar]
- 102.Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9:2643–58. doi: 10.2174/1381612033453640. [DOI] [PubMed] [Google Scholar]
- 103.Matsuo A, Shuto T, Hirata G, Satoh H, Matsumoto Y, Zhao H, et al. Antiinflammatory and chondroprotective effects of the aminobisphosphonate ibadronate (YM 175) in adjuvant induced arthritis. J Rheumatol. 2003;30:1280–90. [PubMed] [Google Scholar]
- 104.Kobayashi A, Donnelly WJ, Scott G, Freeman MA. Early radiological observations may predict the long-term survival of femoral hip prostheses. J Bone Joint Surg Br. 1997;79:583–9. doi: 10.1302/0301-620x.79b4.7210. [DOI] [PubMed] [Google Scholar]
- 105.Meraw SJ, Reeve CM. Qualitative analysis of peripheral peri-implant bone and influence of alendronate sodium on early bone regeneration. J Periodontol. 1999;70:1228–33. doi: 10.1902/jop.1999.70.10.1228. [DOI] [PubMed] [Google Scholar]
- 106.Wang Ching-Jen, Wang Jun-Wen, Ko Jih-yang, et al. Three-Year changes in bone mineral density around the Knee after a six-month course of oral alendronate following total knee arthroplasty. A Prospective, Randomized Study. J Bone Joint Surg. 2006;88A:267–272. doi: 10.2106/JBJS.E.00051. [DOI] [PubMed] [Google Scholar]
- 107.Arabmotlagh M, Pilz M, Warzecha J, et al. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty. J Orthop Res. 2009 Feb;27(2):183–8. doi: 10.1002/jor.20748. [DOI] [PubMed] [Google Scholar]
- 108.Body JJ. Breast cancer: bisphosphonates therapy for metastatic bone disease. Clin Cancer Res. 2006;12:6258s–63s. doi: 10.1158/1078-0432.CCR-06-0840. [DOI] [PubMed] [Google Scholar]
- 109.Goicoechea C, Porras E, Alfaro MJ, Martin MI. Alendronate induces antinociception in mice, not related with its effects in bone. Jpn J Pharmacol. 1999;79:433–7. doi: 10.1254/jjp.79.433. [DOI] [PubMed] [Google Scholar]
- 110.Bonabello A, Galmozzi MR, Bruzzese T, Zara GP. Analgesic effect of bisphosphonates in mice. Pain. 2001;91:269–75. doi: 10.1016/S0304-3959(00)00447-4. [DOI] [PubMed] [Google Scholar]
- 111.Bonabello A, Galmozzi MR, Canaparo R, Serpe L, Zara GP. Longterm analgesic effect of clodronate in rodents. Bone. 2003:567–574. doi: 10.1016/s8756-3282(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 112.Walker K, Medhurst SJ, Kidd BL, Glatt M, Bowes M, Patel S, et al. Disease modifying and anti-nociceptive effects of the bisphosphonate, zoledronic acid in a model of bone cancer pain. Pain. 2002;100:219–29. doi: 10.1016/S0304-3959(02)00040-4. [DOI] [PubMed] [Google Scholar]
- 113.Fleisch H. Bisphosphonates: pharmacology and use in the treatment of tumor induced hypercalcaemic and metastatic bone disease. Drugs. 1991;42:919–44. doi: 10.2165/00003495-199142060-00003. [DOI] [PubMed] [Google Scholar]
- 114.Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 115.McCormack PL, Plosker GL. Ibandronic acid: a review of its use in the treatment of bone metastases of breast cancer. Drugs. 2006;66:711–28. doi: 10.2165/00003495-200666050-00011. [DOI] [PubMed] [Google Scholar]
- 116.Bianchi M, Franchi S, Ferrario P, Sotgiu ML, Sacerdote P. Effects of the bisphosphonate ibandronate on hyperalgesia, substance P, and cytokine levels in a rat model of persistent inflammatory pain. European Journal of Pain. 2008;12:284–292. doi: 10.1016/j.ejpain.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 117.Julius D, Basbaum AI. Molecularmechanismof nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 118.Yoneda T, Hata K, Nakanishi M, Nagae M, Nagayama T, Wakabayashi H, Nishisho T, Sakurai T, Hiraga T. Involvement of acidic microenvironment in the pathophysiology of cancer-associated bone pain. Bone. 2011 Jan;48(1):100–5. doi: 10.1016/j.bone.2010.07.009. Epub 2010 Jul 14. [DOI] [PubMed] [Google Scholar]
- 119.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O’Leary P, Mantyh PW. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroiscience. 2002;113:155–66. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 120.Rousselle AV, Heyman D. Osteoclast acidification pathways during bone resorption. Bone. 2002;30:535–40. doi: 10.1016/s8756-3282(02)00672-5. [DOI] [PubMed] [Google Scholar]
- 121.Mantyh PW, Clohisy DR, Koltzenburg M, Hunt S. Molecular mechanism of cancer pain. Nature Rev Cancer. 2002;2:201–9. doi: 10.1038/nrc747. [DOI] [PubMed] [Google Scholar]
