Abstract
Muscle strength and activation were compared in boys and men during maximal voluntary elbow flexion and extension contractions. Peak torque, peak rate of torque development (dτ/dτmax), rate of muscle activation, and electromechanical delay (EMD) were measured in 15 boys (aged 9.7 ± 1.6 years) and 16 men (aged 22.1 ± 2.8 years). During flexion, peak torque was significantly lower in boys than in men (19.5 ± 5.8 vs. 68.5 ± 11.0 Nm, respectively; p < 0.05), even when controlling for upper-arm cross-sectional area (CSA), and peak electromyography activity. Boys also exhibited a lower normalized dτ/dτmax (7.2 ± 1.7 vs. 9.5 ± 1.6 (Nm·s−1)·(Nm−1), respectively; p < 0.05) and a significantly longer EMD (75.5 ± 28.4 vs. 47.6 ± 17.5 ms, respectively). The pattern was similar for extension, except that group differences in peak torque were no longer significant when normalized for CSA. These results suggest that children may be less able to recruit or fully utilize their higher-threshold motor units, resulting in lower dimensionally normalized maximal torque and rate of torque development.
Keywords: children, coactivation, EMG, maturation, neuromotor, strength
Introduction
Maximal muscle force is lower in children than in adults, even when size-normalized, for example, to body mass (De Ste Croix et al. 1999; Lambertz et al. 2003) or to muscle cross-sectional area (CSA) (Grosset et al. 2008; Halin et al. 2003; Kanehisa et al. 1995a, 1995b; Lambertz et al. 2003; Seger and Thorstensson 2000; Wood et al. 2006). Thus, additional factors, such as neuromuscular activation or muscle composition, must account for the observed strength differences. Very few studies have examined muscle activation when investigating age-related muscle function differences.
Children’s rate of force development (dτ/dτ) also appears to be lower than that of adults (Asai and Aoki 1996; Belanger and McComas 1989; Grosset et al. 2005). As is the case with maximal force, explanations for children’s lower dτ/dτ may involve differences in muscle composition and in muscle activation. Other factors capable of affecting dτ/dτ may include musculo-tendinous stiffness, excitation–contraction coupling, and muscle-fibre conduction velocity. These have been examined in children only to a limited extent, and with inconsistent results (Cornu and Goubel 2001; Grosset et al. 2005; Lambertz et al. 2003).
Muscle-fibre composition in children is believed to be similar to that of adults (Dubowitz 1965). Additionally, during twitch stimulation, contractile characteristics, such as contraction time and half-relaxation time, have been shown to be similar across age groups (Belanger and McComas 1989; Davies 1985, 1983; McComas et al. 1973; Paasuke et al. 2000), supporting the notion of a similar fibre composition in children and adults. Thus, differences in muscle composition, if any, do not seem sufficient to account for the observed child–adult strength differences.
An additional factor that could possibly affect measured strength is agonist–antagonist coactivation. While some studies demonstrate greater coactivation in children (Frost et al. 1997; Grosset et al. 2008; Lambertz et al. 2003), others do not (Bassa et al. 2005; Kellis and Unnithan 1999). All those studies were conducted only on the lower limbs, and their findings are, accordingly, limited.
Asmussen (1973) was one of the first to propose that child–adult strength differences could be related to neuro-motor maturation. Surprisingly, this topic has since been addressed only to a limited extent. Pediatric muscle-activation studies have focused on the lower extremities (Blimkie 1989; Paasuke et al. 2000; Grosset et al. 2008; Lambertz et al. 2003; Streckis et al. 2007). However, age-related strength changes in the upper extremities differ from those in the lower extremities (Parker et al. 1990), and strength changes in elbow flexors differ from those in elbow extensors (Kanehisa et al. 1995a). Moreover, while the lower extremities are commonly used for locomotion and posture control — tasks that do not normally require fast contractions — the upper extremities are more often used for reaching and grabbing, and may involve faster contractions and multiple coordinated steps of precise motor control (Hirschfeld 2007). Such functional differences could possibly manifest themselves as different muscular force–velocity relationships in the upper and lower extremities (Charteris 1999). Thus, muscle performance and activation of the lower extremity may not reflect the corresponding characteristics of the upper extremity.
Only 2 studies have examined children’s muscle strength and electromyography (EMG) activity in the upper extremities, and they reported only elbow flexion (Asai and Aoki 1996; Halin et al. 2003), with no data on extension or on co-activation. Additionally, one of these studies (Asai and Aoki 1996) provided no anthropometric data or any normalization for body size. These studies, therefore, do not elucidate whether children’s lower relative strength of the upper extremity is due only to their smaller muscle mass or whether it is due to differential muscle activation or coactivation as well. Also, since the growth rates of muscular strength and rates of growth size of reciprocal muscle groups differ (Kanehisa et al. 1995a), it is unclear whether flexion and extension forces are similarly affected by maturation.
