Abstract
Objective
High-frequency oscillations (HFOs) can be recorded in epileptic patients with clinical intracranial EEG. HFOs have been associated with seizure genesis because they occur in the seizure focus and during seizure onset. HFOs are also found interictally, partly co-occurring with epileptic spikes. We studied how HFOs are influenced by antiepileptic medication and seizure occurrence, to improve understanding of the pathophysiology and clinical meaning of HFOs.
Methods
Intracerebral depth EEG was partly sampled at 2,000 Hz in 42 patients with intractable focal epilepsy. Patients with five or more usable nights of recording were selected. A sample of slow-wave sleep from each night was analyzed, and HFOs (ripples: 80–250 Hz, fast ripples: 250–500 Hz) and spikes were identified on all artifact-free channels. The HFOs and spikes were compared before and after seizures with stable medication dose and during medication reduction with no intervening seizures.
Results
Twelve patients with five to eight nights were included. After seizures, there was an increase in spikes, whereas HFO rates remained the same. Medication reduction was followed by an increase in HFO rates and mean duration.
Conclusions
Contrary to spikes, high-frequency oscillations (HFOs) do not increase after seizures, but do so after medication reduction, similarly to seizures. This implies that spikes and HFOs have different pathophysiologic mechanisms and that HFOs are more tightly linked to seizures than spikes. HFOs seem to play an important role in seizure genesis and can be a useful clinical marker for disease activity.
High-frequency oscillations (HFOs) in intracranial EEG have been associated with epileptogenesis and seizure genesis and have been studied in humans and rats.1–14 They have been divided into ripples (80 –250 Hz) and fast ripples (FRs; 250 –500 Hz).13 Ripples have been considered more physiologic in nature because they have also been recorded in healthy animal brains, whereas FRs are more frequent in affected hippocampi.3,6,15–20 However, both ripples and FRs have been correlated with the seizure onset zone (SOZ).7,11,21 HFOs were first recorded with microelectrodes but can also be recorded with clinically used macroelectrodes.11,13,21–23
HFOs have been associated with seizure genesis because their localization is related to the seizure focus and they occur at seizure onset1,6,11,24 and with epileptogenesis, as HFOs occurred before spontaneous seizures in kainic acid–injected rats.7 They may result from a γ-aminobuteric acid–mediated feedback imbalance or pathologic neuronal connections.4,25,26 HFOs occur during seizures and interictally, most often in non-REM sleep.6,27 Epileptic spikes in humans fluctuate over time and increase after seizures, suggesting that spikes reflect damaged and epileptogenic cortex, which is worsened by seizures, rather than seizure genesis.28–30 Whereas original observations indicated no effect of medication reduction on spike rates,28,29 recently a decrease has been noted.30 Sixty-two percent of ripples and 48% of FRs co-occur with spikes.21 However, no increase in HFOs after seizures, as occurs in spikes, should be observed if HFOs are part of seizure generation rather than resulting from seizures. In kainic acid rats, the rate and extent of FRs appeared stable over time.31 The effect of anticonvulsant drugs on interictal HFOs has not yet been studied. Assessing whether medication affects the rate, duration, or extent of HFOs can tell us about the pathophysiology of HFOs. This can be studied because the medication dosage is decreased during long-term intracranial EEG monitoring, to increase seizure probability. The aim of the present study was to describe the relation of interictal HFOs to antiepileptic drug reduction and seizure occurrence to improve understanding of their pathophysiology and clinical meaning.
METHODS
Patient selection
Between September 2004 and September 2007, 42 patients with medically intractable focal epilepsy underwent intracranial depth stereo-EEG (SEEG), partly recorded at 2,000 Hz, thus allowing the study of HFOs. The Montreal Neurological Institute and Hospital Research Ethics Committee approved the study, and informed consent was obtained from all patients. Patients with five or more usable night recordings (see below) were selected. All 42 patients showed HFOs, so selection did not depend on their presence. Antiepileptic medication dosage and seizure occurrence were derived from medical charts and EEG reports.
SEEG recordings
Patients were monitored with a 128-channel video-SEEG during 10 to 21 days (Harmonie, Stellate, Montreal, Canada). At times, SEEG was acquired with a 500-Hz low-pass hardware filter and a sampling rate of 2,000 Hz (other times 70-Hz filter, 200-Hz sampling rate). Practical considerations independent of the studied events determined which sampling rate was chosen each night. The intracerebral electrodes were manufactured on site by wrapping a 0.254-mm stainless steel central core with 0.076-mm wire, coated with Teflon and stripped focally yielding electrode contacts. The tip formed the deepest contact (length 1 mm; effective surface 0.85 mm2), whereas the other eight contacts were 5 mm apart (0.5 mm; 0.80 mm2). The implantation consisted of intracerebral depth and epidural cortical surface electrodes.32 Electrode placement depended on clinical decisions. Typically, three temporal depth electrodes were directed orthogonally through the middle temporal gyrus so that the deepest contacts were in the amygdala, anterior hippocampus, and parahippocampal gyrus. The number of extra temporal electrodes varied, and the extra temporal electrodes were directed orthogonally or obliquely, depending on the target and vasculature.