- 122.Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54:2312–7. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Body JJ, Diel IJ, Lichinitzer M, Lazarev A, Pecherstorfer M, Bell R, et al. Oral ibandronate reduces the risk of skeleletal complications in breast cancer patients with metastatic bone disease: results from two randomizes, placebo-controlled phase III studies. Br J Cancer. 2004;90:1133–7. doi: 10.1038/sj.bjc.6601663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heidenreich A, Ohlmann CH. Ibandronate: its pharmacology and clinical efficacy in the management of tumor-induced hypercalcemia and metastatic bone disease. Expert Rev Anticancer Ther. 2004;4:991–1005. doi: 10.1586/14737140.4.6.991. [DOI] [PubMed] [Google Scholar]
- 125.Cameron D, Fallon, Diel I. Ibandronate: its role in metastatic breast cancer. Oncologist. 2006;11(Suppl. 1):27–33. doi: 10.1634/theoncologist.11-90001-27. [DOI] [PubMed] [Google Scholar]
- 126.Croom KF, Scott LJ. Intravenous ibandronate in the treatment of osteoporosis. Drugs. 2006;66:1593–601. doi: 10.2165/00003495-200666120-00005. [DOI] [PubMed] [Google Scholar]
- 127.Coleman R. The use of bisphosphonates in cancer treatment. Ann N Y Acad Sci. 2011 Feb;1218:3–14. doi: 10.1111/j.1749-6632.2010.05766.x. Epub 2010 Sep 28. [DOI] [PubMed] [Google Scholar]
- 128.Dhillon S, Lyseng-Williamson KA. Zoledronic acid: a review of its use in the management of bone metastases of malignancy. Drugs. 2008;68(4):507–34. doi: 10.2165/00003495-200868040-00010. [DOI] [PubMed] [Google Scholar]
- 129.Heidenreich A, Ohlmann C, Body JJ. Ibandronate in metastatic bone pain. Semin Oncol. 2004;31(Suppl. 10):67–72. doi: 10.1053/j.seminoncol.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 130.Zysk SP, Dürr HR, Gebhard HH, Schmitt-Sody M, Refior HJ, Messmer K, et al. Effects of ibandronate on inflammation in mouse antigen-induced arthritis. Inflamm Res. 2003;52:221–6. doi: 10.1007/s000110300075. [DOI] [PubMed] [Google Scholar]
- 131.Santini D, Fratto ME, Vincenzi B, La Cesa A, Dianzani C, Tonini G. Bisphosphonate effects in cancer and inflammatory diseases. In vitro and in vivo modulation of cytokine activities. Biodrugs. 2004;18:269–78. doi: 10.2165/00063030-200418040-00004. [DOI] [PubMed] [Google Scholar]
- 132.Bauss F, Body J. Ibandronate in metastatic bone disease: a review of pre-clinical data. Anti-cancer Drugs. 2005;16:107–18. doi: 10.1097/00001813-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 133.Toussirot É, Wendling D. Bisphosphonates as anti-inflammatory agents in ankylosing spondylitis and spondylarthropathies. Expert Opin Pharmacother. 2005;6:35–43. doi: 10.1517/14656566.6.1.35. [DOI] [PubMed] [Google Scholar]
- 134.Pfister T, Atzpodien E, Bauss F. The renal effects of minimally nephrotoxic doses of ibandronate and zoledronate following single and intermittent intravenous administration in rats. Toxicology. 2003;191:159–67.126. doi: 10.1016/s0300-483x(03)00257-9. [DOI] [PubMed] [Google Scholar]
- 135.Woolf CJ. Generation of acute pain. BMarchand F, Perretti M, Mc Mahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–31.r. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 136.Maggi CA. Tachykinins and calcitonin gene related peptide (CGRP) as co-trasmitters released from peripheral endings of sensory nerves. Prog Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. Med Bull 199147:523–33.127. [DOI] [PubMed] [Google Scholar]
- 137.White DM. Release of substance P from peripheral sensory nerve terminals. J Peripher Nerv Syst. 1997;2:191–201. [PubMed] [Google Scholar]
- 138.Bianchi M, Broggini M, Balzarini P, Baratelli P, Ferrario P, Panerai AE, et al. Effects of tramadol on synovial fluid concentrations of substance P and interleukin-6 in patients with knee osteoarthritis: comparison with paracetamol. Int Immunopharmacol. 2003;3:1901–8. doi: 10.1016/j.intimp.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 139.Bianchi M, Martucci C, Biella G, Ferrario P, Sacerdote P. Increased substance P and tumor necrosis factor alpha in the paws following formalin injection in rat tail. Brain Res. 2004;1019:255–8. doi: 10.1016/j.brainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 140.Delgado AV, Mc Manus AT, Chambers JP. Production of tumor necrosis factor - alpha, interleukin-1b, interleukin-2 and interleukin-6 by rat leukocytes sub-population after exposure to substance P. Neuropeptides. 