The purpose of this study was to compare muscle activation of prepubescent boys with that of men during maximal voluntary isometric contractions of both elbow flexors and extensors. It was hypothesized that size-normalized muscle strength would be lower in the boys and would be associated with less muscle activation.
Materials and methods
Subjects
Fifteen boys (age, 9.6 ± 1.6 years; body mass, 32.7 ± 6.9 kg; height, 1.37 ± 0.09 m) and 16 men (22.1 ± 2.8 years; 81.7 ± 7.2 kg; 1.80 ± 0.07 m) were recruited to participate in this study, which was approved by Brock University’s Research Ethics Board (St. Catharines, Ont.). All participants were right-handed, healthy individuals with no upper-limb injuries. All were physically active, but no one was a competitive athlete or participated in a unilateral sport on a regular basis. Written informed consent was obtained from all participants and the children’s parents prior to the study’s onset.
Anthropometric measurements
Standing and seated heights were measured using a wall-mounted stadiometer (Length Boards, Ellard Instrumentation, Ltd., Monroe, Wash.). Body mass was measured on a digital scale (EKS Electronic Scales, Cedex, France). Skin-fold thickness was measured in triplicate, using skinfold calipers (RH15 916, Harpenden, England) at the biceps, triceps, subscapular, and suprailiac sites on the right side of the body. Body fat percentage was calculated using the equations of Slaughter et al. (1988). Upper-arm CSA was calculated using measures of upper-arm circumference, as well as biceps and triceps skinfold thickness, as described elsewhere (Gurney and Jelliffe 1973). The coefficient of variance of such measurements in another subset of subjects in our lab was 2.0%, with an intraclass correlation coefficient of r = 0.99, which is similar to values reported elsewhere (Knapik et al. 1996).
Experimental protocol
All subjects visited the laboratory on 2 occasions, at least 3 days apart. On the first visit, subjects were informed of the study’s procedures, anthropometric measurements were taken, and questionnaires were filled out (facilitated by a researcher for child subjects). Physical activity levels were determined using the Godin-Shephard Leisure-Time Exercise Questionnaire (Godin and Shephard 1985). The children self-assessed their pubertal stage in accordance with secondary sex characteristics (pubic hair), as described by elsewhere (Tanner 1962; Duke et al. 1980). Then, the complete test protocol was practiced to familiarize the subjects with all test procedures. The actual test protocol was performed on the second visit.
The Biodex System 3 torque dynamometer (Biodex, Shirley, N.Y.) was used to measure torque during isometric contractions of the elbow flexors and extensors. An isometric task was chosen to minimize antagonist involvement (Calder and Gabriel 2007). Thus, child–adult torque differences would be mainly attributable to agonist activation. The subject sat upright in the dynamometer seat, with the right shoulder at 90° of flexion, resting his upper arm on a proprietary armrest adjusted for the subject’s height. With the subject’s hand supinated, grasping the dynamometer’s lever grip, both elbow and the lever arm of the Biodex were set at 90°. The dynamometer’s axis of rotation was visually aligned with the lateral epicondyle of the right humerus. The subject was then stabilized in the dynamometer’s chair with 2 straps secured diagonally across the chest in an X fashion, and 1 strap across the hips.
Warm-up consisted of 3–5 three-second maximal voluntary contractions (MVC) of flexion and extension. Ten seconds separated successive contractions. Subjects were given explicit instructions to contract as hard and as fast as possible from a relaxed state to maximize torque and dτ/dτ. Online visual feedback of the dynamometer’s force signal was available for the subjects on a computer screen. Visual feedback has been shown to be important for torque production (Kellis and Baltzopoulos 1996), especially in young children (Smits-Engelsman et al. 2003). Following 2–3 min of rest, the subject performed 2 sets of contractions, either flexion or extension, in a randomized order among subjects. Instructions were identical to those of the warm-up. Subjects completed 2 sets of 5 MVCs with 30-s rest periods between contractions and at least a 2-min break between sets. While a 30-s rest between contractions is arguably short, it was important for maintaining an appropriate level of concentration in the boys. Subsequent data analysis failed to reveal fatigue or other order effects in either the boys or the men. A minimum of 2 sets was required of each participant (totaling 10 MVCs each of flexion and extension). Additional sets were added, as needed, to reach 10 valid MVCs if some data were deemed unacceptable due to execution errors, deviations in EMG baseline, or abnormal torque or EMG amplitudes or tracing. Throughout each contraction, subjects were verbally encouraged to perform a maximal effort in both torque and contractile speed.
