Abstract
Thousands of kilometers of shelterbelt plantations of Casuarina equisetifolia have been planted to protect the southeast coastline of China. These plantations also play an important role in the regional carbon (C) cycling. In this study, we examined plant biomass increment and C accumulation in four different aged C. equisetifolia plantations in sandy beaches in South China. The C accumulated in the C. equisetifolia plant biomass increased markedly with stand age. The annual rate of C accumulation in the C. equisetifolia plant biomass during 0–3, 3–6, 6–13 and 13–18 years stage was 2.9, 8.2, 4.2 and 1.0 Mg C ha−1 yr−1, respectively. Soil organic C (SOC) at the top 1 m soil layer in these plantations was 17.74, 5.14, 6.93, and 11.87 Mg C ha−1, respectively, with SOC density decreasing with increasing soil depth. Total C storage in the plantation ecosystem averaged 26.57, 38.50, 69.78, and 79.79 Mg C ha−1 in the 3, 6, 13 and 18- yrs plantation, with most of the C accumulated in the aboveground biomass rather than in the belowground root biomass and soil organic C. Though our results suggest that C. equisetifolia plantations have the characteristics of fast growth, high biomass accumulation, and the potential of high C sequestration despite planting in poor soil conditions, the interactive effects of soil condition, natural disturbance, and human policies on the ecosystem health of the plantation need to be further studied to fully realize the ecological and social benefits of the C equisetifolia shelterbelt forests in South China.
Introduction
Forest restorations, including tree plantations, are often proposed as a remedy to combat global climate change. Globally, plantations are being established at an increasing rate, and now accounting for 5% of the global forest cover [1]. Trees can capture atmospheric CO2 through photosynthesis and store it in biomass with a turnover time of several decades. Thus, tree plantations play important roles in global C cycling and the uses of tree products can mediate various anthropogenic C releases [2], [3]. China maintained the largest plantation area in the world, reaching 62 million ha in 2008, and accounting for 35.5% of the total forest area in China [4]. Shelterbelt plantation, established along coastlines, is a special type of well-protected plantation in China due to socio-economic considerations. There is a “zero tolerance” policy on illegal act of cutting and tree harvesting, although local collecting of litter and fallen dead trees are often allowed. China has 18,000 kilometers mainland coastline, corresponding to 11.3 million hectares of coastal land; this provides a wide range of land resource for coastal shelterbelt plantation.
Casuarina equisetifolia is an N-fixing species that has been used extensively for windbreak and coastal stabilization in tropical and sub-tropical areas of the world [5], [6]. The critical ecosystem services of coastal forests, including Casuarina plantations, have gained great recognition recently, particularly after the devastating 2004 Southeast Asian tsunami [6], [7]. Casuarina equisetifolia is naturally distributed in the Oceania, Pacific islands and Southeast Asia [8], [9]. It requires limited growth conditions, likely because of its actinorhizal and mycorrhizal symbioses that fix nitrogen (N) and benefit phosphorus (P) acquisition [9], [10]. Since 1950 s, C. equisetifolia has been planted in the southeast coast of China [11]. The area of the C. equisetifolia plantation in China has currently reached 300,000 ha, with various field and nursery experimental trials been conducted [10], [12].
Casuarina equisetifolia plantation has the potential to sequestrate atmospheric CO2 and contributes to the regional C cycling. Most studies have focused on the ecological functions of C. equisetifolia, such as land reclamation, windbreaks, erosion control, and wood and fuel production [5], [6]. Many N-fixation studies have been done on this plant [13], [14]. In addition, its arbuscular mycorrhizal (AM) and ectomycorrhizal (ECM) symbioses have also been examined [10], [15]. However, the aboveground and belowground biomass increment and C sequestration in C. equisetifolia plantations in South China are largely unknown. Due to its large actual and potential planting areas in China and other parts of the world, the C sequestration potential in C. equisetifolia may greatly account for the regional and global C budget.