Night selections
Only those nights recorded at 2,000 Hz with no seizure between 10 PM and 8 AM, few artifacts, good EKG (assessed for EMG activity), and slow-wave sleep were selected. Only 1 minute each night was selected, because marking the EEG for HFOs is time-consuming (marking 1 minute can take 4 hours). In other studies, we marked 10 minutes but only a subset of channels for each patient.13,21,23 In our experience, HFO rates are stable over time, and a study currently under way shows that in most patients HFO rates can be determined reliably within a shorter period, with most information gained within the first minute (figure e-1 on the Neurology® Web site at www.neurology.org). In this study, several nights were selected for each patient, resulting in marking 5 to 8 minutes per patient (all channels). Selections were made following spectral trend analysis of a selected nonspiking SEEG channel for delta and the EKG channel for EMG activity for the period between midnight and 6 AM. The 1-minute epoch was selected when the delta power was highest, whereas EMG power was regular and low. The EEG was inspected for artifacts and to ensure that there was at least 25% delta. The selection was not influenced by the amount of spikes or HFOs.
Marking HFOs and spikes
For each patient, the 1-minute selections were shuffled and recoded to blind the reviewer (M.Z.) to the order of nights. Then these selections were reviewed in bipolar montage (Harmonie Reviewer). HFOs were marked using a vertically split screen with an 80-Hz high-pass filter on the left and a 250-Hz high-pass filter on the right to distinguish between ripples (80 –250 Hz) and FRs (250 –500 Hz). The time scale was set to maximal resolution, which equals 0.8 seconds/page on a 48-cm-wide monitor. The amplitude scale was raised to 1 μV/mm. Spikes were reviewed on a separate copy of the EEG (filter 0.3–70 Hz, time scale 10 seconds/page, amplitude scale 30 μV/mm). All channels were reviewed and analyzed, except for channels outside the brain or with artifacts in one of the selections.
Analysis
A MATLAB program calculated the rates of spikes, ripples, and FRs and their co-occurrence per channel for each selection. The durations of HFOs were calculated.
It is difficult to assess the influence of medication and seizures independently, because reduced medication increases seizure frequency. To study the influence of seizures, nights after seizures (within 48 hours) were compared with nights with equal medication dosage but with no seizure in the preceding 72 hours (figures 1 and e-2). These nights were selected before examining the events. For each patient, only one pair of nights was chosen, and if there were two possible pairs, the night not directly after the seizure was chosen. The nights were compared for events/minute/channel (the total number of events divided by the number of analyzed channels) of ripples, FRs, spikes, ripples with spikes (R_Sp), ripples without spikes (isolated ripples: R_isol), fast ripples with spikes (FR_Sp), and fast ripples without spikes (FR_isol) using a two-tailed paired t test. The mean durations of HFOs were compared with a two-tailed t test. The number of channels involved (i.e., the number of channels with one or more events during the selected minute) and the numbers of events in the SOZ only were compared before and after seizures to study the effect on spatial distribution of events. The SOZ was derived from the clinical EEG report stating which electrode contacts were involved in the seizure onsets.
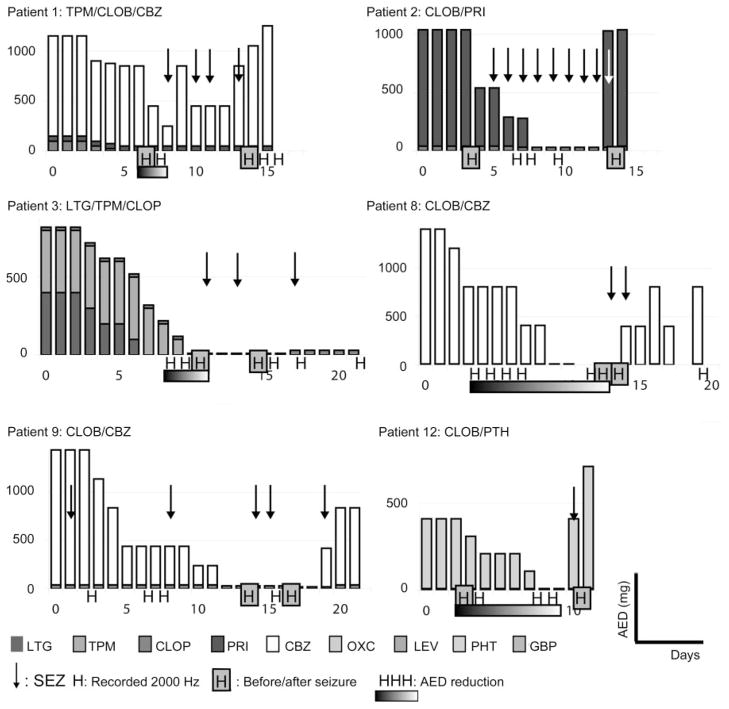
Figure 1. Medication doses (mg/day) and seizure occurrence (arrows) for all patients.
