Abstract
Objective
We aimed to perform a systematic review and meta-analysis examining the impact of TB treatment at the time of combination antiretroviral therapy (cART) initiation on subsequent mortality.
Methods
We searched PubMed, EMBASE, and selected conference proceedings for studies that report adult mortality on cART, stratified by TB treatment status at cART initiation. Stratified random-effects and meta-regression analyses were used to examine the influence of study and population characteristics.
Results
22 eligible cohort studies reported data on 98,350 (range 74-15,225) adults, of whom 14,779 (15%) were receiving TB treatment at cART initiation. Studies of those receiving vs. not receiving TB treatment had an average mortality relative risk of 1.10 (95% confidence interval 0.87-1.40) at 1-3 months (based upon 8 estimates), 1.15 (0.94-1.41) at 6-12 months (11 estimates), and 1.33 (1.02-1.75) at 18-98 months (10 estimates) following cART initiation. However, there was a wide range of estimates and those at later time points were markedly heterogeneous. Meta-regression identified factors associated with elevated average risk estimates: lower median baseline CD4 counts and adjustment for baseline hemoglobin at 1-3 months; longer length of follow-up and women-only studies at 6-12 months; and not adjusting for BMI/weight at 18-98 months.
Conclusions
Patients receiving TB treatment at cART initiation did not have a statistically significant estimated increase in short-term risk of all-cause mortality as compared to those not receiving TB treatment. TB treatment was significantly associated with increased mortality after about a year of cART, suggesting that patients with concurrent TB treatment at cART initiation may benefit from continued support after TB treatment completion.
Introduction
Tuberculosis (TB) continues to threaten the health of people living with HIV (PLWH). Globally in 2011, 13% of incident TB cases were co-infected with HIV and an estimated 0.4 million TB deaths occurred among PLWH [1]. Access to combination antiretroviral therapy (cART) has dramatically increased survival, but a substantial number of PLWH die during the first year of cART. The majority of deaths occur within the first three months [2-5]. Autopsy studies have consistently shown TB to be an important cause of death in PLWH, both in the pre-cART [6-8] and cART eras [9].
In 2010, a meta-analysis of the effect of TB on mortality found PLWH also suffering from TB had a greater risk of mortality than TB-free individuals (hazard ratio [HR]: 1.8, 95% confidence interval [CI]: 1.4-2.3) [10]. Due to the paucity of studies reporting on patients on cART, the authors concluded that the effect of TB on mortality in PLWH exposed to cART must be further evaluated once more cohort study results become available.
Given the World Health Organization’s 2010 recommendation that all PLWH with TB be initiated on cART, regardless of CD4 count [11], and the goal of 100% cART coverage of co-infected patients by 2015 [12], many individuals will be initiating cART while concurrently on treatment for confirmed or clinically suspected active TB. PLWH who are also being treated for TB may experience a differential response to cART due to drug-drug interactions [13,14], an increased risk of drug toxicity [13,14], immune reconstitution inflammatory syndrome [15], and the potential for lower adherence due to the high pill burden [14]. The effect of TB treatment and its associated potential challenges and complications on a patient’s response to cART is not yet clear [16].
We aim to describe the impact of receiving TB treatment at the time of cART initiation on subsequent mortality among HIV-infected adults. We performed separate analyses to identify the effect at 1-3 months, 6-12 months, and 18-98 months.
Methods
Search strategy and selection criteria
To investigate the effect of TB treatment at the time of cART initiation on mortality, we carried out a systematic and sensitive search using an a priori protocol developed according to PRISMA guidelines [17] (Checklist S1). We searched PubMed and EMBASE, as well as abstract databases from the 2009 to 2012 Conferences on Retroviruses and Opportunistic Infections, International Union Against Tuberculosis and Lung Disease World Conferences on Lung Health, and International AIDS Society conferences. The search terms “HIV AND Tuberculosis AND (Viral Load OR CD4 lymphocyte count OR Mortality) AND Antiretroviral therapy” were used to identify relevant articles in PubMed and EMBASE. Searches were performed on March 1, 2013 and included original human subjects cohort studies published since 1997 (the start of the cART era). Additional articles were identified from reference lists, reviews, and Web of Science citation lists.
H.M.S. and A.V.R. independently reviewed titles and abstracts of original studies retrieved by the search. H.M.S. reviewed full-text and references of selected articles. H.M.S. and M.R.P. independently abstracted study data from full reports; discrepancies were resolved by consensus or consultation with A.V.R. and C.P.
Studies were included if they included both antiretroviral-naïve HIV-infected individuals receiving and not receiving TB treatment at cART initiation, and reported mortality after cART initiation stratified by TB treatment status at cART initiation. cART was defined as a treatment regimen containing three or more antiretrovirals. Though we sought reports of cART-naïve patients, studies with ≤10% antiretroviral-experienced patients or patients only previously exposed to a single intrapartum dose of nevirapine were also included. Studies of children <14 years of age were excluded. No additional exclusion criteria or language restrictions were imposed.
Data extraction
The following information, if available, was abstracted from each article: first author surname; publication year; study design; study dates; length of follow-up; geographic location; clinical setting; sample size; number receiving and not receiving TB treatment; if TB treatment was the main exposure of interest; types of TB included; culture confirmation of TB cases; TB site; timing of TB treatment in relation to cART initiation; percentage male; mean or median participant age; proportion treatment-naïve; cART regimen; baseline median CD4 count and HIV-RNA; measure of effect or event counts; covariate adjustment; proportion lost-to-follow-up; and if mortality was confirmed using a national death registry.
