Abstract
Background
We investigated relationships between early growth and proximal femoral geometry at age six years in a prospective population-based cohort, the Southampton Women’s Survey.
Methods
In 493 mother-offspring pairs we assessed linear size (individual measure dependent on developmental stage) using high-resolution ultrasound at 11, 19 and 34 weeks gestation (femur length) and at birth, 1, 2, 3, 4 and 6 years (crown-heel length/height). Standard deviation (SD)-scores were created and conditional regression modelling generated mutually independent growth variables. Children underwent hip DXA (Dual X-ray absorptiometry) at 6 years (Hologic Discovery, Hologic Inc., MA); hip structure analysis software yielded measures of geometry and strength.
Results
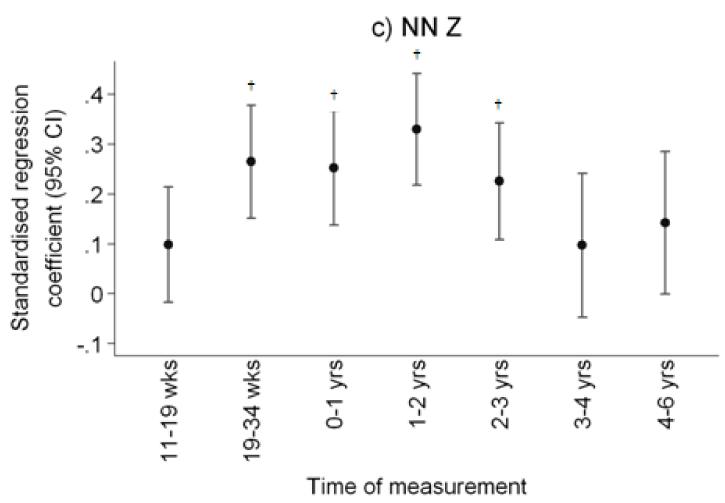
There were strong associations between early linear growth and femoral neck section modulus (Z) at 6 years, with the strongest relationships observed for femur growth from 19-34 weeks gestation (β=0.26 cm3/SD, p<0.0001), and for height growth from birth to 1 year (β=0.25 cm3/SD, p<0.0001) and 1-2 years (β=0.33 cm3/SD, p<0.0001), with progressively weaker relationships over years 3 (β=0.23 cm3/SD, p=0.0002) and 4 (β=0.10 cm3/SD, p=0.18).
Conclusions
These results demonstrate that growth before age 3 years predicts proximal femoral geometry at six years old. The data suggest critical periods in which there is capacity for long term influence on the later skeletal growth trajectory.
Introduction
Risk of osteoporotic fracture in adulthood is strongly associated with bone mass (a composite measure with contributions from both size and volumetric density) (1); more recently it has been recognised that the shape of the proximal femur is an additional risk factor for femoral neck fracture (2-7). There is accumulating evidence that adult bone mass (8) and risk of hip fracture might be partly dependent on growth in early life (9, 10) and that this relationship might be mediated partly through an effect on the geometry of the proximal femur assessed by DXA techniques in older age (11). We reasoned that if this hypothesis were correct, then such relationships between early growth and proximal femoral geometry might already be manifest in childhood. Although data are scant, there is some evidence that proximal femoral shape might be modified by postnatal exposures such as physical activity in young childhood (12); furthermore we have demonstrated that the longer term trajectory of skeletal growth might be influenced by factors acting in utero or in early infancy (13-16). Finally, we have found that growth velocity at different points in development through intrauterine and postnatal life is associated with bone mineral accrual by four years old (17). Taking these childhood findings together with the previously observed associations between early growth and adult hip geometry, we hypothesised that growth velocity in utero and early childhood might positively predict measures of femoral neck strength, assessed by DXA at six years old, in children born to the Southampton Women’s Survey, an ongoing longitudinal mother-offspring cohort.
Methods
Participants
The Southampton Women’s Survey is a prospective cohort study of 12,583 women aged 20-34 years recruited from the general population (18). At enrolment the participants were characterised in detail in terms of diet, lifestyle, health, physical activity (by interviewer administered questionnaire) and anthropometric measurements. 3159 of these women were followed through a subsequent pregnancy and delivered a live born infant. The children are being followed and characterised, with samples of children assessed at birth, 4 and 6 years. Of the 1268 eligible families contacted for a 6 year follow up during the study period (6th birthday up to the end 2009), 530 attended for DXA, forming the cohort presented in this paper.
Prenatal ultrasound scanning
As this was a population survey, no inclusion criteria were set for the pregnancy study other than singleton pregnancy and ability to provide informed consent. At 11, 19 and 34 weeks gestation, the women underwent high-resolution ultrasound scanning using a Kretz Voluson® 730 (GE Kretz Ultrasound, Tiefenbach, Austria) or Acuson Sequoia® 512 (Siemens, Erlangen, Germany) system, which were cross-calibrated. After establishing correct positioning according to standard anatomical landmarks, measurements of crown-rump length (at 11 weeks gestation) and femur length (obtained at 19 and 34 weeks) were made on the frozen images using electronic callipers by the two operators, according to internationally accepted and validated methodology (19). Each measurement was performed in triplicate and the mean value was used for analysis.