2003;37:355–61. doi: 10.1016/j.npep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 141.Hernanz A, Medina S, de Miguel E, Martin-Mola E. Effect of CGRP, neuropeptide Y, substance P and vasoactive intestinal peptide on Interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha production by peripheral whole blood cells from rheumatoid arthritis and osteoarthritis patients. Regul Pept. 2003;115:19–24. doi: 10.1016/s0167-0115(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 142.Safieh-Garabedian B, Poole S, Allchorn A, Winter J, Woolf C. Contribution of IL-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115:1265–75. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marchand F, Perretti M, Mc Mahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–31. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 144.Morioka N, Takeda K, Kumagai K, Hanada T, Ikoma K, Hide I, et al. Interleukin-1b-induced substance P release from rat cultured primary afferents neurons driven by two phospholipase A2 enzymes: secretory type IIA and cytosolic type IV. J Neurochem. 2002;80:989–97. doi: 10.1046/j.0022-3042.2002.00722.x. [DOI] [PubMed] [Google Scholar]
- 145.Pioli G, Hughes DE, Milan A, Aarden L, Meager A, Chapman K. Bisphosphonates stimulate the production of cytokines by human peripheral blood mononuclear cells in vitro. Calcif Tissue Int. 1990;46:A54. [Google Scholar]
- 146.Takagi K, Takagi M, Kanangat S, Warrington KC, Shigemitsu H, Postlewaite A. Modulation of TNF-alpha gene expression by IFNgamma and pamidronate in murine macrophages: regulation by STAT1 dependent pathways. J Immunol. 2005;174:1801–10. doi: 10.4049/jimmunol.174.4.1801. [DOI] [PubMed] [Google Scholar]
- 147.Selander KS, Monkkonnen J, Karhukorpi EK, Harkonen P, Hannuniemi R, Vaananen HK. Characteristics of clodronate-induced apoptosis in osteoclasts and macrophages. Mol Pharmacol. 1996;50:1127–38. [PubMed] [Google Scholar]
- 148.Dehghani F, Conrad A, Kohl A, Korf HW, Hailer NP. Clodronate inhibits the secretion of proinflammatory cytokines and NO by isolated microglial cells and reduces the number of proliferating glial cells in excitotoxically injured hippocampal slice cultures. Exp Neurol. 2004;186:241–51. doi: 10.1016/j.expneurol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 149.Tuominen OM, Ylitalo-heikkala R, Vehmas TI, Mucha I, Ylitalo P, Riutta A. Effect of bisphosphonates on prostaglandin E2 and tromboxane B2 production in human whole blood and monocytes stimulated by lipopolysaccaride and A23187. Methods Find Exp Clin Pharmacol. 2006;28:361–7. doi: 10.1358/mf.2006.28.6.1003551. [DOI] [PubMed] [Google Scholar]
- 150.Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwara K, Yoneda T. Osteoclasts play a part in pain due to inflammation adjacent to bone. Bone. 2006;39:1107–15. doi: 10.1016/j.bone.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 151.Rhee PW, Kress M. Molecular physiology of proton transduction in nociceptors. Curr Opin Pharmacol. 2001;1:45–51. doi: 10.1016/s1471-4892(01)00014-5. [DOI] [PubMed] [Google Scholar]
- 152.Hutter MM, Wick EC, Day AL, Maa J, Zerega EC, Richmond AC, et al. Transient receptor potential vanilloid (TRPV-1) promotes neurogenic inflammation in the pancreas via activation of the neurokinin-1 receptor. Pancreas. 2005;30:260–5. doi: 10.1097/01.mpa.0000153616.63384.24. [DOI] [PubMed] [Google Scholar]
- 153.Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M. Substance Pand CGRP-immunoreactive nerves in bone. Peptides. 1988;9:165–71. doi: 10.1016/0196-9781(88)90023-x. [DOI] [PubMed] [Google Scholar]
- 154.Hukkanen M, Konttinen YT, Rees RG, Santavirta S, Terenghi G, Polak JM. Distribution of nerve ending and sensory neuropeptides in rat synovium, meniscus and bone. Int J Tissue React. 1992;14:1–10. [PubMed] [Google Scholar]
- 155.Fras C, Kravetz P, Mody DR, Heggeness MH. Substance P containing nerve within the human vertebral body: an immunohistochemical study of the basivertebral nerve. Spine. 2003;3:63–7. doi: 10.1016/s1529-9430(02)00455-2. [DOI] [PubMed] [Google Scholar]