Statistical analysis
Reported mortality effect-measure estimates over any length of time were abstracted. If only survival proportions among those receiving or not receiving TB treatment were reported, a risk ratio (RR) and 95% CI were calculated, as the RR approximates the HR for an uncommon outcome. If an effect-measure estimate was reported for those not receiving vs. receiving TB treatment, the inverse of the reported effect-measure estimate and CI were included. If only a p-value from a univariate logistic regression model was presented, the 2x2 table was reconstructed and a RR was calculated. Standard error estimates were inferred from reported CIs by [ln(upper limit) - ln(lower limit)]/3.92 [18].
As tuberculosis is successfully treated in most patients after six months of treatment, and the hazard of mortality following cART initiation is not constant, we grouped available cumulative effect estimates according to length of follow-up: 1-3 months, 6-12 months, and 18-98 months. None of the eligible studies reported estimates at 4-5 months or 13-17 months following cART initiation. If a study reported multiple estimates within a time category, the time frame closest to the category midpoint was included in the primary analysis and other time estimates were examined in sensitivity analyses.
The method of moments estimate of the among-populations variance (τ2) and random-effects summarization using unconditional variances were used to combine relative risks in the three groups [19]. The p-values for a standard chi-square homogeneity test statistic were used to assess overall consistency among the effect estimates. τ2 was used to calculate 95% population effects intervals [20] (where 95% of populations are estimated to have their relative risks), opposite effects proportions [21] (proportion of populations estimated to experience a relative risk on the opposite side of the estimated mean, in this case below unity), and 95% prediction intervals [21] (95% of these intervals will cover the true value estimated by a future study). Stratified and random-effects meta-regression analyses were used to calculate stratum-specific summary measures and 95% CIs, along with ratios of the average RRs as described by Thompson and Sharp [22]. Study characteristics with at least two studies per stratum were eligible for inclusion in the meta-regression.
Funnel plots of ln(mortality relative risk) vs. the inverse-variance weight of studies for each time category were visually examined for asymmetry and statistically assessed using methods proposed by Begg [23] and Egger [24] and the trim-and-fill method of Duval and Tweedie [25]. STATA (version 12, Stata corporation, College Station, TX) was used for these analyses.
Results
Selected studies
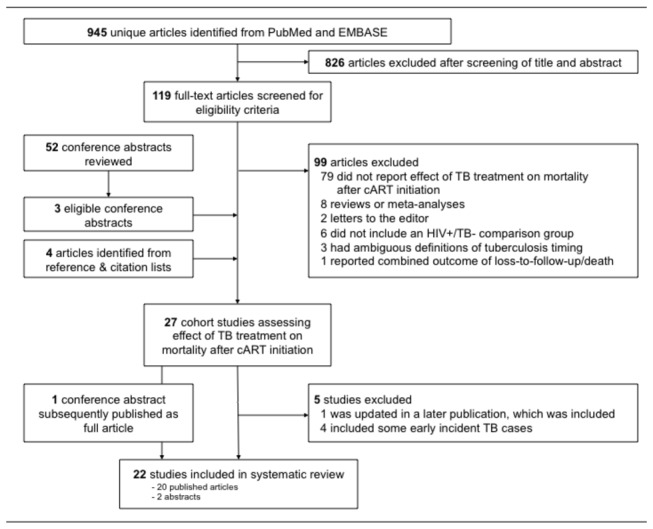
997 unique abstracts were reviewed: 824 from PubMed, 121 additionally from EMBASE, and 52 from conference proceedings (Figure 1). Of these, 119 full-text articles were selected for review. In total, 20 articles [2,26-44] and three conference abstracts [45-47] were eligible. Four additional articles met our inclusion criteria: two from reference lists [48,49] and two from Web of Science citation searches [50,51]. One included abstract [46] was subsequently published as a full article [52]; only data from the full article was included. Five eligible studies were excluded: one [43] because a 2012 paper [29] provided an updated estimate; four because they included some early incident TB cases in their TB treatment-exposed group [28,38,39,42].
Figure 1. Identification and selection of eligible studies.
Study and population characteristics
The 22 final studies provided data on 98,350 PLWH, of which 14,779 (15%) were receiving TB treatment at cART initiation. Selected study and population characteristics are displayed in Table 1. All were cohort studies. Most publications assessed mortality after cART initiation, regardless of regimen type, though some reported estimates specific to nevirapine-[31,41,48,49] or efavirenz-based [26,31,37] therapy. See Table S1 for cART regimens used by each study. All studies used cART initiation as the time origin, except one which began at the commencement of cART education and adherence sessions, with most patients starting cART a month or two later [52]. Four studies included women previously exposed to a single intrapartum dose of nevirapine [30-33], and two studies included 3% [29] and 6% [36] antiretroviral-experienced patients.
Table 1. Characteristics of 22 studies reporting the effect of TB treatment on mortality after cART initiation among HIV-infected adults.