This figure refers to patients 1, 2, 3, 8, 9, and 12; see figure e-2 for patients 4, 5, 6, 7, 10, and 11. Day 0 = day of implantation; H = nights recorded at 2,000 Hz and suitable for analysis (without seizures and without abundant artifacts, with good slow-wave sleep). The 10 night-pairs selected for the comparisons before and after seizure are pointed out by gray squares around the H (patients 1, 2, 3, 4, 5, 7, 8, 9, 10, and 12). In patient 4, the night after seizure was compared with a night that was later in time (bowed arrow). To study the influence of medication reduction, the recorded nights before the first intervening seizure were selected (patients 1, 3, 6, 8, 11, and 12). LTG = lamotrigine; TPM = topiramate; CLOB = clobazam; PRI = primidone; CBZ = carbamazepine; OXC = oxcarbamazepine; LEV = levetiracetam; PTH = phenytoin; GBP = gabapentin; SEZ = one or more seizures; AED = antiepileptic drug.
To study the influence of medication withdrawal, the nights before the first seizure during medication reduction were studied. First, the first and last nights from these selections were compared for events/minute/channel of ripples, FRs, spikes, R_Sp, R_isol, FR_Sp, and FR_isol (two-tailed paired t test) and for mean duration of HFOs (two-tailed t test). The analyses were repeated for the number of channels involved and the number of events in the SOZ. To study not only the first and last but all available nights during medication reduction without intervening seizures, correlation coefficients (Rho) were calculated correlating events to time. To correct for general differences between patients, relative event rates compared with the maximum per patient were calculated, and the nights were recoded such that the first night was 0, the last night was 1, and nights in between were scaled between 0 and 1 (e.g., the fifth of six nights became 0.8). We decided not to study the actual medication dosage, because often several drugs were reduced simultaneously and because blood levels were unknown.
Seizures or medication might influence the depth of slow-wave sleep beyond the selected threshold of greater than 25% delta band power, and the depth of slow-wave sleep might consequently influence event rates. To study this potential bias, the delta band power of a nonspiking channel was calculated per epoch and correlated to the relative event rates (i.e., relative to maximum/patient to correct for general differences between patients). The paired t tests described above were repeated for delta power.
Relative rates of isolated HFOs were correlated to relative rates of spikes (relative to maximum per patient) to study the direct relationship between HFO and spike rates. Last, correlation analysis was performed for consecutively scored epochs (epochs were originally shuffled so this order is random with respect to recorded time) to evaluate the trend in marking events by a reviewer over time.
RESULTS
Twelve patients (one woman) with five to eight nights were selected. All had seizures within the recordings at 2,000 Hz, and all but one underwent medication withdrawal (figures 1 and e-2). Patient characteristics and electrodes positions are given in table 1. Three to nine electrodes were implanted per patient with 20 to 72 bipolar channels (mean 46.8), from which 13 to 62 channels were studied (mean 35.7). In total, 62,231 ripples (11% R_Sp), 13,495 FRs (14% FR_Sp), and 10,736 spikes were marked, with an average duration of 104 ± 54 msec for ripples and 37 ± 15 msec for FRs.
Table 1.
Patient data
| Patient no./age, y/sex | Seizure onset | MRI | Electrodes | Medication | |
|---|---|---|---|---|---|
| n | Positions | ||||
| 1/36/M | Left frontal | Focal cortical dysplasia left frontal | 43 | L+RC, L+ROF, LFp, LFa, LE+RE | TPM/CLOB/CBZ |
| 2/22/M | Left temporal | Resection + cerebral cavernous angioma left temporoparietal | 13 | LH, LP | CLOB/PRI |
| 3/36/M | Left parietal | Infarct + resection left parietal | 29 | LA, LF, LOF, LO, LOP, LT, LCa, LE | LTG/TPM/CLOB |
| 4/46/M | Right temporal | Right hippocampus malrotation | 26 | L+RA, L+RH | CBZ/PTH/GBP |
| 5/19/M | Right parietal | Normal | 45 | RA, RCa, RCs, RH, ROF, ROi, ROs | None |
| 6/43/M | Left frontal | Resection left anteriotemporal | 33 | LCa, LCp, LH, LSMAa, LSMAp, LT | TPM/OXC |
| 7/44/M | Left temporal | Normal | 46 | L+RA, L+RH, L+RP | LTG/PTH |
| 8/47/M | Left parietal | Porencephalic cyst left parietal | 24 | Lai, Las, Lpi, Lps | CLOB/CBZ |
| 9/34/M | Left temporal | Resection left temporal and atrophy left hemisphere | 32 | LH, LP, LT, LO, LE | CLOB/CBZ |
| 10/25/M | Bitemporal | Normal | 62 | L+RA, L+RH, L+RO, L+RS, RP | TPM/CLOB/CBZ |
| 11/49/F | Bitemporal | Atrophy right hippocampus | 36 | L+RA, L+RH, L+RP, LE | OXC/LEV |
| 12/30/M | Bitemporal | Atrophy right hippocampus | 39 | L+RA, L+RH, RC, RP, RE | CLOB/PTH |
The seizure type and presumed lobe of origin, the MRI results, the number of studied electrode contact channels (n) and their positions, the channels showing the first EEG changes during seizures (seizure onset zone [SOZ]), and the antiepileptic medication are shown. All electrodes are depth electrodes except for the epidural electrodes.