Postnatal growth
Crown-heel length at birth was measured using a neonatometer (Harpenden, Wrexham, UK). Children were assessed at 6 months, 1, 2, 3, 4 and 6 years with a home visit from a research nurse. Information on diet, lifestyle, illness and medication was collected and anthropometric measurements were performed. Crown-heel length at one year was measured using an infantometer (Seca Ltd, Birmingham, UK). Height was measured at 2 3, 4 and 6 years using a Leicester height measurer (Seca Ltd).
6 year DXA assessment
The mother and child were invited to visit the Osteoporosis Centre at Southampton General Hospital for assessment of bone mass and body composition. At this visit written informed consent for the DXA scan was obtained from the mother, father or guardian. The child’s height (using a Leicester height measurer, Seca Ltd) and weight, using calibrated digital scales (Seca Ltd) were measured. Whole body (including body composition) and total hip scans were obtained, using a Hologic Discovery A instrument with APEX 3.0 software (Hologic Inc., Bedford, MA). To encourage compliance, a sheet with appropriate pictures was laid on the couch and to help reduce movement artefact, the children were shown a suitable DVD. The total radiation dose for the scans were as follows: whole body (paediatric scan mode) 4.7 microsieverts and hip 7.3 microsieverts. The manufacturer’s coefficient of variation (CV) for the instrument was 0.75% for whole body bone mineral density, and the experimental CV when a spine phantom was repeatedly scanned in the same position 16 times, in a single session with no repositioning, was 0.68%. All scans were checked for movement and clothing artefact resulting in 493 suitable for analysis.
Hip structure analysis
An interactive computer program (Hip Structural Analysis (HSA), Hologic Inc.) was used to derive a number of structural variables from the femoral DXA scans. The HSA software derives geometry of the load supporting surface by employing a projection principle first described by Martin and Burr (20), and the detailed methodology has been published previously, demonstrating a CV approximately 2-4%, depending on exact measure (21). Using this technique, structural indices may be derived at the narrow femoral neck (NN) and intertrochanteric (IT) regions. The hip measurements obtained at six years old, using the standard automated software algorithm to define region of interest, included total hip bone area (BA), bone mineral content (BMC), areal bone mineral density (aBMD) and as the basis for size-corrected bone mineral density (scBMC) (BMC adjusted for BA, height and weight); cortical thickness (CT), cross sectional area (CSA), cross sectional moment of inertia (CSMI) and section modulus (Z) (a measure of bending strength) were estimated at both the narrow femoral neck (NN) and intertrochanteric regions (IT) using the automated hip structure analysis software with minimal operator adjustment.
Statistical analysis
All women had a reliable date of last menstrual period. All variables were checked for normality. Non-normally distributed variables were transformed logarithmically. Differences between boys and girls for all size and outcome variables at each time point were explored using t-test and Wilcoxon rank-sum test where appropriate. Within group z-scores for the ultrasound derived measures of fetal size were generated and adjusted for sex and gestational age at which the measurement was taken. The measures of linear size used were crown-rump length (11 weeks gestation), femur length (19 and 34 weeks gestation), crown heel length (birth and 1 year postnatally) and standing height British Growth Foundation z-scores (2 to 6 years postnatally). Conditional models of change were built using linear regression analysis: thus linear size z-score at 11 weeks was the starting point. Conditional change in linear size z-score from 11 to 19 weeks is equivalent to the standardised residuals resulting from the linear regression model of z-score at 19 weeks on z-score at 11 weeks. The conditional change in z-score from 19 to 34 weeks is given from the standardised residuals obtained from regressing z-score at 34 weeks on both z-score at 19 weeks and z-score at 11 weeks simultaneously. This process was continued for each subsequent time point, resulting in measures of conditional growth which are mutually uncorrelated and yielding standard deviate scores which can be used to compare relationships between growth at different time intervals and bone measures at six years (adjusted for sex and age).
Linear regression methods were used to explore the relationship between the growth measurements and hip geometry at six years old, using Stata V11.1 (Statacorp, College Station, Texas). The resulting β term represents the change in outcome (units dependent on individual measure) per SD change in the predictor.
The Southampton Women’s Survey was approved by the Southampton and South West Hampshire Local Research Ethics Committee. Written consent was obtained from parents/carers of all participants.
Results
Characteristics of the children
There were 493 children (255 boys) with complete usable DXA data. The characteristics of the mothers and children are shown in tables 1a and 1b.
Table 1a.