| Study | Publication year | Geographic location | Sample size |
TB treatment, N (%)
|
Main exposure is TB | Study design | cART regimen a | Naïve, % | Male, % | Mean age, years | Median baseline CD4 count b , cells/μL | Median baseline HIV-RNA, log10 copies/mL | Lost to follow-up, % | Overall mortality c,% | Confirmed mortality with national death registry | Duration of follow-up, months | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bassett[52] | 2012 | South Africa | 951 | 343 | (36) | Yes | P | All | 100 | 41 | 36 | 90 | NS | 7 | 10 | No | 12 |
| Bera[30] | 2009 | South Africa | 385 | 25 | (7) | No | P | All | 100 | 0 | 28 | 173 | 4.6 | 4 | 2 | No | 8 |
| Bhowmik[27] | 2012 | India | 743 | 285 | (38) | No | R | All | 100 | 66 | 35 | 140 | NS | NS | 7 | No | 12 |
| Boulle (a)[31]d | 2008 | South Africa | 1935 | 209 | (11) | Yes | P | NVP | 100 | 21 | 31 | 110 | 5 | 6 | 4 | No | 6 |
| Boulle (b)[31]d | 2008 | South Africa | 2035 | 1074 | (53) | Yes | P | EFV | 100 | 40 | 33 | 78 | 5.2 | 6 | 6 | No | 6 |
| Boulle (a,b)[32] e | 2010 | South Africa | 7323 | 2760 | (38) | No | P | All | 100 | 32 | 33 | 101 | 5.1 | 10 | NS; 16 | Yes | 3; 3-60 |
| Chu[47] | 2011 | Uganda | 15225 | 1177 | (8) | Yes | P | All | 100 | NS | NS | NS | NS | NS | 7 | No | 65 |
| Dao[33] | 2011 | Zambia/Kenya | 661 | 56 | (8) | No | P | All | 100 | 0 | 32 | 147 | 5 | 5 | 8 | No | 12 |
| DeSilva[34] | 2009 | Nigeria | 1552 | 251 | (16) | No | R | All | 100 | 29 | 34 | 112 | NS | 9 | 7 | No | 24 |
| Dronda[35] | 2011 | Spain | 1986 | 110 | (6) | Yes | P | All | 100 | 76 | 38 | 196 | 5.0 | 7 | 3 | No | 47 |
| Greig[36] | 2012 | Sub-Saharan Africa | 14523 | 1159 | (8) | No | R | All | 94 | 35 | 36 | 133 | NS | 12 | 7 | No | 30 |
| Gupta[51] | 2013 | South Africa | 1544 | 464 | (30) | Yes | P | All | 100 | 30 | 34 | 98 | 4.9 | 21 | 13 | No | 100 |
| Lartey[37] | 2011 | Ghana | 74 | 34 | (46) | Yes | P | EFV | 100 | 49 | NS | 83 | 5.4 | 11 | 9 | No | 11 |
| Liechty[40] | 2007 | Uganda | 377 | 32 | (8) | No | P | All | 100 | 29 | 38 | 50 | 5.5 | 0 | 6 | No | 3 |
| Makombe (a,b) [49]e | 2007 | Malawi | 12485 | 1339 | (11) | Yes | R | NVP | 100 | NS | NS | NS | NS | 11 | 12; 13 | No | 6; 12 |
| Manosuthi[41] | 2010 | Thailand | 140 | 70 | (50) | Yes | P | NVP | 100 | 68 | 36 | 31 | 5.6 | 11 | 6 | No | 48 |
| Mugusi (a,b)[26]e | 2012 | Tanzania | 449 | 194 | (43) | Yes | P | EFV | 100 | 42 | 40 | 92 | 5.7 | 12 | 3; 11 | No | 1; 11 |
| Mutevedzi (a,b)[50]f | 2011 | South Africa | 7927 | 1752 | (22) | No | R | All | 100 | 33 | 34 | 117 | 4.4 | 11 | 5; 3 | No | 3; 3-12 |
| Mutevedzi (c,d)[50]f | 2011 | South Africa | 919 | 175 | (19) | No | R | All | 100 | 44 | 54 | 127 | 4.5 | 11 | 6; 6 | No | 3; 3-12 |
| Nguyen[45] | 2011 | Vietnam | 370 | NS | NS | No | R | All | 100 | 66 | 33 | NS | NS | NS | 31 | No | 60 |
| Stringer (a,b)[2]e | 2006 | Zambia | 14306 | 1562 | (11) | No | P | All | 100 | 39 | 35 | 143 | NS | 21 | 6; 9 | No | 3; 18 |
| Westreich (a,b)[29]e | 2012 | South Africa | 7512 | 1197 | (16) | Yes | P | All | 97 | 34 | 35 | 88 | NS | NS | 7; 9 | Yes | 36; 54 |
| Zachariah[44] | 2006 | Malawi | 1507 | 225 | (15) | No | P | All | 100 | 34 | 35 | 123 | NS | 3 | 8 | No | 3 |
| Zachariah[48] | 2009 | Malawi | 2289 | 196 | (9) | No | R | NVP | 100 | 31 | 35 | NS | NS | 5 | 9 | No | 3 |
Abbreviations: cART, combination antiretroviral therapy; EFV, Efavirenz-based cART; HIV, human immunodeficiency virus; NS, not specified; NVP, Nevirapine-based cART; P, prospective study; R, retrospective study; TB, tuberculosis.
See Table S1 for detailed information on cART regimens from each study, if available
See Table S4 for baseline CD4 cell count stratified by TB treatment status from each study, if available
If a study reported relative risks for multiple time periods, an overall mortality estimate is indicated for each respective time period
(a) Nevirapine-based cART; (b) Efavirenz-based cART
For these studies, the letters after the author’s name refer to the estimates reported at differing time points. For example, Makombe (a) refers to the 6-month estimate and Makombe (b) refers to the 12-month estimate.
(a,b) patients <50 years old; (c,d) patients ≥50 years old
Ten studies examined TB treatment at cART initiation as the main exposure of interest [26,29,31,35,37,41,47,49,51,52]. The other twelve studies examined TB treatment as a secondary exposure; five examined any predictors of mortality [27,33,34,44,48], three aimed to describe general cART outcomes [2,30,32], and one each examined the primary exposures of integrated vs. vertical HIV programs [36], positive serum cryptococcal antigen [40], age [50], and hepatitis B and C co-infections [45]. The type of TB being treated varied across studies (Table S2). Only two studies had a substantial subset of bacteriologically-confirmed TB cases [51,52] while others included both confirmed and probable TB cases. Most articles included any TB being treated at the time of cART initiation, whereas others used a specific time period such as new TB diagnosis at study entry [26,38] or diagnosis ≥1 month prior to enrollment [41]. Nine studies reported detail on the duration of TB treatment at the time of cART initiation (Table S3). One study focused solely on pulmonary TB [29], and one censored patients in the reference group that developed incident TB [31].
