Abstract
Background
Aplasia cutis congenita is a disorder of the skin embryonic development characterized by a defect of localized or widespread areas of skin at birth. The lesions are mostly oval, 1-3 cm in diameter, with localization on the parietal part of scalp (60%) and rarely on the face and extremities.
Main observations
Herein, we reported a case of aplasia cutis congenita termly born at 39 weeks of gestation to a 30-year-old mother with bronchial asthma attacks. She was referred for 3 punched-out punctate depressed defective lesions in 0.4 cm’s diameter on the vertex covered with necrotic and hemorrhagic crusts. There was a hypertrichotic area consisting of tufts of terminal hair on the lumbosacral area over a sinus tract. Maternal perinatal drugs included aerosol salbutamol sulfate, ipratropium bromide and oral montelukast sodium for bronchial asthma. The pregnancy was firstly started as a di-chorionic, di-amniotic twin gestation, but deteriorated after the fetal resorption of the co-twin in the 20th gestational week resulting in fetus papyraceus.
Conclusion
In multi-gestational pregnancies, the presence of the fetus papyraceus or the death of the co-twins should make the neonatologists and dermatologists be aware of the possible cutaneous defects like aplasia cutis congenita. We emphasize that the possibility of this rare entity should be kept in mind in the presence of fetus papyraceus, perinatal drug use, maternal cigarette smoke, or maternal diseases like bronchial asthma in multiple gestations.
Keywords: alopecia, aplasia cutis congenita, bronchial asthma, cigarette smoking, faun tail naevus, fetus papyraceus, ipratropium, montelukast, nevoid hypertrichosis, salbutamol
Introduction
Aplasia cutis congenita (ACC) is a rare congenital disease of the embryonic skin development characterized by a defect of the localized or widespread areas of skin at birth where the skin layers and its adnexa are not created.[1] Defective lesions are mostly unique, circular or oval, 1-3 cm in diameter on the midline vertex of the scalp (60%) and rarely on the face and extremities.[1,2] Less often large, symmetric and extensive lesions are seen. It is seen about 1 in 10000 live births.[1,3] To date, more than 500 cases have been reported in the literature. The exact etiology of ACC is still uncertain. Causes of the disease could be genetic, teratogenic substances, placental infarcts, vascular compromise, intrauterine infections, intrauterine trauma, amniogenesis, adhesion of amniotic membrane to fetal skin, amniotic rupture sequence, ectodermal dysplasia, imperfect neural tube closure, mother’s intra-partum drug use.[1,2,4] The disease has been classified into 9 groups by Frieden according to the number and localization of the skin defects, etiopathogenesis like teratogens and presence of some accompanying malformations like limb abnormalities, epidermal and organoid nevi, epidermolysis bullosa or fetus papyraceus.[1]
A vanishing twin or "fetus papyraceus", also known as fetal resorption, is a fetus in a multi-gestation pregnancy which dies in utero and is then partially or completely reabsorbed by the mother or twin.[5] This phenomenon is also referred to as twin embolization syndrome or vanishing twin syndrome by means of ultrasonography. Occasionally, rather than being completely reabsorbed, the dead fetus is compressed by its growing twin to a flattened, parchmentlike state known as fetus papyraceus.[5] There are almost forty cases diagnosed with ACC accompanying with "fetus papyraceus" in the literature.[6-8] Up to now several teratogenic substances like methimazole and diclofenac are also blamed.[9-11] The diagnosis of ACC is based on the clinical features and the prognosis depends on the level of the other organs malfunction and the size of the lesions.