Parental characteristics
| Characteristic | Mean or MedianΦ or n(%) |
SD or IQRΦ |
|---|---|---|
| Age at birth of the child (yrs) | 30.3 | 3.7 |
| Height (cm) | 163.7 | 6.5 |
| BMI prepregnancy (kg/m2) | 26.0 | 24.1-28.8 |
| Smoking prepregnancy (n (%)) | (212 (43%)) | |
| Ethnicity | ||
| White (n (%)) | 474 (96.2%) | |
| Other (n (%)) | 19 (3.9%) | |
| LP Triceps skinfold thickness (mm) | 20.8 Φ | 16.5-25.7 Φ |
| Nulliparous (n (%)) | 224 (45.4) | |
| LP Smoking (n (%)) | 65 (13.3) | |
| LP walking speed (n (%)) | ||
| Very slow | 82 (16.8) | |
| Stroll at an easy pace | 242 (49.5) | |
| Normal speed | 133 (27.2) | |
| Fairly brisk | 30 (6.1) | |
| Fast | 2 (0.4) | |
| Social class | ||
| Professional (I) | 22 (4.6) | |
| Management/ technical (II) | 190 (39.4) | |
| Skilled non-manual (IIIN) | 175 (36.3) | |
| Skilled manual (IIIM) | 35 (7.3) | |
| Partly skilled (IV) | 54 (11.2) | |
| Unskilled (V) | 6 (1.2) | |
| Educational attainment | ||
| None-O levels | 197 (40.0) | |
| A levels-Degree | 296 (60.0) | |
| Paternal height (cm) | 176.6 | 7.6 |
| Paternal BMI (kg/m2) | 24.3 Φ | 22.4-27.5 Φ |
Table 1b.
Children’s characteristics
| [mean (SD unless otherwise stated)] | |||
|---|---|---|---|
| Boys (n=255) | Girls (n=238) | P difference |
|
| 11 week CRL (mm) | 53.0 (8.3) | 53.3 (9.0) | 0.76 |
| 19 week FL (mm) | 30.5 (2.0) | 30.5 (2.0) | 0.95 |
| 34 week FL (mm) | 64.9 (2.6) | 65.2 (2.6) | 0.22 |
| Birth CHL (cm) | 50.4 (1.9) | 49.7 (1.8) | 0.0002 |
| 12 month CHL (cm) | 76.4 (2.5) | 74.7 (2.6) | <0.0001 |
| 24 month HT (cm) | 87.1 (2.8) | 85.9 (3.1) | <0.0001 |
| 36 month HT (cm) | 96.5 (3.2) | 95.5 (3.6) | 0.001 |
| 48 month HT (cm) | 104.1 (3.8) | 103.6 (4.4) | 0.27 |
| 72 month HT (cm) | 119.4 (4.7) | 118.7 (5.2) | 0.11 |
| Hip BA (cm2) | 16.6 (2.1) | 16.9 (2.2) | 0.11 |
| Hip BMC (g) | 11.5 (2.1) | 10.9 (2.0) | 0.007 |
| Hip aBMD (g/cm2) | 0.69 (0.06) | 0.64 (0.06) | <0.0001 |
| NN CSA (cm2) | 1.67 (0.3) | 1.55 (0.2) | <0.0001 |
| NN CT (cm) | 0.15 (0.02) | 0.14 (0.02) | <0.0001 |
| NN CSMI (cm4) | 0.71 (0.2) | 0.66 (0.2) | 0.007 |
| NN Z (cm3) | 0.58 (0.13) | 0.54 (0.11) | 0.0008 |
| Intertrochanter CSA (cm2) | 2.2 (0.3) | 2.1 (0.3) | 0.17 |
| Intertrochanter CSMI (cm4) (median (IQR)) | 1.6 (1.3-2.0) | 1.7 (1.4-2.2) | 0.003 |
| Intertrochanter Z (cm3) | 1.0 (0.2) | 1.0 (0.2) | 0.68 |
| Pints of milk per day | |||
| −0.3 | 52 (45.22) | 63 (54.78) | |
| −0.5 | 95 (48.22) | 102 (51.78) | |
| > 0.5 | 108 (59.67) | 73 (40.33) | 0.024 |
CRL = crown rump length; FL = femur length ; CHL = crown-heel length; HT = standing height; BA = bone area; BMC = bone mineral content; aBMD = areal bone mineral density; scBMC = size-corrected bone mineral content; CSA = cross-sectional area; CT = average cortical thickness; CSMI = cross-sectional moment of inertia; Z = section modulus; LP=late pregnancy
Compared with mothers of children born to the SWS during the same time frame, but who did not have DXA scans at 6 years, the mothers of children who did have DXA assessments were, on average, slightly older at the birth of their child (mean age 30.3 years vs 30.0 years respectively, p=0.07), better educated (60% with higher degree vs 55% respectively, p=0.045) and smoked slightly less (43% smoked before pregnancy vs 48% respectively, p=0.081).