Mortality was mainly assessed using medical records and/or confirmation from family or health workers, however two studies additionally used national death registries [29,32]. In an effort to quantify late mortality, two studies excluded deaths occurring in the first three months [32,50]. Overall loss-to-follow-up was reported by 19 studies and ranged from 0% to 21% (median 9%).
Two studies were limited to women only [30,33]. Mean patient age ranged from 28 to 40 years (median 35 years), with the youngest included age being 14 years [31]. One study stratified mortality estimates according to age [50]. Median baseline HIV-RNA ranged from 4.4 to 5.7 log10 copies/mL (median 5.1), and baseline CD4 count ranged from 31 to 196 cells/μL (median 111). See Table S4 for baseline CD4 count stratified by TB treatment status from each study, if available.
Relative risk of mortality
Thirty-one cumulative mortality relative risks were reported or calculated among those receiving vs. not receiving TB treatment at cART initiation (Figure 2). Most were HRs, though eight RRs [26,30,37,41,47,49,52], four odds ratios [27,40,44,48], and one incidence rate ratio [51] were also included. Among the 21 adjusted estimates, 20 adjusted for baseline CD4 count, 19 for gender, 18 for age, 13 for BMI or weight, eight for hemoglobin, and two for adherence (defined as timeliness of pharmacy attendance). Including only the estimate corresponding to the longest follow-up time from each study, the summarized relative risk of mortality in those receiving vs. not receiving TB treatment across all time periods was 1.24 (95% CI 1.05-1.46, τ2 = 0.097).
Figure 2. Forest plot of mortality relative risks reported by 22 studies.
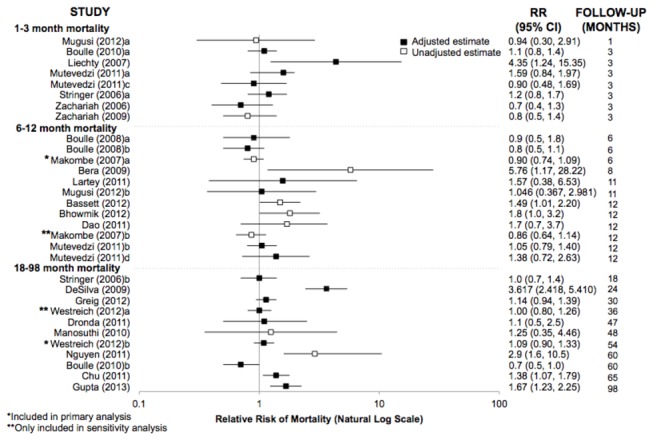
The relative risks correspond to the estimated effect of receiving vs. not receiving TB treatment at the time of cART initiation on subsequent mortality among HIV-infected adults. Estimates are ordered according to length of follow-up time. Estimates were abstracted according to the precision used by the original authors; estimates calculated using available data are reported to 2 decimal places. Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; RR, relative risk; TB, tuberculosis.
Eight relative risks of mortality by 1-3 months were reported, without evidence of substantial heterogeneity (homogeneity p-value = 0.11, Table 2). The random-effects summary estimate was 1.10 (95% CI 0.87-1.40, τ2 = 0.045). The eleven mortality relative risks by 6-12 months produced a random-effects summary estimate of 1.15 (95% CI 0.94-1.41, τ2 = 0.041). By 18-98 months, the summarized relative risk indicated increased mortality among patients receiving TB treatment (random effects relative risk [RRRE] = 1.33, 95% CI 1.02-1.75, τ2 = 0.134), based upon 10 estimates. There was evidence of considerable heterogeneity for the 6-12 and 18-98 month estimates (homogeneity p-values 0.06 and <0.01, respectively).
Table 2. Meta-analysis results for the effect of TB treatment on mortality, by length of follow-up time.
| Length of follow-up time | 1-3 months | 6-12 months | 18-98 months |
|---|---|---|---|
| No. of studies | 8 | 11 | 10 |
| Homogeneity p-value | 0.106 | 0.063 | <0.001 |
| Estimate of between-study variance (τ2) | 0.045 | 0.041 | 0.134 |
| RRRE (95% CI) | 1.10 (0.87, 1.40) | 1.15 (0.94 1.41) | 1.33 (1.02, 1.75) |
| 95% population effects interval | (0.73, 1.67) | (0.77, 1.71) | (0.65, 2.73) |
| Opposite effects proportion | 32.2% | 24.8% | 21.6% |
| 95% prediction interval | (0.62, 1.97) | (0.69, 1.91) | (0.55, 3.23) |
Abbreviations: CI, confidence interval; RRRE, random-effects summary relative risk; TB, tuberculosisRR: risk ratio
Meta-regression
Meta-regression results are displayed in Table S5. For 1-3 month mortality, lower median baseline CD4 counts and adjustment for baseline hemoglobin appear to produce somewhat higher relative risks. Among the 6-12 month estimates, length of follow-up appeared to have the most influence and produced the most homogenous strata, suggesting that this 6-month window may be too wide. At 6 to 9 months following cART initiation, those receiving vs. not receiving TB treatment had a RRRE of 0.93 (95% CI 0.68-1.28, homogeneity p-value = 0.14), whereas at 11 to 12 months the RRRE was 1.29 (95% CI 1.06-1.56, homogeneity p-value = 0.61), indicating an increased risk of mortality after completion of TB treatment. Studies limited to women only also reported higher RRs (RRRE = 2.57, 95% CI 0.83-7.98). Among 18-98 month estimates, a longer follow-up time does not appear to influence the results. However, adjustment for BMI or weight tended to move the relative risk toward the null (RRRE = 1.01, 95% CI 0.84-1.21).