Case Report
A full-term girl in 3600 grams weight was born in the 39th week of gestation via cesarean section to a 30-year old G2P2 mother. She was the second child of non-consanguine parents. Her Apgar score was measured as 7 at 5 min. Five-day-old girl was referred to dermatology clinic for 3 punched-out punctate depressed defective lesions in 0.4 cm’s diameter on the vertex [Fig. 1]. They were hairless, and covered with necrotic and hemorrhagic crusts surrounded by a fibrous tissue. Defects appeared shallow and unattached to skull. The dermoscopic examination of the lesions showed bright yellow dots not associated with the hair follicles, lack of skin appendages with a translucent appearance confirming the presence of an area scaring alopecia in 1.5 cm’s size underneath the lesions. There was also a hypertrichotic area consisting of tufts of terminal hair on the lumbosacral area over a sinus tract called "faun tail naevus" [Fig. 2]. No other abnormalities were noted except than the widespread erythematous rash on her extremities and trunk, the shortness of her neck and the parchment like plantar hyperlinearity. Her nails and mucosae were normal. The family denied any history of familial similar lesions or blistering disorders.
Figure 1.
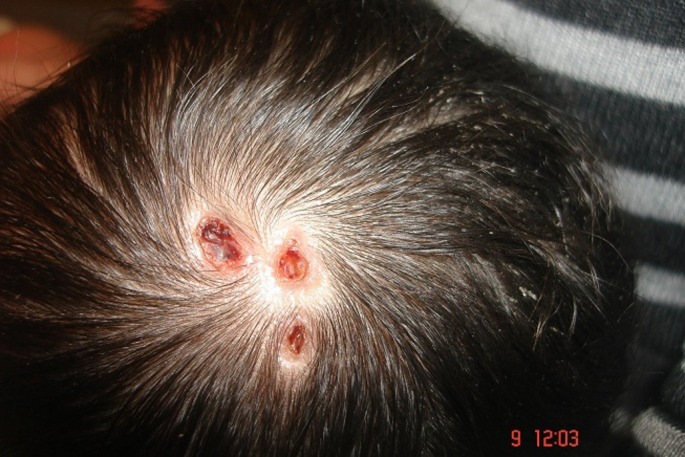
Three defective, punched-out, punctate, depressed lesions in 0.4 cm’s diameter on the vertex.
Figure 2.
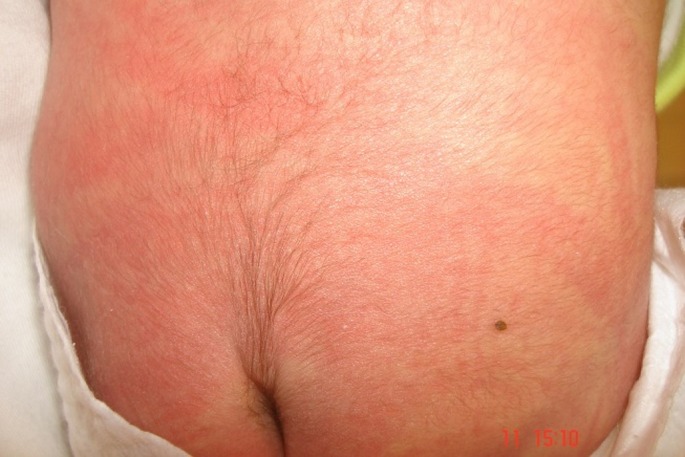
A hypertrichotic area consisting of tufts of terminal hair on the lumbosacral area over a sinus tract called "faun tail naevus".
All the routine laboratory examinations were normal except than a mild hyperbilirubinemia (total/direct bilirubin: 7.62 g/dl / 0.36 mg/dl). Maternal perinatal drugs included the vitamins, folic acid and aerosol 20 mcg/dosage salbutamol sulfates, 20 mcg/dosage ipratropium bromide and 10 mg/day montelukast sodium for bronchial asthma for 10 years. She also admitted smoking one packet of cigarette in a day during her all pregnancy. The maternal history was unremarkable except than several acute asthma attacks during the first trimester. The obstetrical history excluded the presence of any radiation exposure or teratogenic infection. The mother also denoted that her pregnancy was firstly started as a dichorionic-diamniotic twin gestation, and unfortunately deteriorated after the inutero exitus and resorption of one of the fetuses in the 20th gestational week. The fetal demise of the co-twin was detected in the ultrasonographic examination. The physical examination of the placental material also confirmed the presence of a flattened, mummified fetus "fetus papyraceus". The plain radiography and computerized tomography of the brain showed neither skull defect nor any brain malformations. The pediatric and neurological examinations of the newborn were normal for the age of the patient. Lumbosacral, abdomen, transfontanelle cranial ultrasonographies revealed no lesion, tumour, hematoma or hemorrhage excluding the presence of spina bifida or dysraphism. Cytogenetic report has shown karyotypes 46, XX with no changes on the chromosome number and structure. Conservative treatment with topical mupirocin 2% was started for 10 days, and she was discharged from the hospital on the 7th day without any complication. The crusts were disappeared with scaring lesions on the vertex and she has not experienced any new lesions in 6 months follow up.