Linear growth and DXA measurement of hip size, density and strength at six years
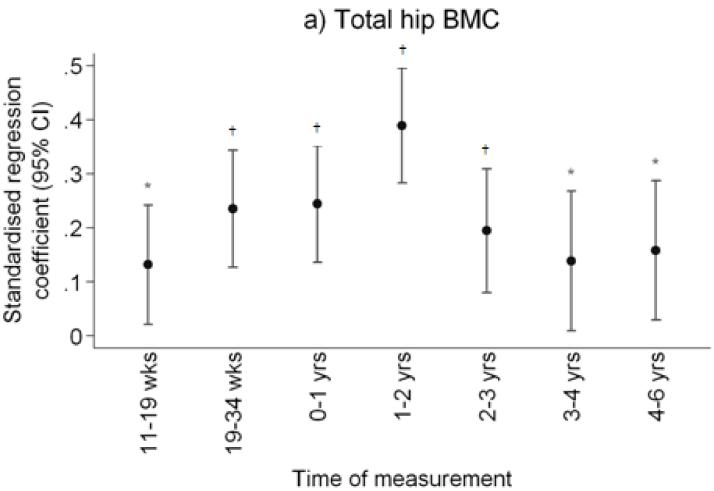
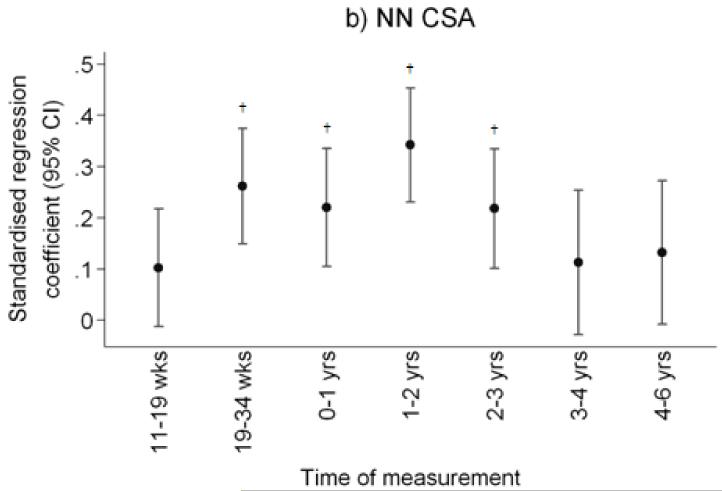
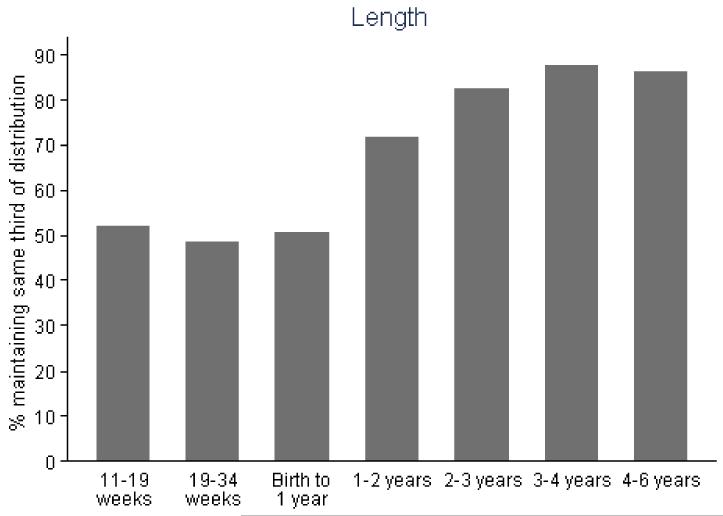
After adjustment for child’s age, sex and milk intake, linear growth at all fetal and infant time intervals was positively related to total hip bone area and bone mineral content, and to femoral narrow neck cross-sectional area, cross-sectional moment of inertia (CSMI) and section modulus (Z). These results are summarised in Table 2 and Figure 1. Thus Z was associated most strongly with growth in late gestation (19-34 week linear growth and Z at 6 years: β=0.26 cm3/SD, p<0.0001 compared with 11-19 week linear growth and Z: β=0.10 cm3/SD, p=0.09), and in the first two years of postnatal life (0-1 year linear growth and Z: β=0.25 cm3/SD, p<0.0001; 1-2 years: β=0.33 cm3/SD, p<0.0001), with progressively weaker relationships over years 3 (β=0.23 cm3/SD, p=0.0002) and 4 (β=0.10 cm4/SD, p=0.18). Relationships between growth and size corrected measures of bone mineral (aBMD and scBMC) were rather weaker, the most robust association being between aBMD and linear growth in the second year of postnatal life. Cortical thickness at the femoral narrow neck was positively associated with linear growth from 19 to 34 weeks (β=0.22cm/SD, p=0.0002) and from 1 to 2 years (β=0.22cm/SD, p=0.0002). Relationships between growth and CSA, CSMI and Z at the intertrochanteric site were similar to those observed at the narrow neck site. These associations were similar when analysed separately by offspring sex. Figure 2 summarises the percentage of subjects who remained in the same third of the length growth distribution over each subsequent growth period; this demonstrates a relatively steady crossing between thirds of the distribution antenatally but then a progressive decrease in crossing postnatally.
Table 2.
Length from birth to 4 years and hip geometry at six years (adjusted for daily milk intake at 6 years). Table shows the standardised β (SD/SD) and significance level.