Funnel plot analysis
For 1-3 month estimates, the funnel plot gives the visual appearance of a slight skew to the left (Document S1), but both tests for asymmetry yielded p-values of 0.8, and only one hypothetical missing result was imputed on the right by trim-and-fill analysis, with little influence on the summary results. In contrast, funnel plots for 6-12 and 18-98 months were skewed more noticeably to the right and the tests for asymmetry yielded lower p-values (Begg p = 0.2, Egger p = 0.03 for 6-12 months, Begg p = 0.3 and Egger p = 0.4 for 18-98 months). Trim-and-fill imputed four hypothetically missing results at 6-12 months and two at 18-98 months, all on the left. The imputation shifted the estimate of the random-effects average RR from 1.15 to 1.02 at 6-12 months and from 1.33 to 1.14 at 18-98 months.
Sensitivity analyses
As Makombe et al. (2007) [49] contained multiple estimates of mortality in the 6-12 month time frame, only the six-month estimate was included in primary analyses. The analysis was then repeated, substituting in the 12-month estimate. As this alternative estimate was similar in point estimate and precision to the 6-month estimate, the substitution did not affect the results or our conclusions. Likewise, Westreich et al. (2012) [29] reported estimates at 36 months and 54 months. Regardless of which was included, the analysis results did not substantially differ. Additionally, as some studies reported asymmetrical confidence intervals, a sensitivity analyses assessed their impact on the summary measures (Document S2).
Discussion
This systematic review and meta-analysis of studies reporting the effect of TB treatment at cART initiation on subsequent mortality among PLWH initiating therapy found that TB treatment did not significant affect mortality on cART in the short-term, but was associated with increased mortality after about a year of cART. These results are important given the many concerns about co-treatment including drug-drug interactions, overlapping drug toxicities, immune reconstitution inflammatory syndrome, and high pill burden. Our findings were not sensitive to geographical study location (Africa vs. Asia or Europe).
Overall, the summary estimates for early mortality were lower than estimates for later mortality. This finding may be contrary to expectations, but could be due to several factors. First, patients on concurrent TB therapy at cART initiation may experience a lower short-term risk of all-cause mortality because TB medications are also effective against infectious diseases other than TB [53]. Second, most PLWH receiving TB treatment often receive co-trimoxazole preventive therapy, which further reduces the risk of death from non-TB infectious diseases. Third, prior to cART initiation, PLWH on TB treatment may have been engaged in care for longer than other PLWH. Fourth, TB deaths can occur early during TB treatment, i.e., prior to the initiation of cART, creating possible left-censoring. However, PLWH not diagnosed with TB also may die prior to cART initiation, and studies did not provide enough information to determine if left-censoring was differential between the two groups. Fifth, undiagnosed and untreated TB among the comparison group may have biased estimates of early mortality toward the null. Autopsy studies consistently show that undiagnosed TB continues to be a major cause of death among HIV-infected adults [6-8], even in the cART era [9].
This meta-analysis has a number of limitations. Since being treated for active TB is not an exposure suitable for a randomized controlled trial, all studies included in our meta-analysis were observational and subject to biases. First, the outcome of mortality may have been misclassified in people lost-to-follow-up. Rates of follow-up loss were variable in the included studies, ranging from 0% to 21%. cART programs with high losses of patients and incomplete death ascertainment can seriously underestimate mortality, with 12% to 87% of patients loss-to-follow-up in fact being deceased [54]. Misclassification of deaths would only produce bias in the estimated relative risks if this misclassification was differential between compared groups [55] or dependent on errors in measuring other variables. This is especially relevant for mortality estimates during the first six months of cART, when loss-to-follow-up may be differential due to regular follow-up for TB treatment. We attempted to examine if mortality confirmation by national death registry affected mortality effect estimates. This was only possible for two studies, both with 18-98 months of follow-up.
Second, the exposure, prevalent TB treatment at cART initiation, captures both confirmed active TB disease and exposure to anti-tuberculosis drugs for clinically suspected TB. This combined exposure is useful from a health systems perspective, particularly in low-resource countries, where active TB cannot always be confirmed, especially in PLWH. The included studies used a variety of methods for determining who had active TB and should receive treatment, and no studies were exclusively limited to bacteriologically-confirmed TB cases. Consequently, active TB could have been misclassified and some patients included in this meta-analysis may have received TB treatment even though they did not have TB. In addition, some patients with active TB may not have been diagnosed.
Third, there was much heterogeneity in the duration of TB treatment prior to cART initiation, with some patients on TB therapy for six months and others beginning TB treatment and cART concurrently. While the timing of TB treatment in relation to cART initiation is an important variable to consider when evaluating mortality [43,56-59], the included studies did not provide enough information on duration of TB treatment to systematically evaluate its effect on our results. Future studies should describe the duration of TB treatment in more detail to facilitate meta-analysis, though a pooled patient-level analysis or randomized controlled trial are study designs better suited to assess this.
Some degree of funnel plot asymmetry was apparent for each time period, possibly due to publication bias or other factors. A direct effect measure was not available from each study, and published estimation methods [18] were used to calculate the effect measure for five studies. These estimation techniques involve a number of assumptions and may have introduced bias or affected variance estimates. Additionally, in 12 of 22 studies, TB treatment was not the primary exposure and covariates included in multivariate models may differ from ideal confounder adjustment for this research question. Some studies only included TB treatment in their multivariable model for mortality because it was a statistically significant predictor, which may explain some funnel plot asymmetry.