L/RC/a/s = left/right cingulate/anterior/superior electrode; L/ROF = left/right orbitofrontal electrode; LF/p/a = left frontal/posterior/anterior electrode; L/RE = left/right epidural electrode; L/RH = left/right hippocampus electrode; L/RP = left/right parahippocampus electrode; L/RA = left/right amygdale electrode; L/RO/i/s = left/right occipital/infracalcine/supracalcine electrode; LOP = left frontal operculum electrode; LT = left anteriotemporal electrode; LSMAa/p = left supplementary motor area anterior/posterior electrode; Lai/s = left anterior inferior/superior electrode (porencephalic cyst); Lpi/s = left posterior inferior/superior electrode; L/RS = left/right supramarginal gyrus electrode; TPM = topiramate; CLOB = clobazam; CBZ = carbamazepine; PRI = primidone; LTG = lamotrigine; PTH = phenytoin; GBP = gabapentin; OXC = oxcarbamazepine; LEV = levetiracetam.
Influence of seizures
In 10 patients (all except nos. 6 and 11), rates before and after seizures could be compared (figure 1, figure e-2, and table 2). The spike rate increased (all channels and SOZ channels), whereas there was no increase in HFO rates. However, the number of involved channels was higher after seizures, not only for spikes, but also for ripples, FRs, and R_isol. The duration of ripples and FRs did not change after seizures. (ripples: 105.0→105.8, p = 0.38; FR: 36.8→37.6, p = 0.08).
Table 2.
Averages with two-tailed paired t test for events before and after seizures
| All events/minute/channel
|
Seizure onset zone events/minute/channel
|
Channels involved, %
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | p Value | Before | After | p Value | Before | After | p Value | |
| R | 22.1 | 22.8 | 0.69 | 66.0 | 61.0 | 0.59 | 46 | 57 | <0.01* |
|
| |||||||||
| FR | 5.1 | 4.7 | 0.66 | 14.3 | 13.0 | 0.66 | 11 | 15 | <0.01* |
|
| |||||||||
| Sp | 3.6 | 4.7 | <0.01* | 11.3 | 13.5 | 0.02* | 37 | 49 | 0.04* |
|
| |||||||||
| R_isol | 19.9 | 20.1 | 0.90 | 58.4 | 52.0 | 0.47 | 44 | 54 | <0.01* |
|
| |||||||||
| FR_isol | 4.4 | 4.0 | 0.64 | 11.3 | 9.4 | 0.51 | 9 | 12 | 0.07 |
|
| |||||||||
| R_Sp | 2.2 | 2.7 | 0.08 | 7.5 | 9.0 | 0.05* | 2 | 3 | 0.53 |
|
| |||||||||
| FR_Sp | 0.8 | 0.7 | 0.85 | 3.0 | 3.6 | 0.18 | 1 | 3 | 0.04* |
Seizure onset zone contained four to nine channels.
p <0.05.
R = ripples; FR = fast ripples; Sp = spikes; R_fisol = ripples without co-occurring spikes; FR_isol = fast ripples without co-occurring spikes; R_Sp = ripples with co-occurring spikes; FR_Sp = fast ripples with co-occurring spikes.
Influence of medication
In six patients (nos. 1, 3, 6, 8, 11, and 12), the influence of medication without intervening seizures could be studied (table 3). After medication reduction, there was an increase in isolated HFOs, whereas HFOs co-occurring with spikes did not increase (no changes in number of channels involved and no significant changes in SOZ channels). Spikes decreased but not significantly. Mean ripple duration increased after medication reduction (ripples: 96.0→98.8, p = 0.05; FR: 36.1→35.6, p = 0.54). HFO rates for all nights before the first seizure are shown in figure 2. During medication reduction, ripples, FRs, R_isol, and FR_isol show linear increase over time (table 3). The correlation for spikes is nonsignificantly negative, similarly for HFOs co-occurring with spikes.
Table 3.