| Total hip | Narrow-neck | Intertrochanter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BA | BMC | aBMD | scBMC | CSA | CT | CSMI | Z | CSA | Z | CSMI | |
| 11-19 week: Conditional length z-score | 0.16** | 0.13* | 0.049 | −0.028 | 0.1 | 0.037 | 0.11 | 0.099 | 0.12* | 0.11 | 0.15* |
| 19-34 week: Conditional length z-score | 0.22† | 0.24† | 0.16** | 0.059 | 0.26† | 0.22† | 0.26† | 0.26† | 0.26† | 0.27† | 0.27† |
| 0-1year: Conditional length z-score | 0.34† | 0.24† | 0.00039 | −0.16** | 0.22† | 0.077 | 0.28† | 0.25† | 0.19** | 0.26† | 0.29† |
| 1-2 year: Conditional length z-score | 0.40† | 0.39† | 0.21† | 0.075 | 0.34† | 0.22† | 0.34† | 0.33† | 0.33† | 0.35† | 0.35† |
| 2-3 year: Conditional length z-score | 0.19** | 0.19† | 0.12* | 0.039 | 0.22† | 0.12* | 0.24† | 0.23† | 0.20† | 0.21† | 0.21† |
| 3-4 year: Conditional length z-score | 0.12 | 0.14* | 0.094 | 0.038 | 0.11 | 0.077 | 0.11 | 0.098 | 0.15* | 0.13 | 0.15* |
| 4-6 year: Conditional length z-score | 0.18** | 0.16* | 0.076 | −0.02 | 0.13 | 0.093 | 0.11 | 0.14 | 0.16* | 0.18** | 0.19** |
BA = bone area; BMC = bone mineral content; aBMD = areal bone mineral density; scBMC = size-corrected bone mineral content; CSA = cross-sectional area; CT = average cortical thickness; CSMI = cross-sectional moment of inertia; Z = section modulus;
p<0.05;
p<0.01;
p<0.001
Figure 1.
Plot of standardised regression coefficients for a) total hip BMC, and narrow femoral neck b) cross-sectional area (NN-CSA) and c) section modulus (NN-Z) on conditional linear growth 11 weeks to 4 years. Growth variables are all independent. *p<0.05; **p<0.01; †p<0.001
Figure 2.
Percentage of participants remaining in the same third of linear size over each subsequent time interval.
Influence of maternal anthropometry and lifestyle, and child’s body composition
We investigated the role of parental factors, some of which have been previously associated with offspring intrauterine bone mineral accrual, that might influence fetal growth and childhood skeletal development through genetic and/or environmental effects (maternal height, ethnicity, social class, education, pre-pregnancy smoking and body mass index, and triceps skinfold thickness, walking speed and smoking in late pregnancy; paternal height and body mass index) together with offspring birth order. Of these, associations with early growth and 6-year DXA-derived hip indices were only observed for maternal height, and more weakly, for paternal height (Table 3). Thus maternal height was positively related to growth up to three years, and to 6-year hip size, shape and strength; paternal height was positively associated with growth in late pregnancy and the first year of postnatal life. The inclusion of parental height, however, as with all other parental measures and offspring birth order, did not materially alter the observed relationships between growth and 6-year hip bone outcomes. In further analyses including childhood body composition at age 6 year (percentage lean or percentage fat) as a covariate, relationships between early growth and six year hip structure remained robust.
Table 3.
Parental height and offspring growth and 6 year hip measures. Table shows the standardised β (SD/SD) and significance level.
| Linear growth | Total hip | Narrow-neck | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-19 wks | 19-34 wks | 0-1 yr | 1-2 yrs | 2-3 yrs | 3-4 yrs | 4-6 yrs | BA | BMC | aBMD | scBMC | CSA | CT | CSMI | Z | |
| Maternal height | 0.03** | 0.02** | 0.03† | 0.02* | 0.02** | 0.0 | 0.02 | 0.04† | 0.03† | 0.0 | -0.02* | 0.03† | 0.01 | 0.04† | 0.03† |
| Paternal heigh† | 0.01 | 0.02* | 0.02* | 0.01 | 0.02* | 0.0 | 0.0 | 0.01* | 0.01 | 0.0 | 0.0 | 0.01 | 0.01 | 0.01 | 0.01* |
BA = bone area; BMC = bone mineral content; aBMD = areal bone mineral density; scBMC = size-corrected bone mineral content; CSA = cross-sectional area; CT = average cortical thickness; CSMI = cross-sectional moment of inertia; Z = section modulus;
p<0.05;
p<0.01;
p<0.001
Discussion
We have demonstrated that early growth predicts proximal femoral size, mineralisation, geometry and strength at six years old, independent of current body composition. The relationships are particularly strong for linear growth in late pregnancy and in the first two to three years of postnatal life suggesting that these might be critical periods in which there is capacity for long term influence on the later skeletal growth trajectory.
We recruited children from a free-living population cohort and used objective measures of body composition and bone size and density. However, there are several limitations to our study. Intrauterine ultrasound measurements are prone to a certain amount of error, but data were collected by two experienced operators following internationally agreed guidelines (19) and repeatability was good (coefficient of variation 0.6% for femur length). We were only able to study a proportion of the original cohort, but the children who underwent the 6 year assessment did not differ at birth or 1 year old from those who did not. Mothers of children who underwent 6 year assessment were broadly similar to mothers of those children who did not, but tended to have higher levels of education. However, as the analysis is based on internal comparisons it is difficult to envisage how this would have spuriously shown an association between growth and hip geometry. Secondly, measurement of bone mineral in young children by DXA is hampered by their tendency to move and also by their low absolute BMC. However, we used specific paediatric software, and movement artefact was modest and uniform across the cohort; those few children with excessive movement were excluded from the analysis. DXA measures of bone mass have been shown to correlate well with whole body calcium content in ashing studies of piglets (22, 23). The hip structure analysis software assumes a constant volumetric bone mineral density at the tissue level; this will systematically influence the absolute measurements obtained from the software, but should have no effect on the ranking of the individuals, and thus should not have influenced our results. Indeed this technique has been used successfully in a young paediatric cohort (12). It is not possible, in this observational cohort study, to deduce whether observed relationships are causal, or whether they are explained by genetic or environmental factors, or an interaction between the two. Finally, owing to the high percentage of white mothers in the cohort (96%), our study was not powered to enable elucidation of any ethnicity-specific differences in the relationships between early growth and childhood hip geometry.