It was difficult to examine the influence of study or population characteristics on the effect estimates, as grouping studies by specific characteristics produced small strata with imprecise estimates. Furthermore, some characteristics are correlated. For example, risk ratios and odds ratios tended to be higher than hazard ratios, but the former also tended to be unadjusted estimates. Also, as studies adjusted for sets of key covariates, it was difficult or impossible to separate out the influence of adjusting for a specific covariate (see footnotes of Table S5).
Given the patient characteristics of the included studies, the results are most generalizable to therapy-naïve adults in Sub-Saharan Africa with relatively low CD4 counts at cART initiation. More studies in populations outside of sub-Saharan Africa, particularly in North America, would be useful additions to the literature. This review only includes studies among adults; it is unknown whether the relationship holds in children. Similarly, it is possible that cART regimen would modify the effect of TB treatment due to drug-drug interactions, and regimen-specific estimates would be of most use to clinicians treating co-infected patients.
In conclusion, our results reinforce the concept [29] that TB treatment does not increase early mortality after cART initiation — the issue is undiagnosed and untreated TB — underscoring the need for intensified case finding to reduce early mortality associated with undiagnosed TB in PLWH. After about a year of cART, TB treatment was associated with increased mortality, despite the possibility of downward biases. This association should be elucidated in future studies, with an emphasis on separating possible effects of TB treatment from effects of active TB itself. In the meantime, patients receiving concurrent TB treatment at cART initiation may benefit from continued support after TB treatment completion.
Supporting Information
Combination antiretroviral therapy regimens utilized in each study.
(PDF)
Types of TB included, by study.
(PDF)
Timing of TB treatment in relation to cART initiation, by study.
(PDF)
Median (IQR) baseline CD4 cell count by TB treatment status, if available.
(PDF)
Meta-regression results for the effect of TB treatment on mortality.
(PDF)
Funnel plots of mortality relative risks and inverse-variance weights.
(PDF)
Sensitivity analysis of asymmetrical confidence intervals.
(PDF)
PRISMA checklist.
(PDF)
Acknowledgments
We are grateful to Mellanye Lackey for her assistance with developing our search strategy, and to Joseph Eron, Jr., Sonia Napravnik, Harry Moultrie and Alan Brookhart for their thoughtful comments and guidance. Preliminary study results were presented as a poster at the 17th Annual Conference of the International Union Against Tuberculosis and Lung Disease – North America Region, Vancouver, February 28 – March 2, 2013.
Funding Statement
HMS and MRP were partially supported by National Institutes of Health training grant 2T32AI070114. No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. World Health Organization (2012) Global Tuberculosis Report. Geneva: WHO. [Google Scholar]
- 2. Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A et al. (2006) Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA 296: 782-793. doi: 10.1001/jama.296.7.782. PubMed: 16905784. [DOI] [PubMed] [Google Scholar]
- 3. Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A et al. (2006) Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet 367: 817-824. doi: 10.1016/S0140-6736(06)68337-2. PubMed: 16530575. [DOI] [PubMed] [Google Scholar]
- 4. Lawn SD, Harries AD, Anglaret X, Myer L, Wood R (2008) Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS 22: 1897-1908. doi: 10.1097/QAD.0b013e32830007cd. PubMed: 18784453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker AS, Prendergast AJ, Mugyenyi P, Munderi P, Hakim J et al. (2012) Mortality in the year following antiretroviral therapy initiation in HIV-infected adults and children in Uganda and Zimbabwe. Clin Infect Dis 55: 1707-1718. doi: 10.1093/cid/cis797. PubMed: 22972859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rana FS, Hawken MP, Mwachari C, Bhatt SM, Abdullah F et al. (2000) Autopsy study of HIV-1-positive and HIV-1-negative adult medical patients in Nairobi, Kenya. J Acquir Immune Defic Syndr 24: 23-29. doi: 10.1097/00042560-200005010-00004. PubMed: 10877491. [DOI] [PubMed] [Google Scholar]
- 7. Lucas SB, Hounnou A, Peacock C, Beaumel A, Djomand G et al. (1993) The mortality and pathology of HIV infection in a west African city. AIDS 7: 1569-1579. doi: 10.1097/00002030-199312000-00005. PubMed: 7904450. [DOI] [PubMed] [Google Scholar]
- 8. Murray J, Sonnenberg P, Nelson G, Bester A, Shearer S et al. (2007) Cause of death and presence of respiratory disease at autopsy in an HIV-1 seroconversion cohort of southern African gold miners. AIDS 21 Suppl 6: S97-S104. doi: 10.1097/QAD.0b013e3280117cb5. PubMed: 18032945. [DOI] [PubMed] [Google Scholar]
- 9. Wong EB, Omar T, Setlhako GJ, Osih R, Feldman C et al. (2012) Causes of death on antiretroviral therapy: a post-mortem study from South Africa. PLOS ONE 7: e47542. doi: 10.1371/journal.pone.0047542. PubMed: 23094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Straetemans M, Bierrenbach AL, Nagelkerke N, Glaziou P, van der Werf MJ (2010) The effect of tuberculosis on mortality in HIV positive people: a meta-analysis. PLOS ONE 5: e15241. doi: 10.1371/journal.pone.0015241. PubMed: 21209936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization (2010) Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva: WHO. [PubMed] [Google Scholar]
- 12.(2010) The Global Plan to Stop TB 2011-2015. Geneva: World. Health Organization and Stop TB Partnership. [Google Scholar]