- 156.Goto T, Tanaka TM. Tachykinin and tachykinin receptors in bone. Microsc Res Tech. 2002;58:91–7. doi: 10.1002/jemt.10123. [DOI] [PubMed] [Google Scholar]
- 157.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: How hot is the link? Biochem Pharmacol. 2006;72:1605–21. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 158.Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwara K, Yoneda T. Osteoclasts play a part in pain due to inflammation adjacent to bone. Bone. 2006;39:1107–15. doi: 10.1016/j.bone.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 159.Bauss F, Body J. Ibandronate in metastatic bone disease: a review of pre-clinical data. Anti-cancer Drugs. 2005;16:107–18. doi: 10.1097/00001813-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 160.Barrett J, Worth E, Bauss F, Epstein S. Ibandronate: a clinical pharmacological and pharmacokinetic update. J Clin Pharmacol. 2004;44:951–65. doi: 10.1177/0091270004267594. [DOI] [PubMed] [Google Scholar]
- 161.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006 May 13;367(9522):1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 162.Jeffery AE, Wylde V, Blom AW, Horwood JP. It’s there and I’m stuck with it: patients’ experiences of chronic pain following total knee replacement surgery. Arthritis Care Res. 2011 Feb;63(2):286–92. doi: 10.1002/acr.20360. [DOI] [PubMed] [Google Scholar]
- 163.Stusser RJ. Reflections on the scientific method in medicine. Encyclopedia of Life Support Systems (EOLSS); available at http://www.eolss.net/Sample-Chapters/C03/E6-59-01-02.pdf. [Google Scholar]
- 164.Chesney D, Sales J, Elton R, Brenkel IJ. Infection after knee arthroplasty a prospective study of 1509 cases. J Arthroplasty. 2008 Apr;23(3):355–9. doi: 10.1016/j.arth.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 165.Biant Leela C, Bruce Warwick JM, van der Wall Hans, Walsh William R. Infecion or allergy in paiful metal-on-metal total hip arthroplasty. J Arthroplasty. 2010 Apr;25(2):334e11–334e16. doi: 10.1016/j.arth.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 166.Palestro CJ, Kim CK, Swyer AJ, Capozzi JD, Solomon RW, Goldsmith SJ. Total1-hip arthroplasty: periprosthetic Indium-111-labeled leukocyte activity and complementary technetium-99m-Sulfur Colloid imaging in suspected infection. J Nucl Med. 1990;31:1950–5. [PubMed] [Google Scholar]
- 167.Wukich DK, Abreu SH, Callaghan JJ, Van Nostrand D, Savory CG, Eggli DF, Garcia JE, Berrey BH. Diagnosis of infection by preoperative scintigraphy with indium-labeled white blood cells. J Bone Joint Surg Am. 1987 Dec;69(9):1353–60. [PubMed] [Google Scholar]
- 168.Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999 Oct;81(10):1434–45. doi: 10.2106/00004623-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 169.Haddad FS, Muirhead-Allwood SK, Manktelow AR, Bacarese-Hamilton I. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg (Br) 2000;82:689–94. doi: 10.1302/0301-620x.82b5.9668. [DOI] [PubMed] [Google Scholar]
- 170.Meek RM, Dunlop D, Garbuz DS, McGraw R, Greidanus NV, Masri BA. Patient satisfaction and functional status after aseptic versus septic revision total knee arthroplasty using the PROSTALAC articulating spacer. J Arthroplasty. 2004 Oct;19(7):874–9. doi: 10.1016/j.arth.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 171.Breuer B, Pappagallo M, Ongseng F, Chen CI, Goldfarb R. An open-label pilot trial of ibandronate for complex regional pain syndrome. Clin J Pain. 2008 Oct;24(8):685–9. doi: 10.1097/AJP.0b013e318175920f. [DOI] [PubMed] [Google Scholar]
- 172.Cassisi G, Sartori L. Efficacy of intramuscular clodronate in Complex Regional Pain Syndrome type I: description of a case located in the astragalus in a patient with psoriatic arthritis. Acta Biomed. 2009;80(3):268–77. [PubMed] [Google Scholar]
- 173.Robinson JN, Sandom J, Chapman PT. Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med. 2004 Sep;5(3):276–80. doi: 10.1111/j.1526-4637.2004.04038.x. [DOI] [PubMed] [Google Scholar]
- 174.Thillemann TM, Pedersen AB, Mehnert F, Johnsen SP, Søballe K. Postoperative use of bisphosphonates and risk of revision after primary total hip arthroplasty: a nationwide population-based study. Bone. 2010 Apr;46(4):946–51. doi: 10.1016/j.bone.2010.01.377. Epub 2010 Jan 25. [DOI] [PubMed] [Google Scholar]