To our knowledge, this is the first longitudinal study to assess hip geometry assessed by DXA in relation to growth from early gestation to late infancy using objective measures such as ultrasound. Given the difficulties of setting up suitable prospective cohorts, many previous studies, particularly those of intrauterine growth, have been cross-sectional in nature. Much of the seminal work in this area has been carried out by Tanner and colleagues (24, 25), using a combination of cross-sectional measurements of post-natal fetal size at various gestations and longitudinal measurements of children. The patterns of growth deduced from such studies are compatible with the findings of our current and previous work (17), in which we demonstrated positive associations between growth in linear size from 19 weeks gestation to 3 years postnatal life, and whole body bone size, mineral content and areal density. Similar to the relationships observed in our current study, the magnitude of the association reduced with each successive postnatal year. However in this earlier study we were not able to examine structural parameters at the hip.
Based on these and other studies, after peak growth velocity is achieved in the last trimester there appears to be a period of growth velocity reduction, particularly for weight, in the very last part of pregnancy, followed by a further modulation of growth velocity over the first couple of years of life. This slowing in late pregnancy is thought to be a result of the increasing fetal size outstripping placental capacity and acts to limit fetal size, allowing the baby to be safely delivered through the birth canal (24). Thus if a Shire horse is crossed with a Shetland pony, the offspring is born small when the mother is a Shetland pony and large when the mother is a Shire horse (26). When the offspring is fully grown it is a similar size from either combination, which is midway between the two parents. This late pregnancy deviation from the earlier growth trajectory appears to be sensitive to environmental factors such as maternal fat stores, physical activity, smoking (14, 27); over the first two years of postnatal life studies have demonstrated that a considerable proportion of infants change position in the size distribution relative to their peers (25, 28). Clinical observations, results from analytical investigations such as the Karlberg infant-child-puberty model (29, 30) and our current and previous data all suggest that the first two to three years of postnatal life are critical for setting up the later growth trajectory. Indeed, in our study, as in our previous work (17), the percentage of children who remained in the same third of the length distribution relative to their peers across each subsequent time interval increased progressively with each subsequent postnatal time interval, consistent with gradual settling onto a sustained childhood growth trajectory.
Our previous work has suggested that growth in early life, both in utero and during infancy, predicts adult bone mineral content (31, 32), as well as the risk of hip fracture (9, 10). Furthermore, in a cohort of 333 adults from Hertfordshire aged 60 to 75 years, we found positive associations between weight at one year and femoral neck width and intertrochanteric cross-sectional moment of inertia, the former relationship persisting after adjustment for adult body size (11). These results suggest that the associations between early growth and risk of hip fracture might be mediated by alterations to proximal femoral geometry. The shape of the proximal femur has been shown to predict fracture risk in adult populations (2-7); greater femoral neck length has consistently been associated with increased fracture risk, but relationships between incident fracture and femoral neck width have been reported in both directions (2-5). In those studies which have included derived measurements of mechanical strength, such as section modulus, these have generally been negatively related to future fracture risk (3-5), as has cortical thickness (2-4). We believe that ours is the first study to look at growth prenatally and in early childhood in relation to later childhood bone geometry. Our current results are consistent with relationships between early growth and proximal femoral geometry manifesting at an early stage in postnatal life, and taken with the results from the Hertfordshire cohort (11), these associations may have important implications for risk of hip fracture in late adulthood.
There is some evidence that habitual physical activity and body composition during postnatal life may influence hip geometry (12), but data from exercise intervention studies, whilst sometimes demonstrating a positive effect on bone mineral accrual (33, 34), have not consistently shown effects on proximal femoral geometry (35-38). It is likely that, in contrast to mineralisation, the shape of the hip requires a much longer time to change in response to modulation of physical activity than allowed for in these studies; alterations might therefore reflect ongoing loading from muscle related to habitual physical activity. However, the results we observed were not materially influenced by adjustment for whole body percentage lean or fat mass at 6 years, consistent with a mechanism at least partly independent of current body composition.
In conclusion, we have identified periods of linear growth in late intrauterine and early postnatal life which are particularly strongly related to later proximal femoral size, mineralisation, geometry and strength at six years old, independent of childhood body composition. These findings are consistent with our previous data and both clinical and theoretical models of infant growth, and suggest that these might be critical periods in which there is capacity for long term influence on the later skeletal growth trajectory and thus adult fracture risk.
ACKNOWLEDGEMENTS
We thank the mothers who gave us their time; and a team of dedicated research nurses and ancillary staff for their assistance. We thank Mrs G Strange and Mrs R Fifield for helping prepare the manuscript.