The patterns of C stock during forest development have gained great attention since one century ago [16]. Many studies have reported changes in biomass and production with stand age using chronosequence method [17]. Generally, plant biomass would increase gradually with stand age. However, soil C might perform differently. The decomposition of soil C might proceed more rapidly than the plant C input in the initial stage, resulting in a net loss of soil C at early stage of stand development. In this paper, we investigated the plant biomass increment and soil C change in four different aged C. equisetifolia plantations growing at a sandy beach site in South China. The objectives of this study were to: 1) quantify aboveground and belowground biomass of C. equisetifolia at different aged plantations; 2) estimate C sequestration in plant biomass and soil. Our hypotheses were that (1) ecosystem C storage (including biomass C and soil C) would increase with the stands ages; and (2) soil might contribute to carbon storage less than plant biomass as a result of unfavorable texture of sandy soil.
Materials and Methods
Ethics Statement
This research was conducted in South China Botanical Garden, Chinese Academy of Sciences. This study was also supported by this institute. We confirmed that the location is not privately-owned and the sampling of soils and plants was approved by Forestry Agency of Maogang District, the local administrator of coastal shelterbelt plantations. We also confirmed that the field studies did not involve endangered or protected species.
Site Description
The study area is located in the coastal Maogang District, southeast of Maoming City (110°54′E, 21°27′N), Guangdong Province, South China. The region has a tropical monsoon climate, including a rainy and warm season (April to October: precipitation 1400 mm) and a dry and cool season (November to March: precipitation 160 mm). Typhoons occur in this area from June to late October. The annual mean temperature is 23°C, with an average July high of 30°C and January low of 18°C.
Casuarina equisetifolia has been planted continuously in this area since 1960 s. Due to the disturbance of typhoon, the plantations were frequently destroyed and replanted again. However, as a substitute for fuel material, the litterfalls of C. equisetifolia were collected regularly by the local residents, so were the fallen trees after the typhoon disturbance.
Experiment Design
We applied a chronosequence approach. Four different age classes of C. equisetifolia plantations were selected, which were established in 1994, 1999, 2006 and 2009, respectively. Soil texture was very homogenous in these plantations, with sand accounting for 92–93% and clay for 4–5% of the soil mass (Table S1). The original number of trees planted at all sites is 2500 plant ha−1. Four 10 m×10 m plots were established in each of the four plantations in March 2012. Species density, tree height (H) and tree diameter at breast height (DBH) were recorded at each plot (Table 1). We used a C. equisetifolia growth model developed specifically for the coastal South China region to estimate the plant biomass [18]. Plant biomass included root, stem, branch, and branchlet was estimated in each plot (Table S2).
Table 1. The general status of four age classes of C. equisetifolia plantations in 2011 (mean±S.E.).
| Stand age(yrs) | Density(plants ha−1) | DBH(cm) | Height(m) |
| 3 | 2350a±87 | 4.49d±0.28 | 4.92c±0.63 |
| 6 | 2200a±82 | 9.46c±0.14 | 8.16b±0.40 |
| 13 | 1250b±144 | 15.88b±0.40 | 12.67a±0.16 |
| 18 | 975b±63 | 19.35a±0.90 | 10.63b±0.57 |
Note: DBH: Diameter at breast height. Means in a column followed by different lower-case letters are significantly different at P<0.05 (one-way ANOVA and LSD test).
Field Sampling and Measurements
We collected samples of C. equisetifolia branchlets, branches, stems and fine roots at each individual plot, and analyzed each component separately for their relative concentration of C. Samples were dried to a constant weight at 65°C, ground and passed through a fine screen (0.5 mm). The C concentration was determined using the potassium dichromate oxidation method [19], and then applied to the growth model to estimate biomass C accural.
Roots were also sampled directly using a 25 cm diameter PVC pipe. Root cores were taken at 3 random places in each plot at the 0–10, 10–20, 20–40, 40–60, 60–80 and 80–100 cm soil depths. Roots were washed free of soil with a spray of water. Fine root (<2 mm) was separated from coarse root (>2 mm) and dried at 65°C to a constant weight.
Soil samples were collected from 3 positions in each plot by using a 5 cm diameter PVC tube at the 0–10, 10–20, 20–40, 40–60 and 60–100 cm soil depths. Bulk density was determined by taking an adjacent core. All of soil samples within the same soil depth in each plot were pooled together and air-dried. Before analyzing total N (TN) and organic matter content, soils were ground to pass through a 0.25-mm sieve. Total N concentration was determined by the micro-Kjeldahl digestion followed by the colorimetric determination [19] on a flow injection autoanalyzer (FIA) (Lachat Instruments, USA). Soil organic carbon (SOC) was determined by the potassium dichromate oxidation method with SOM calculated as SOM = 1.724×SOC [19].