- 13. Volberding P, Sande M, Lange J, Greene W (2008) Global HIV/AIDS Medicine; Gallant J. Philadelphia: Elsevier. [Google Scholar]
- 14. Curran A, Falcó V, Pahissa A, Ribera E (2012) Management of Tuberculosis in HIV-Infected Patients. AIDS Res 14: 231-246. PubMed: 23258298. [PubMed] [Google Scholar]
- 15. Breton G, Bourgarit A, Pavy S, Bonnet D, Martinez V et al. (2012) Treatment for tuberculosis-associated immune reconstitution inflammatory syndrome in 34 HIV-infected patients. Int J Tuberc Lung Dis 16: 1365-1370. doi: 10.5588/ijtld.11.0693. PubMed: 23107635. [DOI] [PubMed] [Google Scholar]
- 16. Lawn SD, Harries AD, Wood R (2010) Strategies to reduce early morbidity and mortality in adults receiving antiretroviral therapy in resource-limited settings. Curr Opin HIV Aids 5: 18-26. doi: 10.1097/COH.0b013e328333850f. PubMed: 20046144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greenland S, O'Rourke K (2008) Meta-analysis. In: Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed ed. Philadelphia: Lippincott Williams & Wilkins; pp. 652-682. [Google Scholar]
- 19. Egger M, Smith GD, Altman DG (2001) Systematic reviews in health care: Meta-analysis in context. London: BMJ Publishing Group. [Google Scholar]
- 20. Borenstein M, Hedges L, Higgins JP, Rothstein H (2009) Introduction to Meta-Analysis. West Sussex: John Wiley & Sons, Ltd. [Google Scholar]
- 21. Higgins JP, Thompson SG, Spiegelhalter DJ (2009) A re-evaluation of random-effects meta-analysis. J R Stat Soc A 172: 137-159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson SG, Sharp SJ (1999) Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 18: 2693-2708. doi: 10.1002/(SICI)1097-0258(19991030)18:20. PubMed: 10521860. [DOI] [PubMed] [Google Scholar]
- 23. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088-1101. doi: 10.2307/2533446. PubMed: 7786990. [DOI] [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629-634. doi: 10.1136/bmj.315.7109.629. PubMed: 9310563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duval S, Tweedie R (2000) Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56: 455-463. doi: 10.1111/j.0006-341X.2000.00455.x. PubMed: 10877304. [DOI] [PubMed] [Google Scholar]
- 26. Mugusi SF, Ngaimisi E, Janabi MY, Mugusi FM, Minzi OM et al. (2012) Risk factors for mortality among HIV-positive patients with and without active tuberculosis in Dar es Salaam, Tanzania. Antivir Ther 17: 265-274. PubMed: 22293579. [DOI] [PubMed] [Google Scholar]
- 27. Bhowmik A, Bhandari S, De R, Guha SK (2012) Predictors of mortality among HIV-infected patients initiating anti retroviral therapy at a tertiary care hospital in Eastern India. Asian Pacific journal of tropical medicine 5: 986-990. [DOI] [PubMed]
- 28. Siika AM, Yiannoutsos CT, Wools-Kaloustian KK, Musick BS, Mwangi AW et al. (2013) Active tuberculosis is associated with worse clinical outcomes in HIV-infected African patients on antiretroviral therapy. PLOS ONE 8: e53022. doi: 10.1371/journal.pone.0053022. PubMed: 23301015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Westreich D, Fox MP, Van Rie A, Maskew M (2012) Prevalent tuberculosis and mortality among HAART initiators. AIDS 26: 770-773. doi: 10.1097/QAD.0b013e328351f6b8. PubMed: 22313956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bera E (2009) Maternal outcomes following introduction of antiretroviral therapy in the public sector: A prospective study at a tertiary hospital in the Eastern Cape. S Afr J Obstet Gynaecol 15: 26-33. [Google Scholar]
- 31. Boulle A, Van Cutsem G, Cohen K, Hilderbrand K, Mathee S et al. (2008) Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA 300: 530-539. doi: 10.1001/jama.300.5.530. PubMed: 18677025. [DOI] [PubMed] [Google Scholar]
- 32. Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M et al. (2010) Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS 24: 563-572. doi: 10.1097/QAD.0b013e328333bfb7. PubMed: 20057311. [DOI] [PubMed] [Google Scholar]
- 33. Dao CN, Peters PJ, Kiarie JN, Zulu I, Muiruri P et al. (2011) Hyponatremia, hypochloremia, and hypoalbuminemia predict an increased risk of mortality during the first year of antiretroviral therapy among HIV-infected Zambian and Kenyan women. AIDS Res Hum Retrovir 27: 1149-1155. doi: 10.1089/aid.2010.0345. PubMed: 21417949. [DOI] [PubMed] [Google Scholar]
- 34. DeSilva MB, Merry SP, Fischer PR, Rohrer JE, Isichei CO et al. (2009) Youth, unemployment, and male gender predict mortality in AIDS patients started on HAART in Nigeria. AIDS Care 21: 70-77. doi: 10.1080/09540120802017636. PubMed: 19085222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dronda F, Sobrino P, Hernández-Novoa B, Caro-Murillo AM, Montero M et al. (2011) Response to HAART in treatment-naive HIV-infected patients with a prior diagnosis of tuberculosis or other opportunistic infections. Curr HIV Res 9: 229-236. doi: 10.2174/157016211796320324. PubMed: 21631429. [DOI] [PubMed] [Google Scholar]
- 36. Greig J, O'Brien DP, Ford N, Spelman T, Sabapathy K et al. (2012) Similar mortality and reduced loss to follow-up in integrated compared with vertical programs providing antiretroviral treatment in sub-saharan Africa. J Acquir Immune Defic Syndr 59: e92-e98. doi: 10.1097/QAI.0b013e31824206c7. PubMed: 22134144. [DOI] [PubMed] [Google Scholar]
- 37. Lartey M, Sagoe KW, Yang H, Kenu E, Xexemeku F et al. (2011) Viral decay rates are similar in HIV-infected patients with and without TB coinfection during treatment with an Efavirenz-based regimen. Clin Infect Dis 52: 547-550. doi: 10.1093/cid/ciq196. PubMed: 21252140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker LG et al. (2010) Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS 24: 1323-1328. PubMed: 20386425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lawn SD, Myer L, Bekker LG, Wood R (2006) Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS 20: 1605-1612. doi:10.1097/01.aids.0000238406.93249.cd. PubMed: 16868441. [DOI] [PubMed] [Google Scholar]