Funding Sources: Medical Research Council, British Heart Foundation, Arthritis Research UK, National Osteoporosis Society, International Osteoporosis Foundation, Cohen Trust, NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, and NIHR Musculoskeletal Biomedical Research Unit, University of Oxford.
Footnotes
Conflict of interest: all authors report no conflict of interest
Reference
- 1.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Kaissi S, Pasco JA, Henry MJ, Panahi S, Nicholson JG, Nicholson GC, Kotowicz MA. Femoral neck geometry and hip fracture risk: the Geelong osteoporosis study. Osteoporos Int. 2005;16:1299–1303. doi: 10.1007/s00198-005-1988-z. [DOI] [PubMed] [Google Scholar]
- 3.Kaptoge S, Beck TJ, Reeve J, Stone KL, Hillier TA, Cauley JA, Cummings SR. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23:1892–1904. doi: 10.1359/JBMR.080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito M, Wakao N, Hida T, Matsui Y, Abe Y, Aoyagi K, Uetani M, Harada A. Analysis of hip geometry by clinical CT for the assessment of hip fracture risk in elderly Japanese women. Bone. 2010;46:453–457. doi: 10.1016/j.bone.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 5.LaCroix AZ, Beck TJ, Cauley JA, Lewis CE, Bassford T, Jackson R, Wu G, Chen Z. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int. 2010;21:919–929. doi: 10.1007/s00198-009-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Im GI, Lim MJ. Proximal hip geometry and hip fracture risk assessment in a Korean population. Osteoporos Int. 2011;22:803–807. doi: 10.1007/s00198-010-1301-7. [DOI] [PubMed] [Google Scholar]
- 7.Center JR, Nguyen TV, Pocock NA, Noakes KA, Kelly PJ, Eisman JA, Sambrook PN. Femoral neck axis length, height loss and risk of hip fracture in males and females. Osteoporos Int JID - 9100105. 1998;8:75–81. doi: 10.1007/s001980050051. [DOI] [PubMed] [Google Scholar]
- 8.Dennison EM, Aihie-Sayer A, Syddall H, Arden N, Gilbody H, Cooper C. Birthweight is associated with bone mass in the seventh decade: the Hertfordshire 31-39 Study. Pediatric Research. 2003;53:S25A. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Eriksson JG, Forsen T, Osmond C, Tuomilehto J, Barker DJ. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporos Int JID - 9100105. 2001;12:623–629. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- 10.Javaid MK, Eriksson JG, Kajantie E, Forsen T, Osmond C, Barker DJ, Cooper C. Growth in childhood predicts hip fracture risk in later life. Osteoporos.Int. 2011;22:69–73. doi: 10.1007/s00198-010-1224-3. [DOI] [PubMed] [Google Scholar]
- 11.Javaid MK, Lekamwasam S, Clark J, Dennison EM, Syddall HE, Loveridge N, Reeve J, Beck TJ, Cooper C. Infant growth influences proximal femoral geometry in adulthood. J Bone Miner Res. 2006;21:508–512. doi: 10.1359/jbmr.051214. [DOI] [PubMed] [Google Scholar]
- 12.Janz KF, Burns TL, Levy SM, Torner JC, Willing MC, Beck TJ, Gilmore JM, Marshall TA. Everyday activity predicts bone geometry in children: the iowa bone development study. Med Sci Sports Exerc. 2004;36:1124–1131. doi: 10.1249/01.mss.0000132275.65378.9d. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, Cooper C. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res JID - 8610640. 2001;16:1694–1703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 14.Harvey NC, Javaid MK, Arden NK, Poole JR, Crozier SR, Robinson SM, Inskip HM, Godfrey KM, Dennison EM, Cooper C. Maternal predictors of neonatal bone size and geometry: the Southampton Women’s Survey. Journal of Developmental Origins of Health and Disease. 2010;1:35–41. doi: 10.1017/S2040174409990055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 16.Harvey NC, Javaid MK, Poole JR, Taylor P, Robinson SM, Inskip HM, Godfrey KM, Cooper C, Dennison EM. Paternal skeletal size predicts intrauterine bone mineral accrual. J Clin Endocrinol.Metab. 2008;93:1676–1681. doi: 10.1210/jc.2007-0279. [DOI] [PubMed] [Google Scholar]
- 17.Harvey NC, Mahon PA, Kim M, Cole ZA, Robinson SM, Javaid K, Inskip HM, Godfrey KM, Dennison EM, Cooper C. Intrauterine growth and postnatal skeletal development: findings from the Southampton Women’s Survey. Paediatr.Perinat.Epidemiol. 2012;26:34–44. doi: 10.1111/j.1365-3016.2011.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. Cohort profile: The Southampton Women’s Survey. Int J Epidemiol. 2005 doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 4. Femur length. Br.J.Obstet.Gynaecol. 1994;101:132–135. doi: 10.1111/j.1471-0528.1994.tb13078.x. [DOI] [PubMed] [Google Scholar]
- 20.Martin RB, Burr DB. Non-invasive measurement of long bone cross-sectional moment of inertia by photon absorptiometry. J Biomech. 1984;17:195–201. doi: 10.1016/0021-9290(84)90010-1. [DOI] [PubMed] [Google Scholar]