Statistical Analysis
Statistical analyses were performed using SAS 8.1 for Windows (SAS Institute, Cary, NC, USA.). Biomass accumulation, carbon concentrations and C storage of plant materials and soils, as well as fine root distribution among 4 age classes of plantations were compared by one-way ANOVA, followed by the LSD method to test among different age groups.
Results
Plant Biomass and C Concentration
Aboveground biomass (AGB), belowground biomass (BGB), and total plant biomass (PB) of the C. equisetifolia plantations increased markedly with stand age. The plantation followed a classic self-thinning growth with the steady increases of DBH and height along with the decrease of tree density (Table 1). The AGB and BGB of C. equisetifolia plantation increased from 14.22 and 7.77 Mg ha−1 at the 3 yrs-old plots to 130.34 and 21.13 Mg ha−1 at the 18 yrs-old plots, respectively (Table 2). The PB of C. equisetifolia plantation in our study site increased from 21.99 Mg ha−1 at the 3 yrs-old plots to 151.46 Mg ha−1 at the 18 yrs-old plots, with the most rapid increase occurred in the 3–6 years stage (Table 2). The biomass of stem and root accounted for more than 70% of the total plant biomass. In the four age classes plantations, we found that the proportion of stem biomass was the largest and the branchlet biomass was the smallest. The BGB/AGB ratio was 0.61, 0.21, 0.16, and 0.16 at the 3, 6, 13, and 18 yrs old plantation, respectively.
Table 2. Biomass of four different age classes of C. equisetifolia plantations (Mg ha−1, mean ± S.E.).
| Stand age(yrs) | Stem | Branch | Branchlet | AGB | Root (BGB) | PB |
| 3 | 8.80c±2.06 | 2.59c±0.52 | 2.83b±0.37 | 14.22c±2.96 | 7.77c±0.43 | 21.99c±3.33 |
| 6 | 46.05b±2.79 | 10.65b±0.61 | 7.01a±0.35 | 63.71b±3.74 | 13.1b±0.60 | 76.9b±4.32 |
| 13 | 96.0a±6.70 | 18.3a±1.35 | 8.12a±0.69 | 122.5a±8.69 | 19.6a±1.44 | 142.1a±10.1 |
| 18 | 104.6a±21.9 | 18.5a±3.31 | 7.20a±0.91 | 130.3a±26.1 | 21.1a±4.32 | 151.5a±30.4 |
Note: AGB refers to aboveground biomass, PB refers to total plantation biomass. Means in a column followed by different lower-case letters are significantly different at P<0.05 (one-way ANOVA and LSD test).
There was significant difference in the C concentrations among different parts of C. equisetifolia tree (P<0.05, data not shown). Across all age groups, stems had the highest C concentration with mean value of 45.69%. In contrast, fine roots had the lowest C concentration with a mean of 35.32%. The mean C concentration of branches and branchlets was 45.28% and 42.08%, respectively.