Averages with two-tailed paired t test for events of first and last night during medication reduction without intervening seizures
| All events/minute/channel
|
Correlation and slope (all)
|
Seizure onset zone events/minute/channel
|
Channels involved, %
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First | Last | p Value | Rho | Slope | p Value | First | Last | p Value | First | Last | p Value | |
| R | 12.5 | 15.6 | 0.04* | 0.63 | 1.3 | <0.01* | 38.8 | 45.5 | 0.38 | 42 | 42 | 0.90 |
|
| ||||||||||||
| FR | 2.3 | 2.5 | 0.47 | 0.50 | 0.6 | <0.02* | 3.2 | 3.7 | 0.63 | 7 | 7 | 1.00 |
|
| ||||||||||||
| Sp | 3.6 | 3.0 | 0.27 | −0.40 | −0.3 | 0.08 | 16.2 | 8.9 | 0.14 | 36 | 28 | 0.14 |
|
| ||||||||||||
| R_isol | 10.6 | 14.3 | 0.01* | 0.71 | 1.5 | <0.01* | 28.2 | 39.9 | 0.17 | 38 | 39 | 0.83 |
|
| ||||||||||||
| FR_isol | 1.7 | 2.1 | <0.01* | 0.69 | 0.8 | <0.01* | 0.8 | 2.9 | 0.15 | 5 | 5 | 0.76 |
|
| ||||||||||||
| R_Sp | 1.9 | 1.2 | 0.43 | −0.31 | −0.4 | 0.18 | 10.6 | 5.5 | 0.30 | 3 | 2 | 0.59 |
|
| ||||||||||||
| FR_Sp | 0.6 | 0.4 | 0.41 | −0.22 | −0.2 | 0.35 | 2.4 | 0.7 | 0.37 | 2 | 1 | 0.63 |
For all nights during medication reduction before the occurrence of the first seizure, a correlation coefficient (Rho) was calculated between event rates (as a percentage of the maximum rate) and the nights (corrected for the number of nights). Seizure onset zone contained four to six channels.
p < 0.05.
R = ripples; FR = fast ripples; Sp = spikes; R_isol = ripples without co-occurring spikes; FR_isol = fast ripples without co-occurring spikes; R_Sp = ripples with co-occurring spikes; FR_Sp = fast ripples with co-occurring spikes.
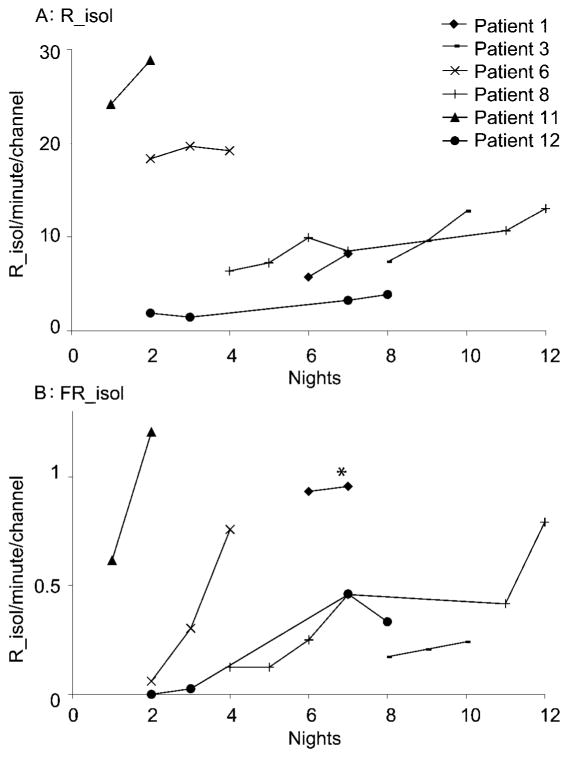
Figure 2. Rates of isolated high-frequency oscillations during medication reduction but without intervening seizures (six patients).
(A) Rates of R_isol/minute/channel. In all six patients, an overall increase of ripple rates is seen. (B) Rates of FR_isol/minute/channel. In all patients, an overall increase in fast ripple rates is seen. *The y values of patient 1 are divided by 10 to improve visualization of the other data. R_isol = ripples without co-occurring spikes; FR_isol = fast ripples without co-occurring spikes.
Other calculations
There was no correlation between the relative delta band power (percentage of maximum per patient) and the relative event rates for ripples, FRs, and spikes (rho = 0.09, −0.10, and 0.12 [p > 0.10]). Also, there was no change in relative delta power during medication change (62→60%, p = 0.91) or after seizures (66→62%, p = 0.75).
Analyzing consecutively scored epochs yielded no significant correlation (rho = 0.01, −0.07, and 0.06 [p > 0.10] for ripples, FRs, and spikes).
There was no correlation between relative rates of spikes and of isolated HFOs (rho = 0.20 [p = 0.10] and −0.01 [p = 0.93] for spikes-R_isol and spikes-FR_isol), which was compared to assess any direct relationship between these rates.