Discussion
Hypertrichosis is one of the most frequent cutaneous marker of a spinal cord malformation (SCM) with an incidence varying between 20 and 50%.[12] In this case the presence of hypertrichosis, the underlying lumbosacral dermal sinus tract and the midline aplastic lesion on the scalp first made us suspect of the presence of a SCM. However our case was hopefully neurologically intact and did not undergo any surgery. As we know, this is the sixth reported case of ACC presented with a solitary limited scalp defect, accompanying with a faun tail naevus.[12-15] However, in most of the other reported cases with ACC accompanying with faun tail naevus an occult SCM like diastematomyelia was represented which was remarkably different from our case.[12-15]
According to Freiden, our case may be classified in both groups V and VIII as it was ACC associated with both fetus papyraceus and caused by teratogens.[1] There are almost forty cases diagnosed with ACC accompanying with "fetus papyraceus" in the literature.[6-8] There is a proposed hypothesis that the disruptive mechanical forces of tension and stretching on the embryonic skin and the underlying mesenchyme affects the separation of the neuroectoderm from the epithelial ectoderm which normally occurs along the mid-dorsal aspect of the embryo between the third and fifth weeks of the intrauterine life.[16] These compressive and disruptive effects which may cause a vascular compromise resulting in placental vascular anastomoses are the mostly considered pathogenic factors in fetus papyraceus-associated ACC, observed in almost 95% of the reported cases.[6-8] In the setting of the mono-chorionic gestation the vascular shunts between the two halves of the placenta resulting in transfusion syndrome cause the thromboplastic material formed after the in-utero death of the co-twin spread through the surviving fetus’s blood stream.[6] It is believed that the development of disseminated intravascular coagulation, thrombotic events and embolization cause abnormal skin development in the living fetus.[6] However, the presence of di-chorionic and di-amniotic gestation in this case excluded the probability of twin-to-twin transfusion syndrome and it was also exactly distinct from the other reported cases of fetus papyraceus-associated ACC cases. The abnormalities seen in this case may also be due to the presence of hypoxia caused by some vascular abnormalities like ischemia of the placental artery in twin gestation resulting in fetal demise and aplasia.[7] We also think that the history of maternal excessive cigarette smoking might also have facilitated this situation in this case. It is also believed that the demise of the co-twin might have given rise to the surviving fetus suffered from acute hypervolemia which leads to the ischemia of the skin and the internal organs.[7] However, the reason of the variabilities in the severity of the cases of ACC associated with fetus papyraceus is still unexplained. The fetal death time may also determine the severity and size of the aplasia seen in the surviving fetus.[7,8] If the death occurs in the late first to early second trimester; before 14 weeks gestation the lesions tend to be more in trunk rather than in extremities.[7] Also smaller, linear, actuate, triangular and well-demarcated lesions are expected according to the current consensus. On the other hand, the presence of large, stellate or angulated lesions mostly located on the extremities is a useful marker to predict the time of the fetal late demise.