Soil C, N Concentration and Fine Root Distribution
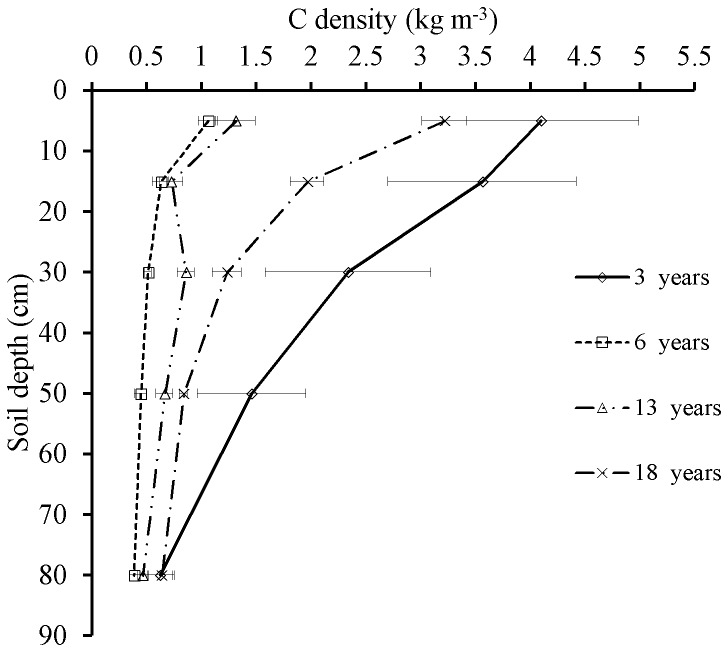
In the 0–10 cm soil, mean SOC concentrations ranged from 0.71 g kg−1 in the 6-yrs plots to 2.67 g kg−1 in the 3-yrs plots (Table 3). In all age plantations, SOC decreased with increasing soil depth (Fig. 1). There was a significant difference in the SOC density among the four age classes C. equisetifolia plantations respectively (P<0.05, Fig. 1), with the highest values in 3-yrs plots and lowest ones in 6-yrs plots in all soil layers. Soil total N (TN) was also low in these plantations. In the 0–10 cm soil, TN ranged from just 0.09 g kg−1 in the 6-yrs plots to 0.39 g kg−1 in the 3-yrs plots. Soil TN followed a similar decline with increasing soil depth like the SOC (Table 3). Like SOC, TN was highest in the 3-yrs plots, declined to the lowest concentration in the 6-yrs plots, and then gradually recovered in the 18-yrs plots. C/N ratios of the 0–10 cm soils ranged from 6.94 to 11.33, and the ratios typically decreased with increasing soil depth (Table 3).
Table 3. Properties of the soil in four different age classes of C. equisetifolia plantations (only 0–10 and 10–20 cm soil data were presented here, mean ± S.E.).
| Variables | Stand age | ||||
| Soildepth | 3 | 6 | 13 | 18 | |
| BulkDensity(g cm−3) | 0–10 cm | 1.54±0.02 | 1.50±0.01 | 1.49±0.03 | 1.53±0.02 |
| 10–20 cm | 1.50±0.02 | 1.50±0.01 | 1.47±0.01 | 1.48±0.02 | |
| SOC(g kg−1) | 0–10 cm | 2.67a±0.59 | 0.71b±0.06 | 0.87b±0.11 | 2.10a±0.14 |
| 10–20 cm | 2.35a±0.55 | 0.43c±0.05 | 0.49c±0.07 | 1.31b±0.11 | |
| TN (g kg−1) | 0–10 cm | 0.39a±0.02 | 0.09c±0.01 | 0.13c±0.02 | 0.19b±0.02 |
| 10–20 cm | 0.32a±0.04 | 0.09c±0.01 | 0.09b±0.02 | 0.15b±0.02 | |
| C/N | 0–10 cm | 6.94b±1.66 | 7.62b±0.68 | 6.68b±0.27 | 11.33a±1.38 |
| 10–20 cm | 7.12ab±1.39 | 4.84c±0.59 | 5.85bc±0.62 | 9.44a±1.37 | |
Note: Means in a row followed by different lower-case letters are significantly different at P<0.05 (one-way ANOVA and LSD test).
Figure 1. The soil carbon density of four different age classes of C. equisetifolia plantations.
Note: Error bar indicating SE.
Mean fine root biomass at 1 m soil depth averaged 2.62, 4.48, 2.69 and 2.82 Mg ha−1 in the 3, 6, 13 and 18 yrs old C. equisetifolia plantations, respectively (Table 4). Six yrs plantation had the highest fine root biomass among the four age classes plantations (P<0.05). The fine root biomass decreased with an increase in soil depth, with a large proportion located in the top 40 cm soil (70.1%, 46.7%, 62.8%, and 78.9% of the total in the 3-yrs, 6-yrs, 13-yrs, and 18-yrs old plantations, respectively). Fine roots were sparse in the 3-yrs old C. equisetifolia plantation, especially in the deep soil; then it increased in the 6-yrs old plantation and declined again in the 13- and 18-yrs plantation (Table 4), despite the increase of total plant biomass over time.