Individual graphs
Ten patients showed overall increase in ripples during the investigation. Patients 5 (no medication) and 11 showed an overall decrease, which in patient 11 occurred when medication was stable, but several seizures intervened (figure e-3). In patients 1 and 2, antiepileptic drugs were restarted at the end of the recording with EEG recorded at 2,000 Hz, enabling the study of HFOs after medication increase. Patient 2 showed a decrease in ripples, though with daily intervening seizures. In patient 1, ripples (with spikes) increased, related to an increase in spikes after an intervening seizure, and FRs decreased. The seizures make it impossible to conclude about the effect of reinstating medication. The number of patients is too small to enable statistical comparison between temporal and extratemporal cases or between different antiepileptic drugs. However, an increase in HFOs after medication withdrawal was seen in both temporal and extratemporal patients, medicated with different antiepileptic drugs.
DISCUSSION
This study offers new insight into the pathophysiology of HFOs in epilepsy. In contrast to spikes, HFOs did not increase after seizures, whereas they clearly increased after medication reduction. This means that, although HFOs often co-occur with spikes,21 they behave more like seizures, which increase after medication withdrawal. This supports the idea that HFOs are important in understanding seizure genesis and HFOs may be a better biologic marker of epileptogenic tissue than spikes.
The increase in HFOs after medication reduction might also result from the anesthetics used for implantation (general anesthesia decreases physiologic HFOs33) or from the electrode implantation disrupting local networks. However, mostly the increase was not regular and continued until the end of the recording, whereas there was no increase in patient 5 (no medication) and in patient 11 while medication was stable. The medication effect would be proven if HFOs decrease after drugs are restarted. As we study sleep, this requires 2,000-Hz recording one full day after restarting medication, without intervening seizures. This was not available within the whole group of 42 patients.
HFOs co-occurring with spikes showed a nonsignificant decrease after medication reduction like spikes themselves, suggesting that HFOs with and without spikes behave differently. HFOs with spikes behave like spikes, and their pathophysiology is possibly different and can be viewed similarly to HFOs with somatosensory evoked potentials.20 It has been suggested that spikes with and without HFOs are different.21 Spike rates were too low to study this.
FRs are generally considered pathologic but can be evoked in the somatosensory cortex.20 It is currently not possible to distinguish normal from pathologic ripples, except in dentate gyrus, where these do not occur normally. Ripples and FRs showed no difference in their changes with seizures and medication: differences in significance probably resulted from lower FR rates.
Marking spikes is subjective and depends on signal-to-noise ratios. Sharp waves were not marked, and small spikes in changing background are easily missed. Also, spikes seen on one channel might be related to HFOs on adjacent channels. It is therefore possible that some HFOs without a marked co-occurring spike behave like HFOs with spikes.
The selection of nights around seizures was based on a stable medication dose in the 24 hours preceding the selections. However, a bias might exist in actual blood levels because there is a trend toward more medication before than after seizures beyond these 24 hours. For example, when looking at 72 hours preceding the selections, the medication dose was equal in two patients, larger before the seizure in seven patients, and larger after the seizure in one patient. This bias could lead to more HFOs after seizures. Considering this, there might actually be a relative decrease of HFO rates after seizures. Epileptic spikes have been suggested to be inhibitory.34–37 This might explain why HFOs decrease immediately after spikes.21,23 Likewise, seizures consisting of multiple rhythmic spikes might cause a similar decrease after seizures for a longer period. For almost all events, more channels were involved after seizures. This might be explained by the theory described above that some HFOs without a spike might show “HFOs-within-spikes–like” behavior.
No correlation was found between delta power and event rates, meaning that differences in delta above the 25% cutoff did not influence the rates. Also, we found no change in reviewer sensitivity as marking progresses. The proportion of HFOs showing simultaneous spikes is lower than in a previous study.21 This is because of different channel selections (fewer channels with spikes), patients, and reviewer.
There are two limitations. First, only 1 minute per night was analyzed. One minute was chosen because most information about HFO rates is gained within 1 minute. However, more stability would be achieved with a longer period. If 1 minute were not enough, however, results would be noisy and unlikely to be significant. Spikes are less stable over time and less frequent, so spike rates may have been too low to reach significance. Also, the substudies assessing HFO spread and HFOs with or without spikes might have been limited by this. As automatic detection programs are developed, longer time frames can be studied.5,38 We chose not to use automatic detection because it has not been validated enough (on our data) and because separation of HFOs with and without spikes by such methods is not possible yet. Second, the limited number of patients did not allow distinction between different kinds of epilepsies (different locations or etiologies) and medication types. Probably it is more feasible to study this in animals. The selection of patients included strikingly more men, which seems to be by chance because we could not find any explanation.