Electromyography
Surface EMG signals were collected from 2 sites: at the muscle-belly midsections of the biceps brachii, and at the lateral head of the triceps brachii. These were determined visually during a resisted static contraction. The bipolar surface electrodes (DE-2.1, DelSys Inc., Boston, Mass.) were placed in line with the muscle fibres, away from the estimated motor point (Delagi and Perotto 1980). The reference electrode was placed over the middle of the right clavicle. Electrode sites were prepared by shaving the skin, when necessary, and thoroughly rubbing the skin with alcohol. Raw EMG signals were amplified by a DelSys amplifier (10–500 Hz band-pass filter, Bagnoli-4 EMG System, Boston, Mass.), converted from analog to digital (DI-205-C, DATAQ Instruments, Akron, Ohio), and recorded at an acquisition rate of 1000 Hz (WinDaq Pro Data Acquisition, DATAQ Instruments).
Data analysis
Using MatLab (The MathWorks, Natick, Mass.), several variables were calculated separately for flexion and extension. Peak agonist and antagonist EMG amplitude were determined and averaged over the best 10 trials. Mean traces were created for torque, agonist EMG, and antagonist EMG. These traces were used to calculate dτ/dτ, rate of muscle activation (Q30) of both muscle groups, and electromechanical delay (EMD). Peak rate of torque development (dτ/dτmax) was calculated by taking the maximum of the first derivative of the torque signal (Gabriel et al. 2001). Rate of muscle activation was defined by the area under the EMG curve of the linear envelope of the detected EMG signal during the first 30 ms (Gottlieb et al. 1989; Gabriel and Boucher 2000). EMD was defined as the time lapse between the onsets of EMG and torque generation, and was calculated in the agonist muscle. The time of dτ/dτmax was calculated as the time delay between the onset of torque generation and the occurrence of dτ/dτmax. Coactivation was calculated as the ratio between the antagonist’s EMG amplitude divided by its EMG amplitude as an agonist (i.e., for flexion: (triceps EMG amplitude in flexion)/(triceps EMG amplitude in extension); for extension: (biceps EMG amplitude in extension)/(biceps EMG amplitude in flexion)).
Statistical analysis
Data for both child and adult groups are presented as means ± standard deviations. An unpaired 2-tailed Student’s t test was used to compare the means between the 2 groups. Possible confounding factors (e.g., EMG signal amplitude) were entered as covariates in an analysis of covariance. Differences between groups were considered significant at p < 0.05. All analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, Ill.).
Results
Subject characteristics appear in Table 1. While body size was significantly (p < 0.001) smaller in the boys, no differences were observed in body-fat percentage or in skinfold thickness over the biceps or triceps. Thus, the possible effect of subcutaneous fat on the EMG signal (De la Barrera and Milner 1994) was similar in the 2 groups.
Table 1.
Subject characteristics (data are means ± SD).
| Variables | Men (n = 16) | Boys (n = 15) |
|---|---|---|
| Age (years)* | 22.1±2.8 | 9.6±1.6 |
| Body mass (kg)* | 81.7±7.2 | 32.8±6.9 |
| Height (cm)* | 180.9±7.2 | 137.5±8.7 |
| Body fat (%) | 18.3±5.5 | 16.4±6.2 |
| Lean body mass (kg)* | 68.7±5.5 | 27.1±4.6 |
| Biceps skinfold (mm) | 5.0±1.8 | 4.9±2.1 |
| Triceps skinfold (mm) | 10.4±3.2 | 10.6±4.0 |
| Upper-arm CSA (cm2)* | 69.4±9.0 | 24.6±4.3 |
| Years from PHV | na | −3.6±1.0 |
Note: CSA, cross-sectional area; na, not applicable; PHV, peak height velocity.
Significant difference between groups, p < 0.05.
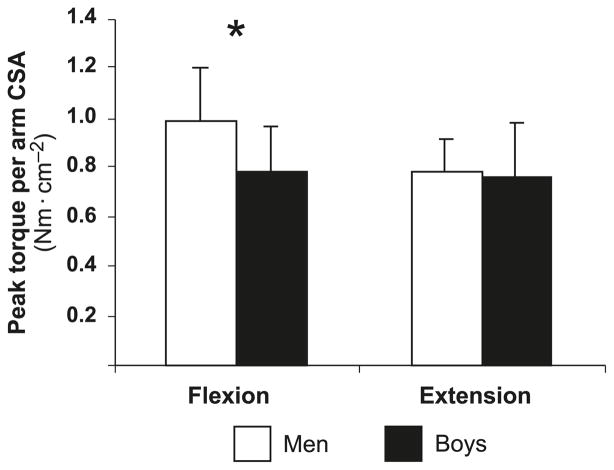
As expected, in absolute terms, men were significantly stronger than boys in both flexion (68.5 ± 11.0 vs. 19.5 ± 5.8 Nm, respectively; p < 0.001) and extension (55.0 ± 10.1 vs. 18.4 ± 5.7 Nm, respectively; p < 0.001). Normalized to body mass, peak torque remained significantly higher in the men in flexion (0.84 ± 0.15 vs. 0.60 ± 0.15 Nm·kg−1, respectively; p < 0.001), and not significantly higher in extension (0.68 ± 0.14 vs. 0.58 ± 0.20 Nm·kg−1, respectively; p = 0.11). Similar findings were obtained when peak torque was normalized for upper-arm CSA (Fig. 1). Peak torque was moderately correlated with peak EMG amplitude (r = 0.48, p = 0.01 and r = 0.36, p = 0.05 for flexion and extension, respectively). Controlling for EMG amplitude (as a covariate) did not change the pattern of results. That is, child–adult differences in body-size-normalized peak flexion torque remained significantly lower in boys than in men, while no such difference could detected in extension peak torque.