Table 4. The depth distribution of fine root biomass at four age classes of C. equisetifolia plantations (Mg ha−1, mean ± S.E.).
| Stand age (yrs) | 0–10 cm | 10–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | 80–100 cm | Total |
| 3 | 0.68±0.09 | 0.54±0.06 | 0.62±0.09 | 0.34±0.12 | 0.29b±0.13 | 0.16b±0.07 | 2.62b±0.37 |
| 6 | 0.59±0.04 | 0.64±0.11 | 0.86±0.27 | 0.80±0.09 | 0.65a±0.13 | 0.94a±0.22 | 4.48a±0.40 |
| 13 | 0.59±0.20 | 0.40±0.10 | 0.71±0.16 | 0.41±0.06 | 0.23b±0.08 | 0.36b±0.12 | 2.69b±0.55 |
| 18 | 1.03±0.25 | 0.55±0.06 | 0.64±0.23 | 0.38±0.19 | 0.09b±0.04 | 0.13b±0.06 | 2.82b±0.67 |
Note: Means in a column followed by different lower-case letters are significantly different at P<0.05(LSD test).
Ecosystem C Sequestration
Most plant C was accumulated in the aboveground biomass, especially in stems. Carbon storage in the aboveground plant biomass averaged 6.3, 28.8, 55.9, and 59.7 Mg C ha−1 in the 3, 6, 13, and 18 yrs old plantations respectively (Table 5). Root biomass C increased from 2.5 Mg C ha−1 in the 3-yrs plots to 8.3 Mg C ha−1 in the 18-yrs plots, representing 28.2%, 13.7%, 11.0%, and 12.2% of the total plant biomass C in the four age classes of plantations. The C accumulated in the C. equisetifolia plant biomass increased markedly with stand age. The annual rate of C accumulation in the C. equisetifolia plant biomass during 0–3, 3–6, 6–13 and 13–18 yrs stage was 2.9, 8.2, 4.2 and 1.0 Mg C ha−1 yr−1, respectively (Table 6).
Table 5. Carbon storage in biomass and soils in four C. equisetifolia plantations (Mg ha−1, mean ± S.E.).
| Stand age(yrs) | Stem | Branch | Leaf | AGCS | Root | TBCS | SOC | TCS |
| 3 | 3.94c±0.92 | 1.18c±0.24 | 1.22b±0.16 | 6.34c±1.32 | 2.49c±0.14 | 8.83c±1.44 | 17.74a±3.21 | 26.57c±2.80 |
| 6 | 21.04b±0.00 | 4.82b±0.27 | 2.93a±0.14 | 28.78b±0.42 | 4.58bc±0.21 | 33.37b±0.63 | 5.14c±0.24 | 38.51b±2.11 |
| 13 | 44.19a±3.09 | 8.34a±0.61 | 3.41a±0.29 | 55.94a±3.97 | 6.91ab±0.51 | 62.85a±4.48 | 6.93c±0.24 | 69.78a±4.68 |
| 18 | 48.35a±10.1 | 8.31a±1.49 | 2.99a±0.38 | 59.65a±12.0 | 8.26a±1.69 | 67.91a±13.6 | 11.87b±0.76 | 79.78a±13.91 |
Note: SOC, TBCS, AGCS and TCS refer to soil organic carbon (0–100 cm), total biomass C storage, aboveground C storage and total C storage, respectively. Means in a column followed by different lower-case letters are significantly different at P<0.05(one-way ANOVA and LSD test).
Table 6. Average annual rate of biomass carbon accumulation at four age classes of C. equisetifolia plantations (Mg C ha−1 yr−1).
| Stand stage | AGC | BGC | TBC |
| 0–3 yrs | 2.1 | 0.8 | 2.9 |
| 3–6 yrs | 7.5 | 0.7 | 8.2 |
| 6–13 yrs | 3.9 | 0.3 | 4.2 |
| 13–18 yrs | 0.7 | 0.3 | 1.0 |
Note: AGB refers to aboveground biomass C; BGC refers to belowground biomass C; TBC refers to total biomass C.
Soil organic C storage in the top 100 cm soil was 17.74, 5.14, 6.93, 11.87 Mg ha−1 at the 3, 6, 13 and 18 yrs plantation, respectively (Table 5). The highest soil carbon storage existed at the 3 yrs plantation and the lowest at the 6 yrs plantation, with SOC storage increased markedly from 6-yrs plots to 13-yrs and 18-yrs plots (Table 5).
Total C storage in the plantation ecosystem averaged 26.57, 38.50, 69.78, and 79.79 Mg C ha−1 in the 3, 6, 13 and 18- yrs plantation respectively, with most of the C accumulated during the 18 yr growth stored in the aboveground biomass rather than in the belowground root biomass and soil organic C (Table 5).