The number of HFOs seems to represent disease activity and might be used for seizure prediction and therapy evaluation. In cats, carbamazepine and phenytoin reduce the ictal high-frequency component, whereas phenobarbital and diazepam shorten seizure duration.39 Some drugs might affect HFO rates and decrease seizure probability, whereas others affect seizure propagation. We found no difference between drugs. In scalp EEG, clobazam is known to increase beta activity, and it would be interesting to know how this relates to higher frequencies. However, clobazam was reduced in only two patients, simultaneously with carbamazepine, so the effect could not be studied.
Future study will determine whether differences exist between ripples and FRs and between spikes with and without HFOs. A more extensive follow-up study can include the influence of seizure severity and different drugs. A prospective study on the effect of increasing medication or the acute effect of clobazam is feasible. When studying interictal HFOs, one should realize that HFO rates and ratios to spikes can differ.
Supplementary Material
Acknowledgments
Supported by grant MOP-10189 from the Canadian Institutes of Health Research and by the Netherlands Organization for Scientific Research AGIKO grant no. 92003481, the University Medical Center Utrecht (internationalization grant), and the “Stichting de drie lichten” (M.Z.).
GLOSSARY
- AED
antiepileptic drug
- CBZ
carbamazepine
- CLOB
clobazam
- FR
fast ripple
- FR_isol
fast ripples without co-occurring spikes
- FR_Sp
fast ripples with co-occurring spikes
- GBP
gabapentin
- HFO
high-frequency oscillation
- Lai/s
left anterior inferior/superior electrode (porencephalic cyst)
- LEV
levetiracetam
- LF/p/a
left frontal/posterior/anterior electrode
- LOP
left frontal operculum electrode
- Lpi/s
left posterior inferior/superior electrode
- L/RA
left/right amygdale electrode
- L/RC/a/s
left/right cingulate/anterior/superior electrode
- L/RE
left/right epidural electrode
- L/RH
left/right hippocampus electrode
- L/ROF
left/right orbitofrontal electrode
- L/RO/i/s
left/right occipital/infracalcine/supracalcine electrode
- L/RP
left/right parahippocampus electrode
- L/RS
left/right supramarginal gyrus electrode
- LSMAa/p
left supplementary motor area anterior/posterior electrode
- LT
left anteriotemporal electrode
- LTG
lamotrigine
- OXC
oxcarbamazepine
- PRI
primidone
- PTH
phenytoin
- R
ripple
- R_isol
ripples without co-occurring spikes
- R_Sp
ripples with co-occurring spikes
- SEEG
stereo-EEG
- SEZ
one or more seizures
- SOZ
seizure onset zone
- Sp
spike
- TPM
topiramate
Footnotes
Disclosure: J.G. was a major shareholder of Stellate. The other authors report no disclosures.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by M. Zijlmans and R. Zelmann.
References
- 1.Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol. 1992;9:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Allen PJ, Fish DR, Smith SJ. Very high-frequency rhythmic activity during SEEG suppression in frontal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1992;82:155–159. doi: 10.1016/0013-4694(92)90160-j. [DOI] [PubMed] [Google Scholar]
- 3.Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41 (suppl 6):S144–S152. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 5.Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- 6.Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- 7.Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- 8.Akiyama T, Otsubo H, Ochi A, et al. Focal cortical high-frequency oscillations trigger epileptic spasms: confirmation by digital video subdural EEG. Clin Neurophysiol. 2005;116:2819–2825. doi: 10.1016/j.clinph.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Khosravani H, Pinnegar CR, Mitchell JR, Bardakjian BL, Federico P, Carlen PL. Increased high-frequency oscillations precede in vitro low-Mg seizures. Epilepsia. 2005;46:1188–1197. doi: 10.1111/j.1528-1167.2005.65604.x. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama T, Otsubo H, Ochi A, et al. Topographic movie of ictal high-frequency oscillations on the brain surface using subdural EEG in neocortical epilepsy. Epilepsia. 2006;47:1953–1957. doi: 10.1111/j.1528-1167.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 11.Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- 12.Ochi A, Otsubo H, Donner EJ, et al. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 13.Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100 –500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- 14.Staba RJ, Frighetto L, Behnke EJ, et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- 15.Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high-frequency oscillations (80 –500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002;52:407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- 16.Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 17.Draguhn A, Traub RD, Bibbig A, Schmitz D. Ripple (approximately 200-Hz) oscillations in temporal structures. J Clin Neurophysiol. 2000;17:361–376. doi: 10.1097/00004691-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Clemens Z, Janszky J, Szucs A, Bekesy M, Clemens B, Halasz P. Interictal epileptic spiking during sleep and wakefulness in mesial temporal lobe epilepsy: a comparative study of scalp and foramen ovale electrodes. Epilepsia. 2003;44:186–192. doi: 10.1046/j.1528-1157.2003.27302.x. [DOI] [PubMed] [Google Scholar]