- 40. Liechty CA, Solberg P, Were W, Ekwaru JP, Ransom RL et al. (2007) Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health 12: 929-935. doi: 10.1111/j.1365-3156.2007.01874.x. PubMed: 17697087. [DOI] [PubMed] [Google Scholar]
- 41. Manosuthi W, Tantanathip P, Chimsuntorn S, Eampokarap B, Thongyen S et al. (2010) Treatment outcomes of patients co-infected with HIV and tuberculosis who received a nevirapine-based antiretroviral regimen: a four-year prospective study. Int J Infect Dis 14: e1013-1017 PubMed: 20880733. [DOI] [PubMed] [Google Scholar]
- 42. Moore DM, Yiannoutsos CT, Musick BS, Tappero J, Degerman R et al. (2011) Determinants of early and late mortality among HIV-infected individuals receiving home-based antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr 58: 289-296. doi: 10.1097/QAI.0b013e3182303716. PubMed: 21857358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Westreich D, MacPhail P, Van Rie A, Malope-Kgokong B, Ive P et al. (2009) Effect of pulmonary tuberculosis on mortality in patients receiving HAART. AIDS 23: 707-715. doi: 10.1097/QAD.0b013e328325d115. PubMed: 19279444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L et al. (2006) Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS 20: 2355-2360. doi: 10.1097/QAD.0b013e32801086b0. PubMed: 17117022. [DOI] [PubMed] [Google Scholar]
- 45. Nguyen VTT, Pham NM, Pham DT, Kato M, Nguyen TTM et al. (2011) HBV and HCV co-infection as predictors for survival in antiretroviral therapy patients in Vietnam. Antivir Ther 16: A51-A52. doi: 10.3851/IMP1720. [DOI] [Google Scholar]
- 46. Bassett IV, Wang B, Chetty S, Giddy J, Losina E et al. (2010) Loss to follow-up and mortality among those co-infected with TB at ART initiation in Durban, South Africa. Vienna, Austria: XVIII INTERNATIONAL AIDS Conference; (p. TUPDB303). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chu R, Mills E, Beyene J, Bakanda C, Nachega J et al. (2011) Impact of tuberculosis on mortality among HIV-infected patients receiving antiretroviral therapy. Am J Epidemiol 173: S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zachariah R, Harries K, Moses M, Manzi M, Line A et al. (2009) Very early mortality in patients starting antiretroviral treatment at primary health centres in rural Malawi. Trop Med Int Health 14: 713-721. doi: 10.1111/j.1365-3156.2009.02291.x. PubMed: 19497082. [DOI] [PubMed] [Google Scholar]
- 49. Makombe SD, Harries AD, Yu JKL, Hochgesang M, Mhango E et al. (2007) Outcomes of tuberculosis patients who start antiretroviral therapy under routine programme conditions in Malawi. Int J Tuberc Lung Dis 11: 412-416. PubMed: 17394687. [PubMed] [Google Scholar]
- 50. Mutevedzi PC, Lessells RJ, Rodger AJ, Newell M-L (2011) Association of age with mortality and virological and immunological response to antiretroviral therapy in rural South African adults. PLOS ONE 6: e21795. doi: 10.1371/journal.pone.0021795. PubMed: 21747959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD (2013) Prevalent and incident tuberculosis are independent risk factors for mortality among patients accessing antiretroviral therapy in South Africa. PLOS ONE 8: e55824. doi: 10.1371/journal.pone.0055824. PubMed: 23418463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bassett IV, Chetty S, Wang B, Mazibuko M, Giddy J et al. (2012) Loss to follow-up and mortality among HIV-infected people co-infected with TB at ART initiation in Durban, South Africa. J Acquir Immune Defic Syndr 59: 25-30. doi: 10.1097/QAI.0b013e31823d3aba. PubMed: 22027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zar HJ, Cotton MF, Strauss S, Karpakis J, Hussey G et al. (2007) Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ 334: 136. doi: 10.1136/bmj.39000.486400.55. PubMed: 17085459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brinkhof MW, Pujades-Rodriguez M, Egger M (2009) Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLOS ONE 4: e5790. doi: 10.1371/journal.pone.0005790. PubMed: 19495419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rodgers A, MacMahon S (1995) Systematic underestimation of treatment effects as a result of diagnostic test inaccuracy: implications for the interpretation and design of thromboprophylaxis trials. Thromb Haemost 73: 167-171. PubMed: 7792725. [PubMed] [Google Scholar]
- 56. Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C et al. (2010) Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 362: 697-706. doi: 10.1056/NEJMoa0905848. PubMed: 20181971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C et al. (2011) Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 365: 1492-1501. doi: 10.1056/NEJMoa1014181. PubMed: 22010915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C et al. (2011) Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 365: 1471-1481. doi: 10.1056/NEJMoa1013911. PubMed: 22010913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S et al. (2011) Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 365: 1482-1491. doi: 10.1056/NEJMoa1013607. PubMed: 22010914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Combination antiretroviral therapy regimens utilized in each study.
(PDF)
Types of TB included, by study.
(PDF)
Timing of TB treatment in relation to cART initiation, by study.
(PDF)
Median (IQR) baseline CD4 cell count by TB treatment status, if available.
(PDF)
Meta-regression results for the effect of TB treatment on mortality.
(PDF)
Funnel plots of mortality relative risks and inverse-variance weights.
(PDF)
Sensitivity analysis of asymmetrical confidence intervals.
(PDF)
PRISMA checklist.
(PDF)