Discussion
Biomass C Accumulation in Casuarina Equisetifolia Plantations
This study supports our hypothesis that C. equisetifolia biomass and C storage increased quickly with plantation age in both aboveground and belowground parts. A number of studies have reported similar trends of forest growth [20], [21]. Moreover, the accumulation rate was fast in the early stages of plantation and then slowed down as the plantation aged. The highest accumulation rate was observed in the 3–6 yrs old stage (Table 6). The biomass of the fine root also reached maximum values in this stage (Table 4). These results were consistent with other studies, which found the largest fine root biomass in the fast-growth stage stand [22], [23].
The C. equisetifolia plantation also accumulated more biomass than many other secondary tropical forests. Harmand et al. [24] reported that total biomass in the 7 yrs old Eucalyptus camaldulensis and 6 yrs old Senna siamea was 62.35 and 45.39 Mg ha−1, respectively. Similarly, aboveground biomass in the 7 yrs old secondary forests in the Uxpanapa Region of Veracruz, Mexico was 52.7 Mg ha−1 [25]. Miao et al. [26] examined biomass of different mangrove forests in South China and found that total forest biomass in the 5 yrs old Aegiceras corniculatum, Avicennia marina, and Kandelia candel forests was 5.5, 16.4, and 62.6 Mg ha−1, respectively. These values were smaller than the 63.71 Mg ha−1 (6 yrs old plantation) found in our study. Our data thus suggest that the C. equisetifolia plantations can accumulate large amount of biomass in both of aboveground and belowground parts, despite growing in the sandy coastal soils with low nutrient and low organic matter.
Most tropical forests accumulate large amounts of biomass in their roots [24], [26]. In this study, however, we found average BGB (Belowground biomass)/AGB (Aboveground biomass) ratio was just 0.27 in the four C. equisetifolia plantations. The ratios reported here was much lower than those reported in other tropical forests. Harmand et al. [24] reported that the BGB/AGB ratio was 0.76, 0.90, and 0.44 in the 5 yrs old Acacia polyacantha, S. siamea and E. camaldulensis forests, respectively. However, the average BGB/AGB ratio was 0.29 in the humid tropical forests in Costa Rica [27], a value much closer to ours. In the mangrove forests, Miao et al. [26] reported that BGB/AGB ratio for the three 5 yrs old mangrove forests was 0.6, 0.7, and 0.8, respectively.
Soil Organic C and TN in Casuarina Equisetifolia Plantations
Our study did not support the hypothesis that C storage in the soil increased with plantation age. Davis et al. [28] reported an increase of SOC from 29.8 Mg ha−1 to 42.0 Mg ha−1 in the mineral soil (0–10 cm) along a stand development sequence in a New Zealand Nothofagus forest. Sartori et al. [29] conducted a chronosequence study of poplar plantations in USA. They found that C concentration in the upper 5 cm of the mineral soil increased with plantation age but decreased with age at 5–15 cm and 15–25 cm depths. Similar results had been reported in other studies [30], [31]. In our study, SOC decreased in the early stage after the reforestation and then gradually increased with the stand age. Paul et al. [32] showed the similar result in an afforestation site in Australia. They found that surface soil (<10 or <30 cm depth) C generally decreased during the first 5 years but then increased, and recovered after about 30 yrs afforestation. It is well known in ecological literature that during early stages of forest development, fast litter decomposition and SOC mineralization could lead to SOC decrease (before its eventual increase), a phenomena termed “Covington curve” [33], [34]. Such initial decrease of SOC followed by SOC increase have been shown in many other studies [35]–[38].
Land use history had a significant effect on changes in soil C, so had the soil disturbance during the site preparation for forest growth [20], [39], [40]. In the first several years of reforestation, there is relatively little input of C from aboveground [41]. However, C from residues of the preceding plantation continues to decompose during this time. In addition to the land use history, the methods of cultivation and management also have significant effects on the C content [32]. During many plantation site preparations in China, soils were often heavy disturbed, sometimes including root excavation, which can greatly accelerate SOC loss in the early years of reforestation.