Fig. 1.
Peak torque during flexion and extension, corrected for size (upper-arm cross-sectional area (CSA)). *, p < 0.05.
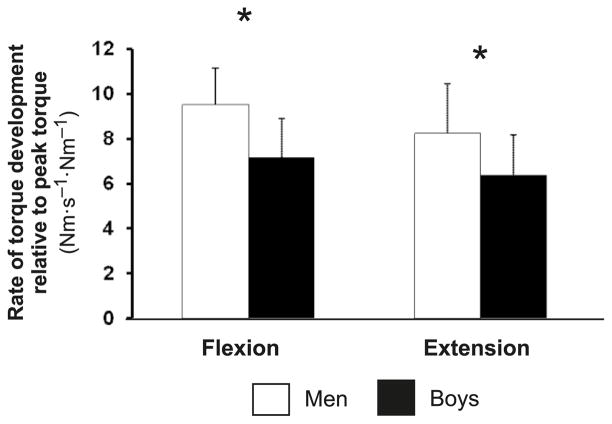
Men exhibited significantly higher absolute dτ/dτmax values than boys in both flexion and extension (652 ± 154 vs. 141 ± 53 and 445 ± 113 vs. 113 ± 32 Nm·s−1, respectively; p < 0.001). This difference persisted in both contractions when dτ/dτmax was normalized for peak torque (Fig. 2). Normalized dτ/dτmax was moderately correlated with Q30 (r = 0.40, p = 0.02) for flexion, but not for extension. Controlling for Q30 as a covariate, dτ/dτmax remained lower in boys than in men, although the difference did not reach significance in flexion (p = 0.08). The time from torque onset to dτ/dτmax was significantly longer in boys during flexion (71.0 ± 29.1 vs. 51.2 ± 9.7 ms, respectively; p = 0.015), but not during extension (58.3 ± 31.7 vs. 58.8 ± 23.7 ms, respectively; p = 0.96).
Fig. 2.
Peak rate of torque development during flexion and extension normalized for peak torque. *, p < 0.05.
Flexion Q30 was significantly lower in the boys than in the men (0.097 ± 0.080 vs. 0.366 ± 0.168 mV·s, respectively; p < 0.001). A similar pattern was observed during extension, but the difference did not reach statistical significance (0.070 ± 0.046 vs. 0.082 ± 0.047 mV·s, respectively; p = 0.46). Q30 was moderately correlated with agonist EMG amplitude during flexion (r = 0.55, p = 0.01), but not during extension. Controlling for root mean squares amplitude as a covariate, the boys’ Q30 values remained lower than the men’s.
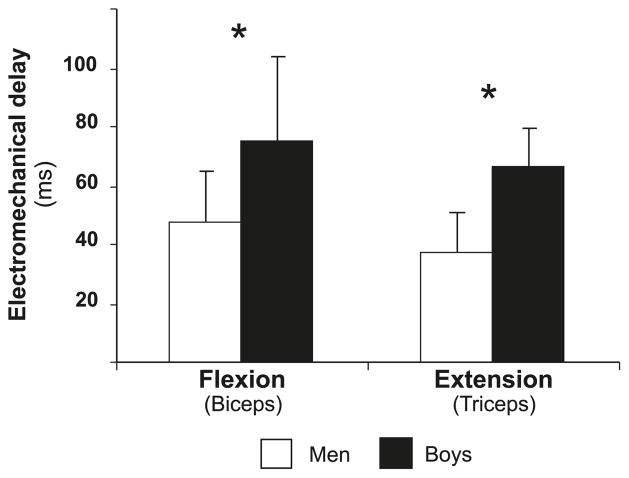
Agonist EMD was significantly shorter in men than in boys in both flexion (p = 0.002) and extension (p = 0.002) (Fig. 3).
Fig. 3.
The delay between electromyography onset of the agonist and force onset in flexion and extension. *, p < 0.05.
The coactivation index was not significantly different between boys and men (0.59 ± 0.44 vs. 0.44 ± 0.27 in flexion, p = 0.26; and 0.09 ± 0.06 vs. 0.12 ± 0.07 in extension, p = 0.41, respectively). It should be noted that during extension, antagonist activation could not be detected in 7 of the men or in 6 of the boys.