- 19.Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131(pt 7):1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- 20.Curio G, Mackert BM, Burghoff M, Koetitz R, Abraham-Fuchs K, Harer W. Localization of evoked neuromagnetic 600 Hz activity in the cerebral somatosensory system. Electroencephalogr Clin Neurophysiol. 1994;91:483–487. doi: 10.1016/0013-4694(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs J, Levan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80 –500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worrell GA, Gardner AB, Stead SM, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urrestarazu E, Jirsch JD, LeVan P, et al. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. Epilepsia. 2006;47:1465–1476. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 24.Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80 –200 Hz) during seizures: intracellular correlates. J Neurophysiol. 2003;89:841–852. doi: 10.1152/jn.00420.2002. [DOI] [PubMed] [Google Scholar]
- 25.Jones MS, MacDonald KD, Choi B, Dudek FE, Barth DS. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2000;84:1505–1518. doi: 10.1152/jn.2000.84.3.1505. [DOI] [PubMed] [Google Scholar]
- 26.Moschovos C, Kostopoulos G, Papatheodoropoulos C. Long-term potentiation of high-frequency oscillation and synaptic transmission characterize in vitro NMDA receptor-dependent epileptogenesis in the hippocampus. Neurobiol Dis. 2008;29:368–380. doi: 10.1016/j.nbd.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2008 doi: 10.1111/j.1528-1167.2008.01784.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotman J, Koffler DJ. Interictal spiking increases after seizures but does not after decrease in medication. Electroencephalogr Clin Neurophysiol. 1989;72:7–15. doi: 10.1016/0013-4694(89)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Gotman J, Marciani MG. Electroencephalographic spiking activity, drug levels, and seizure occurrence in epileptic patients. Ann Neurol. 1985;17:597–603. doi: 10.1002/ana.410170612. [DOI] [PubMed] [Google Scholar]
- 30.Spencer SS, Goncharova II, Duckrow RB, Novotny EJ, Zaveri HP. Interictal spikes on intracranial recording: behavior, physiology, and implications. Epilepsia. 2008;49:1881–1892. doi: 10.1111/j.1528-1167.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- 31.Bragin A, Wilson CL, Engel J. Spatial stability over time of brain areas generating fast ripples in the epileptic rat. Epilepsia. 2003;44:1233–1237. doi: 10.1046/j.1528-1157.2003.18503.x. [DOI] [PubMed] [Google Scholar]
- 32.Olivier A, Germano IM, Cukiert A, Peters T. Frameless stereotaxy for surgery of the epilepsies: preliminary experience. Technical note J Neurosurg. 1994;81:629–633. doi: 10.3171/jns.1994.81.4.0629. [DOI] [PubMed] [Google Scholar]
- 33.Urasaki E, Genmoto T, Yokota A, Maeda R, Akamatsu N. Effects of general anesthesia on high-frequency oscillations in somatosensory evoked potentials. J Clin Neurophysiol. 2006;23:426–430. doi: 10.1097/01.wnp.0000186217.15904.99. [DOI] [PubMed] [Google Scholar]
- 34.Engel J, Jr, Ackermann RF. Interictal EEG spikes correlate with decreased, rather than increased, epileptogenicity in amygdaloid kindled rats. Brain Res. 1980;190:543–548. doi: 10.1016/0006-8993(80)90296-6. [DOI] [PubMed] [Google Scholar]
- 35.Avoli M. Do interictal discharges promote or control seizures? Experimental evidence from an in vitro model of epileptiform discharge. Epilepsia. 2001;42 (suppl 3):2–4. doi: 10.1046/j.1528-1157.2001.042suppl.3002.x. [DOI] [PubMed] [Google Scholar]
- 36.Swartzwelder HS, Lewis DV, Anderson WW, Wilson WA. Seizure-like events in brain slices: suppression by interictal activity. Brain Res. 1987;410:362–366. doi: 10.1016/0006-8993(87)90339-8. [DOI] [PubMed] [Google Scholar]
- 37.Bragdon AC, Kojima H, Wilson WA. Suppression of interictal bursting in hippocampus unleashes seizures in entorhinal cortex: a proepileptic effect of lowering [K+]o and raising [Ca2+]o. Brain Res. 1992;590:128–135. doi: 10.1016/0006-8993(92)91088-v. [DOI] [PubMed] [Google Scholar]
- 38.Nelson R, Myers SM, Simonotto JD, et al. Detection of high frequency oscillations with Teager energy in an animal model of limbic epilepsy. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2578–2580. doi: 10.1109/IEMBS.2006.259694. [DOI] [PubMed] [Google Scholar]
- 39.Ito T, Hori M, Yoshida K, Shimizu M. Effect of anticonvulsants on cortical focal seizure in cats. Epilepsia. 1977;18:63–71. doi: 10.1111/j.1528-1157.1977.tb05588.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.