The limited defective lesions seen in our case was exactly smaller than the most other reported cases with fetus papyraceus-associated ACC, which demonstrated classical large and symmetrical lesions most widely distributed on the trunk and extremities.[6-8] However, the central, small, punched-out lesions seen in our case on the midline was completely incompatible with the literature, as the fetal death was seen in 20th week of gestation. We believe that this case is a rare variant of Type V ACC among the other published cases due to the presence of an isolated aplastic scalp lesion.[6-8] The reason why ACC is mostly seen in the vertex area of the scalp and why some cases are affected phenotypically mild and some severe is still controversial. The two different type of scalp lesions; are known as the "mild" form of the neural tube closure defect or "membranous type" like in our case and the "irregular" or "non-membranous type".[17] The presence of possible different etiopathogenetic mechanisms and abnormalities in formation of the embryonic scalp skin may cause those clinical different presentations. We also believe that the dose, time and duration of the perinatal drug exposure may also have an effect on the severity of the lesions.
Up to now, cases diagnosed with ACC secondary to drugs like methimazole and diclofenac sodium are already reported.[9-11] However, perinatal exposures of montelukast sodium, salbutamol or ipratropium bromide have not been blamed before for the development of ACC. However, none of these drugs are reported to be teratogenic up to now. Although montelukast refer to FDA pregnancy category B, salbutamol or ipratropium bromide refer to FDA pregnancy category C. Ipratropium has been shown to increase fetal resorption and decrease conception rates when only administered at doses above approximately 5400 times the maximum recommended human inhalation dose. Rare reports of various congenital anomalies following intrauterine exposure to salbutamol (including cleft palate, limb defects and cardiac disorders) have been also reported, but none of them are evidence based.[18] On the other hand in a recent clinical study investigating the influence of salbutamol, a beta-2-receptor-stimulating agent, on the blood flow through the utero-placental unit evaluated in the human serial placenta scintigrams were analyzed quantitatively.[19] It was found that in the third trimester of pregnancy salbutamol infusion causes a decrease in blood flow in the absence of uterine contractions.[19]
The recent increase in the in-vitro fertilization technics caused a remarkable rise in the incidence of multiple gestation pregnancies nowadays. We believe that all the dermatologists and neonatologists should be aware of this rare entity in multi-gestational pregnancies in the presence of the fetus papyraceus or the death of the co-twins. In this case we believe that both the resulting hypoxemia and the vascular ischemia of the utero-placental artery caused by the possible vasoconstructive effects of maternal cigarette smoking, bronchial asthma attacks, and fetus papyraceus might give rise to the development of a skin aplasia. In the presence of ACC or any midline malformation cranial, lumbosacral and abdominal ultrasonography and magnetic resonance imaging studies should be performed to exclude the presence of a SCM and prevent the neurological further sequels and morbidities.
References
- Frieden IJ. Aplasia cutis congenita: a clinical review and proposal for classification. J Am Acad Dermatol. 1986;14:646–660. doi: 10.1016/s0190-9622(86)70082-0. [DOI] [PubMed] [Google Scholar]
- Demmel U. Clinical aspects of congenital skin defects. I. Congenital skin defects on the head of the newborn. Eur J Pediatr. 1975;121:21–50. doi: 10.1007/BF00464392. [DOI] [PubMed] [Google Scholar]