Discussion
This study examined maximal voluntary isometric torque and rate of torque development, along with agonist and antagonist EMG activity, during elbow flexion and extension in prepubertal boys and men. The main findings are the lower peak torque and dτ/dτmax in boys than in men, not only in absolute terms, but also when corrected for body size and controlled for EMG activity. The elbow-extension peak torque, corrected for arm CSA, was no longer different between groups, while dτ/dτmax remained lower in boys, even after correcting for body size and controlling for EMG activity. The EMD was consistently longer in the boys, during both flexion and extension.
Normalizing peak torque for lean upper-arm CSA (Fig. 1) greatly reduced peak torque age differences, but the normalized torque remained lower in the boys than in the men. This was clearly apparent in flexion, but not in extension. Anthropometrically derived CSA, as determined in this study, is limited in its accuracy and resolution. Nevertheless, our results agree with previous studies, which also demonstrated a lower CSA-normalized torque in children than in adults in elbow flexion, as well as in other muscle groups, using anthropometry or magnetic resonance imaging (Kanehisa et al. 1995b; Seger and Thorstensson 2000; Davies 1985; Grosset et al. 2008; Halin et al. 2003; Tonson et al. 2008). It should be noted that Tonson et al. (2008) recently suggested that the appropriate body-size parameter for normalizing muscle strength should be muscle volume (determined by magnetic resonance imaging). The authors showed that when using the volume parameter, no child–adult strength difference could be detected. However, Bamman et al. (2000) demonstrated that muscle CSA was a better parameter than volume (both determined by magnetic resonance imaging), since strength better correlated with CSA and regressed to the origin. Furthermore, the study did not find physiological CSA to be more precise than the anatomically determined CSA, leading the authors to recommend anatomical CSA for estimating specific tension in vivo. Thus, using various indices, body size does not appear to fully explain the child–adult differences in maximal flexion strength. It is interesting to note that, similar to the current study’s findings, Kanehisa et al. (1995a) reported an age-related increase in CSA-normalized elbow-flexor, but not in elbow-extensor, strength in 7- to 9- vs. 16- to 18-year-old boys. Therefore, as suggested by Kanehisa et al. (1995a), it is possible that strength development in reciprocal muscle groups may not follow the same pattern during growth and maturation.
Age-group differences in size-normalized-flexion peak torque were still apparent after controlling for EMG activity (root mean squares peak amplitude). Our results agree with previously reported age-related differences in peak torque, relative to EMG amplitude, in the lower extremities (plantar flexion) (Grosset et al. 2008; Lambertz et al. 2003; Moritani et al. 1989), and extend them to the upper extremities. These data suggest that other factors, such as possible differences in moment arm or muscle composition, may also partly explain age-related differences in elbow-flexion strength.
The lower absolute dτ/dτmax observed in the boys is partly explained by the dependence of dτ/dτ on peak torque. Thus, normalizing dτ/dτ to peak torque can be useful in searching for other factors that might determine dτ/dτ (Holtermann et al. 2007). Surprisingly, only 1 previous study normalized children’s rate of force development to peak force; it reported lower values for elbow flexion in 6-year-old boys, compared with men (Asai and Aoki 1996). Our findings (Fig. 2) correspond to those of Asai and Aoki (1996), and complement them with elbow-extension data. Thus, children’s lower rate of force development is a persistent finding, independent of their lower maximal strength.
The rate of force development depends on the rate of muscle activation (Corcos et al. 1989). To our knowledge, no previous study has related children’s rate of force development to any index of muscle activation. In both flexion and extension, when Q30 was taken into account, dτ/dτmax, absolute or corrected for peak torque, remained lower in boys. These results suggest that factors other than muscle size and muscle activation may be involved in determining the rate of force development, as is the case for peak torque (see below).
EMD reflects muscle-tendon stiffness, excitation–contraction coupling, and muscle-fibre conduction velocity (Cavanagh and Komi 1979; Halin et al. 2003). EMD in the current study was consistently longer in boys than in men during flexion and extension (Fig. 3), as was the time to maximal rate of torque development. An age-related decrease in EMD has previously been reported in maximal elbow flexion and in plantar-flexion twitch contraction (Asai and Aoki 1996; Grosset et al. 2005). Using different types of muscle contractions, Cavanagh and Komi (1979) demonstrated that, in adults, it is mainly the series-elastic component (muscle–tendon stiffness), and not the excitation–contraction coupling, that determines EMD. Indeed, lower musculo-tendinous stiffness has been reported in 7-to 10-year-old boys compared with adults during plantar flexion (Lambertz et al. 2003). However, Cornu and Goubel (2001) could not show these differences during elbow flexion. Moreover, in a recent study (Grosset et al. 2009), musculo-tendinous stiffness changes could account only for <20% of the variance in EMD changes. Thus, it is unlikely that the boys’ longer EMD in the current study is solely due to their more compliant muscle–tendon complex. More likely is the proposition that factors such as lower muscle activation and lower muscle-fibre conduction velocity in boys (Halin et al. 2003) are also significant determinants of EMD. Further study is needed to elucidate this issue.