- 21.Khoo BC, Beck TJ, Qiao QH, Parakh P, Semanick L, Prince RL, Singer KP, Price RI. In vivo short-term precision of hip structure analysis variables in comparison with bone mineral density using paired dual-energy X-ray absorptiometry scans from multi-center clinical trials. Bone. 2005;37:112–121. doi: 10.1016/j.bone.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Brunton JA, Bayley HS, Atkinson SA. Validation and application of dual-energy x-ray absorptiometry to measure bone mass and body composition in small infants. Am J Clin Nutr. 1993;58:839–845. doi: 10.1093/ajcn/58.6.839. [DOI] [PubMed] [Google Scholar]
- 23.Brunton JA, Weiler HA, Atkinson SA. Improvement in the accuracy of dual energy x-ray absorptiometry for whole body and regional analysis of body composition: validation using piglets and methodologic considerations in infants. Pediatr Res. 1997;41:590–596. doi: 10.1203/00006450-199704000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Tanner JM. Growth before birth. Foetus into Man: Physical growth from conception to maturity. Castlemead Publications, Ware. 1989:36–50. [Google Scholar]
- 25.Tanner JM. The organisation of the growth process. Foetus into Man: Physical growth from conception to maturity. Castlemead Publications, Ware. 1989:165–177. [Google Scholar]
- 26.Walton A, Hammond J. The maternal effects on growth and conformation in Shire horse-Shetland pony crosses. Proc R Soc Lond (Biol) 1938;125:311–335. [Google Scholar]
- 27.Harvey NC, Poole JR, Javaid MK, Dennison EM, Robinson S, Inskip HM, Godfrey KM, Cooper C, Sayer AA. Parental determinants of neonatal body composition. J Clin Endocrinol.Metab. 2007;92:523–526. doi: 10.1210/jc.2006-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith DW, Truog W, Rogers JE, Greitzer LJ, Skinner AL, McCann JJ, Harvey MA. Shifting linear growth during infancy: illustration of genetic factors in growth from fetal life through infancy. J Pediatr. 1976;89:225–230. doi: 10.1016/s0022-3476(76)80453-2. [DOI] [PubMed] [Google Scholar]
- 29.Karlberg J. A biologically-oriented mathematical model (ICP) for human growth. Acta Paediatr.Scand.Suppl. 1989;350:70–94. doi: 10.1111/j.1651-2227.1989.tb11199.x. [DOI] [PubMed] [Google Scholar]
- 30.Karlberg J, Engstrom I, Karlberg P, Fryer JG. Analysis of linear growth using a mathematical model. I. From birth to three years. Acta Paediatr.Scand. 1987;76:478–488. doi: 10.1111/j.1651-2227.1987.tb10503.x. [DOI] [PubMed] [Google Scholar]
- 31.Cooper C, Cawley M, Bhalla A, Egger P, Ring F, Morton L, Barker D. Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res. 1995;10:940–947. doi: 10.1002/jbmr.5650100615. [DOI] [PubMed] [Google Scholar]
- 32.Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr.Res. 2005;57:582–586. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 33.Morris FL, Naughton GA, Gibbs JL, Carlson JS, Wark JD. Prospective ten-month exercise intervention in premenarcheal girls: positive effects on bone and lean mass. J Bone Miner Res. 1997;12:1453–1462. doi: 10.1359/jbmr.1997.12.9.1453. [DOI] [PubMed] [Google Scholar]
- 34.Bradney M, Pearce G, Naughton G, Sullivan C, Bass S, Beck T, Carlson J, Seeman E. Moderate exercise during growth in prepubertal boys: changes in bone mass, size, volumetric density, and bone strength: a controlled prospective study. J Bone Miner Res. 1998;13:1814–1821. doi: 10.1359/jbmr.1998.13.12.1814. [DOI] [PubMed] [Google Scholar]
- 35.Alwis G, Linden C, Stenevi-Lundgren S, Ahlborg HG, Dencker M, Besjakov J, Gardsell P, Karlsson MK. A school-curriculum-based exercise intervention program for two years in pre-pubertal girls does not influence hip structure. Dyn.Med. 2008;7:8. doi: 10.1186/1476-5918-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weeks BK, Young CM, Beck BR. Eight months of regular in-school jumping improves indices of bone strength in adolescent boys and Girls: the POWER PE study. J Bone Miner.Res. 2008;23:1002–1011. doi: 10.1359/jbmr.080226. [DOI] [PubMed] [Google Scholar]
- 37.Alwis G, Linden C, Stenevi-Lundgren S, Ahlborg HG, Besjakov J, Gardsell P, Karlsson MK. A one-year exercise intervention program in pre-pubertal girls does not influence hip structure. BMC.Musculoskelet.Disord. 2008;9:9. doi: 10.1186/1471-2474-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKay HA, MacLean L, Petit M, MacKelvie-O’Brien K, Janssen P, Beck T, Khan KM. “Bounce at the Bell”: a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br.J Sports Med. 2005;39:521–526. doi: 10.1136/bjsm.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]