- Lataifeh IM, Khriesat WM, Baqain EB, Al Qarqaz FA, Abuekteish F. Aplasia cutis congenita associated with coarctation of the aorta: case report and review of the literature. Int J Dermatol. 2009;48:1222–1224. doi: 10.1111/j.1365-4632.2009.04158.x. [DOI] [PubMed] [Google Scholar]
- Gorlin RJ, Cohen MM Jr, Hennekam RCM. Syndromes affecting the skin and mucosa. In: Syndromes of the Head and Neck, 4th edn. New York: Oxford University Press; 2001. pp. 494–498. [Google Scholar]
- Landy HJ, Weiner S, Corson SL, Batzer FR, Bolognese RJ. The "vanishing twin": ultrasonographic assessment of fetal disappearance in the first trimester. Am J Obstet Gynecol. 1986;155:14–19. doi: 10.1016/0002-9378(86)90068-2. [DOI] [PubMed] [Google Scholar]
- Blouin MM, Bernard J, Caron F, Auger I. Aplasia cutis congenita of the trunk and scalp associated with fetus papyraceus. Int J Dermatol. 2011;50:733–735. doi: 10.1111/j.1365-4632.2010.04619.x. [DOI] [PubMed] [Google Scholar]
- Klein RQ, Robinson DM, Lieber CD, Antaya RJ. Symmetric aplasia cutis congenita associated with fetus papyraceus: report of two cases. Pediatr Dermatol. 2011;28:467–469. doi: 10.1111/j.1525-1470.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- Schaffer JV, Popiolek DA, Orlow SJ. Symmetric truncal aplasia cutis congenita following multifetal reduction of a sextuplet pregnancy. J Pediatr. 2008;153:860–863. doi: 10.1016/j.jpeds.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Rodríguez-García C, González-Hernández S, Hernández-Martín A, Pérez-Robayna N, Sánchez R, Torrelo A. Aplasia cutis congenita and other anomalies associated with methimazole exposure during pregnancy. Pediatr Dermatol. 2011;28:743–745. doi: 10.1111/j.1525-1470.2011.01572.x. [DOI] [PubMed] [Google Scholar]
- Abe M, Syuto T, Yokoyama Y, Ishikawa O. Aplasia cutis congenita after methimazole exposure in utero successfully treated with basic fibroblast growth factor. Int J Dermatol. 2010;49:334–335. doi: 10.1111/j.1365-4632.2009.04254.x. [DOI] [PubMed] [Google Scholar]
- Pajaziti L, Rexhepi S, Shatri-Muça Y, Ferizi M. The role of diclofenack on inducing of aplasia cutis congenita: a case report. Cases J. 2009;2:150. doi: 10.1186/1757-1626-2-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Singal A, Pandhi D. Faun tail naevus: a cutaneous marker of spinal dysraphism. Indian Pediatr. 2005;42:67–69. [PubMed] [Google Scholar]
- Cho AY, Lee SS, Lee Y, Kim CD, Lee JH, Seo YJ. Aplasia cutis congenita with hair collar sign and dermal melanocytosis. Int J Dermatol. 2012;51:745–747. doi: 10.1111/j.1365-4632.2010.04641.x. [DOI] [PubMed] [Google Scholar]
- Calikoğlu E, Oztaş P, Yavuzer Anadolu R, Catal F, Görpelioğlu C. Faun tail with aplasia cutis congenita and diastematomyelia. Dermatology. 2004;209:333–334. doi: 10.1159/000080859. [DOI] [PubMed] [Google Scholar]
- Mittler MA, McComb JG. Idiopathic thoracolumbar syrinx with cutaneous marker. Pediatr Neurosurg. 1999;30:100–101. doi: 10.1159/000028771. [DOI] [PubMed] [Google Scholar]
- Stephan MJ, Smith DW, Ponzi JW, Alden ER. Origin of scalp vertex aplasia cutis. J Pediatr. 1982;101:850–853. doi: 10.1016/s0022-3476(82)80346-6. [DOI] [PubMed] [Google Scholar]
- Cambiaghi S, Maffeis L, Restano L, Gelmetti C. Hypertrophic scarring is the usual outcome of non-membranous aplasia cutis of the scalp. Pediatr Dermatol. 2009;26:362–363. doi: 10.1111/j.1525-1470.2009.00928.x. [DOI] [PubMed] [Google Scholar]
- Munsie JW, Lin S, Browne ML, Campbell KA, Caton AR, Bell EM, Rasmussen SA, Romitti PA, Druschel CM. et al. Maternal bronchodilator use and the risk of orofacial clefts. Hum Reprod. 2011;26:3147–3154. doi: 10.1093/humrep/der315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnäs S, Joelsson I, Lewander R, Lundqvist H, Lunell NO, Sarby B, Aström H. The effect of beta-receptor-stimulating agents on the utero-placental blood flow. Acta Obstet Gynecol Scand. 1977;56:297–301. doi: 10.3109/00016347709154982. [DOI] [PubMed] [Google Scholar]