We suggest that the boys’ longer EMD is partly explained by their lesser recruitment or utilization of the faster, higher-threshold motor units. Two studies have examined muscle strength and activation during elbow flexion in children and adults (Asai and Aoki 1996; Halin et al. 2003). In both studies, it is argued that children involve fewer type-II fibres during maximal voluntary contractions than do adults. Halin et al. (2003) reported that during 30 s of isometric maximal voluntary elbow flexion, force decrement was lower and the decline in the EMG mean power frequency was slower in boys than in men. Additionally, during the 30-s contraction, there was no change in the boys’ muscle-fibre conduction velocity, while the men’s conduction velocity decreased. Based on these findings, the authors suggest that the boys’ lower maximal force and fatigability were due to lower involvement of type-II fibres. Asai and Aoki (1996) reported that increased elbow-flexor pretension resulted in a decreased rate of isometric force development in men but not in 6-year-old boys. The authors argue that the difference between children and adults in response to pretension may be explained by children’s lower reliance on type-II muscle-fibre recruitment (Asai and Aoki 1996). This contention is supported by our findings, where lower recruitment or utilization of type-II muscle fibres could explain the observed lower peak torque and dτ/dτmax, as well as the longer EMD, in the boys. This differential recruitment or utilization of higher-threshold motor units is also consistent with the lower size-normalized anaerobic power (Falk and Bar-Or 1993) and the faster recovery from intense short-term exercise (Falk and Dotan 2006; Hebestreit et al. 1993) observed in children.
Systematic group differences in coactivation could not be discerned in the current study. In fact, the EMG amplitude of the antagonist muscle was consistently low, or was nonexistent, in both groups. This finding upholds the choice of isometric testing as a methodological means of isolating group differences in muscle function. Thus, in isometric contraction, coactivation does not appear to be a substantial contributor to the child–adult differences observed in measured force or power generation. Therefore, our findings of consistently lower elbow-flexion peak torque and rate of torque development in boys provide further support to the notion of lower agonist activation in children during maximal force generation.
In conclusion, the results of the current study suggest that, during maximal voluntary isometric elbow flexion, children are less able to recruit or utilize their higher-threshold motor units, resulting in lower maximal force and rate of force development. Further research is needed to better understand children’s force generation and motor-unit recruitment patterns in maximal, as well as submaximal, isometric and dynamic contractions of reciprocal muscle groups. The possibility of affecting the rate and magnitude of age-related changes should be investigated in training studies.
Acknowledgments
We thank the participants and their parents for their cooperation in and enthusiasm for the project. We also thank Stephanie MacIntosh and Katie Banks for their technical assistance in data collection and reduction. The study was partly funded by the Canadian Institute for Health Research.
References
- Asai H, Aoki J. Force development of dynamic and static contractions in children and adults. Int J Sports Med. 1996;17:170–174. doi: 10.1055/s-2007-972827. [DOI] [PubMed] [Google Scholar]
- Asmussen E. Growth in muscular strength and power. In: Rarick G, editor. Physical activity, human growth and development. Academic Press; London: 1973. pp. 60–79. [Google Scholar]
- Bamman MM, Newcomer BR, Larson-Meyer DE, Weinsier RL, Hunter GR. Evaluation of the strength-size relationship in vivo using various muscle size indices. Med Sci Sports Exerc. 2000;32:1307–1313. doi: 10.1097/00005768-200007000-00019. [DOI] [PubMed] [Google Scholar]
- Bassa H, Kotzamanidis C, Patikas D. Activation of antagonist knee muscles during isokinetic efforts in prepubertal and adult males. Pediatr Exerc Sci. 2005;17:65–75. [Google Scholar]
- Belanger AY, McComas AJ. Contractile properties of human skeletal muscle in childhood and adolescence. Eur J Appl Physiol Occup Physiol. 1989;58:563–567. doi: 10.1007/BF00418500. [DOI] [PubMed] [Google Scholar]
- Blimkie CJ. Age- and sex-associated variation in strength during childhood: anthropometric, morphologic, neurologic, biomechanical, endocrinologic, genetic, and physical activity correlates. In: Gisolfi CV, Lamb DR, editors. Perspectives in exercise science and sports medicine. Vol. 2: Youth, exercise and sports. Benchmark Press; Indianapolis, Ind: 1989. pp. 99–161. [Google Scholar]
- Calder KM, Gabriel DA. Adaptations during familiarization to resistive exercise. J Electromyogr Kinesiol. 2007;17:328–335. doi: 10.1016/j.jelekin.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42:159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Charteris J. Effects of velocity on upper to lower extremity muscular work and power output ratios of intercollegiate athletes. Br J Sports Med. 1999;33:250–254. doi: 10.1136/bjsm.33.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcos DM, Gottlieb GL, Agarwal GC. Organizing principles for single-joint movements. II A speed-sensitive strategy. J Neurophysiol. 1989;62:358–368. doi: 10.1152/jn.1989.62.2.358. [DOI] [PubMed] [Google Scholar]
- Cornu C, Goubel F. Musculo-tendinous and joint elastic characteristics during elbow flexion in children. Clin Biomech (Bristol, Avon) 2001;16:758–764. doi: 10.1016/S0268-0033(01)00076-6. [DOI] [PubMed] [Google Scholar]
- Davies CT. Strength and mechanical properties of muscle in children and young adults. Scand J Sports Sci. 1985;7:11–15. [Google Scholar]
- Davies CT, White MJ, Young K. Muscle function in children. Eur J Appl Physiol Occup Physiol. 1983;52:111–114. doi: 10.1007/BF00429036. [DOI] [PubMed] [Google Scholar]
- De la Barrera EJ, Milner TE. The effects of skinfold thickness on the selectivity of surface EMG. Electroencephalogr Clin Neurophysiol. 1994;93:91–99. doi: 10.1016/0168-5597(94)90071-X. [DOI] [PubMed] [Google Scholar]
- De Ste Croix MBA, Armstrong N, Welsman JR. Concentric isokinetic leg strength in pre-teen, teenage and adult males and females. Biol Sport. 1999;16:75–86. [Google Scholar]
- Delagi EF, Perotto A. Anatomic guide for the electromyographer. CC Thomas; Springfield, Ill: 1980. [Google Scholar]
- Dubowitz V. Enzyme histochemistry of skeletal muscle. J Neurol Neurosurg Psychiatry. 1965;28:516–524. doi: 10.1136/jnnp.28.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- Falk B, Bar-Or O. Longitudinal changes in peak aerobic and anaerobic mechanical power of circumpubertal boys. Pediatr Exerc Sci. 1993;5:318–331. [Google Scholar]
- Falk B, Dotan R. Child-adult differences in the recovery from high-intensity exercise. Exerc Sport Sci Rev. 2006;34:107–112. doi: 10.1249/00003677-200607000-00004. [DOI] [PubMed] [Google Scholar]
- Frost G, Dowling J, Dyson K, Bar-Or O. Cocontraction in three age groups of children during treadmill locomotion. J Electromyogr Kinesiol. 1997;7:179–186. doi: 10.1016/S1050-6411(97)84626-3. [DOI] [PubMed] [Google Scholar]
- Gabriel DA, Boucher JP. Practicing a maximal performance task: a cooperative strategy for muscle activity. Res Q Exerc Sport. 2000;71:217–228. doi: 10.1080/02701367.2000.10608902. [DOI] [PubMed] [Google Scholar]
- Gabriel DA, Basford JR, An K. Training-related changes in the maximal rate of torque development and EMG activity. J Electromyogr Kinesiol. 2001;11:123–129. doi: 10.1016/S1050-6411(00)00041-9. [DOI] [PubMed] [Google Scholar]
- Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- Gottlieb GL, Corcos DM, Agarwal GC. Organizing principles for single-joint movements. I A speed-insensitive strategy. J Neurophysiol. 1989;62:342–357. doi: 10.1152/jn.1989.62.2.342. [DOI] [PubMed] [Google Scholar]
- Grosset JF, Mora I, Lambertz D, Perot C. Age-related changes in twitch properties of plantar flexor muscles in prepubertal children. Pediatr Res. 2005;58:966–970. doi: 10.1203/01.PDR.0000181375.61935.7D. [DOI] [PubMed] [Google Scholar]
- Grosset JF, Mora I, Lambertz D, Perot C. Voluntary activation of the triceps surae in prepubertal children. J Electromyogr Kinesiol. 2008;18:455–465. doi: 10.1016/j.jelekin.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Grosset JF, Piscione J, Lambertz D, Perot C. Paired changes in electromechanical delay and musculo-tendinous stiffness after endurance or plyometric training. Eur J Appl Physiol. 2009;105:131–139. doi: 10.1007/s00421-008-0882-8. [DOI] [PubMed] [Google Scholar]
- Gurney JM, Jelliffe DB. Arm anthropometry in nutritional assessment: nomogram for rapid calculation of muscle circumference and cross-sectional muscle and fat areas. Am J Clin Nutr. 1973;26:912–915. doi: 10.1093/ajcn/26.9.912. [DOI] [PubMed] [Google Scholar]
- Halin R, Germain P, Bercier S, Kapitaniak B, Buttelli O. Neuromuscular response of young boys versus men during sustained maximal contraction. Med Sci Sports Exerc. 2003;35:1042–1048. doi: 10.1249/01.MSS.0000069407.02648.47. [DOI] [PubMed] [Google Scholar]
- Hebestreit H, Mimura K, Bar-Or O. Recovery of muscle power after high-intensity short-term exercise: comparing boys and men. J Appl Physiol. 1993;74:2875–2880. doi: 10.1152/jappl.1993.74.6.2875. [DOI] [PubMed] [Google Scholar]
- Hirschfeld H. Motor control of every day motor tasks: guidance for neurological rehabilitation. Physiol Behav. 2007;92:161–166. doi: 10.1016/j.physbeh.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Holtermann A, Roeleveld K, Vereijken B, Ettema G. The effect of rate of force development on maximal force production: acute and training-related aspects. Eur J Appl Physiol. 2007;99:605–613. doi: 10.1007/s00421-006-0380-9. [DOI] [PubMed] [Google Scholar]
- Kanehisa H, Ikegawa S, Tsunoda N, Fukunaga T. Strength and cross-sectional areas of reciprocal muscle groups in the upper arm and thigh during adolescence. Int J Sports Med. 1995a;16:54–60. doi: 10.1055/s-2007-972964. [DOI] [PubMed] [Google Scholar]
- Kanehisa H, Yata H, Ikegawa S, Fukunaga T. A cross-sectional study of the size and strength of the lower leg muscles during growth. Eur J Appl Physiol Occup Physiol. 1995b;72:150–156. doi: 10.1007/BF00964130. [DOI] [PubMed] [Google Scholar]
- Kellis E, Baltzopoulos V. Resistive eccentric exercise: effects of visual feedback on maximum moment of knee extensors and flexors. J Orthop Sports Phys Ther. 1996;23:120–124. doi: 10.2519/jospt.1996.23.2.120. [DOI] [PubMed] [Google Scholar]
- Kellis E, Unnithan VB. Co-activation of vastus lateralis and biceps femoris muscles in pubertal children and adults. Eur J Appl Physiol Occup Physiol. 1999;79:504–511. doi: 10.1007/s004210050545. [DOI] [PubMed] [Google Scholar]
- Knapik JJ, Staab JS, Harman EA. Validity of an anthropometric estimate of thigh muscle cross-sectional area. Med Sci Sports Exerc. 1996;28:1523–1530. doi: 10.1097/00005768-199612000-00013. [DOI] [PubMed] [Google Scholar]
- Lambertz D, Mora I, Grosset JF, Perot C. Evaluation of musculotendinous stiffness in prepubertal children and adults, taking into account muscle activity. J Appl Physiol. 2003;95:64–72. doi: 10.1152/japplphysiol.00885.2002. [DOI] [PubMed] [Google Scholar]
- McComas AJ, Sica RE, Petito F. Muscle strength in boys of different ages. J Neurol Neurosurg Psychiatry. 1973;36:171–173. doi: 10.1136/jnnp.36.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritani T, Oddsson L, Thorstensson A, Astrand PO. Neural and biomechanical differences between men and young boys during a variety of motor tasks. Acta Physiol Scand. 1989;137:347–355. doi: 10.1111/j.1748-1716.1989.tb08763.x. [DOI] [PubMed] [Google Scholar]
- Paasuke M, Ereline J, Gapeyeva H. Twitch contraction properties of plantar flexor muscles in pre- and post-pubertal boys and men. Eur J Appl Physiol. 2000;82:459–464. doi: 10.1007/s004210000236. [DOI] [PubMed] [Google Scholar]
- Parker DF, Round JM, Sacco P, Jones DA. A cross-sectional survey of upper and lower limb strength in boys and girls during childhood and adolescence. Ann Hum Biol. 1990;17:199–211. doi: 10.1080/03014469000000962. [DOI] [PubMed] [Google Scholar]
- Seger JY, Thorstensson A. Muscle strength and electromyogram in boys and girls followed through puberty. Eur J Appl Physiol. 2000;81:54–61. doi: 10.1007/PL00013797. [DOI] [PubMed] [Google Scholar]
- Slaughter MH, Lohman TG, Boileau BA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- Smits-Engelsman BC, Westenberg Y, Duysens J. Development of isometric force and force control in children. Brain Res Cogn Brain Res. 2003;17:68–74. doi: 10.1016/S0926-6410(03)00081-8. [DOI] [PubMed] [Google Scholar]
- Streckis V, Skurvydas A, Ratkevicius A. Children are more susceptible to central fatigue than adults. Muscle Nerve. 2007;36:357–363. doi: 10.1002/mus.20816. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at adolescence. Blackwell Scientific Publications; Oxford: 1962. [Google Scholar]
- Tonson A, Ratel S, Le Fur Y, Cozzone P, Bendahan D. Effect of maturation on the relationship between muscle size and force production. Med Sci Sports Exerc. 2008;40:918–925. doi: 10.1249/MSS.0b013e3181641bed. [DOI] [PubMed] [Google Scholar]
- Wood LE, Dixon S, Grant C, Armstrong N. Elbow flexor strength, muscle size, and moment arms in prepubertal boys and girls. Pediatr Exerc Sci. 2006;18:457–469. doi: 10.1123/pes.18.4.457. [DOI] [PubMed] [Google Scholar]