The litterfalls of C. equisetifolia were collected regularly by the local residents as biofuel materials. As an important aboveground C input into soils, the harvesting of litter usually results in additional losses of C from the soil. Many studies have suggested that retaining residues on site could reduce soil C loss [42], and the removing or burning of litters and residues always lead to a large loss of C. Thus, our study suggests that given appropriate management practices (i.e., retaining litterfall), the C. equisetifolia plantations would have a higher potential to sequester more C.
Nitrogen is one of the most common limiting elements in terrestrial ecosystems that limit the primary production and other ecological processes [43], [44]. Soil N would vary considerably during soil development as N accumulates through N-fixation and deposition [45]. In our study, the concentration of soil TN ranged from 0.09 to 0.39 g kg−1 in the 0–10 soil layer, which was much lower than the data reported in nearby soils. For example, Mo et al. [46] reported that total N of top soil (0–20 cm) in the disturbed, rehabilitated and mature forests in tropical China was 0.9, 1.0, and 1.9 g kg−1, respectively. The specific soil type may partially explain the low N status in this study. More than 92% of sand fraction of the soil might enhance the soil N loss through leaching during N mineralization processes. In addition, the collection of litterfall by the local residents might be another reason for the low concentration in soil N, because litterfall is a major pathway of nutrient return to the soil [47].
C Accrual in Casuarina Equisetifolia Plantations
Our results suggested that organic C can accumulate rapidly in the C. equisetifolia plantations in South China. Furthermore, most C accrual was due to high biomass accumulation. The estimation of annual biomass C accumulation rate of C. equisetifolia plantation reached 8.2 Mg/ha in 3–6 yrs stage (Table 6). This value was higher than the data in 3 yrs old A. crassicarpa plantation (6.5 Mg/ha) in tropical China [48]. Our estimation was also consistent with previous study [49]. Yang and Guan [49] have reported annual biomass C accumulation rate in various forests of Pearl River Delta, and found that C. equisetifolia plantation (7.25 Mg/ha) had the fastest rate among different forest types (ie: Pinus elliottii plantation: 4.8 Mg/ha, Acacia plantation: 4.7 Mg/ha, broadleaf forests: 6.5 Mg/ha).
In consistent with our hypotheses, the contribution of SOC to total ecosystem C storage decreases with the stand age. SOC was 17.74, 5.14, 6.93, and 11.87 Mg C ha−1 at the 3, 6, 13 and 18 yrs old plantation, respectively, representing 66.8%, 13.3%, 9.9% and 14.9% of the total ecosystem carbon. The percentage was smaller than many other studies. Fonseca et al. [27] reported that the amount of carbon stored in the soil represented 74.3% of the total carbon in forest, 51.5% higher than the biomass C in the humid tropics of Costa Rica. The rapid accrual of ecosystem C in the C. equisetifolia plantations has benefited from the coastal protection policy of “zero tolerance” on tree cutting and harvesting. Conversely, the low soil nutrient (TN) and SOC content in the coastal sandy soil may limit soil microbial mediated nutrient turnover, subsequently, may limit the C accumulation potential of this ecosystem. The C accumulation potential is further constrained by the routine litter removal by local residents. The litter removal not only reduced direct SOC flow belowground, but also diminished essential soil nutrients (N, P) for plant growth and further C accumulation in the system.
Since there is a large planting area reaching 300,000 ha for this species in the coastal area of China [12], C. equisetifolia plantations might have played great roel in sequestering C. To fully realize such potential, we need to understand the interactive effects of soil nutrient, natural disturbance, and human decisions on the ecosystem health of the C. equisetifolia plantations. The large-scale Casuarina plantations as shelterbelt forest along the coastline in South China has important roles beyond C sequestration, including restoring degraded coastal land and protecting coastal community, thus the social and ecological factors that affecting such critical ecosystem services need to be further investigated.
Conclusions
Casuarina equisetifolia plantations (3–18 years old) in this study could rapidly accumulate large quantities of biomass. The total plant biomass of C. equisetifolia plantation at 3, 6, 13, and 18 yrs old plantations was 22.0, 76.8, 142.1, and 151.5 Mg ha−1, respectively, greater than many other tropical forests of similar ages. The SOC content was 17.74, 5.14, 6.93, and 11.87 Mg ha−1, representing 66.8%, 13.3%, 9.9% and 14.9% respectively of the total ecosystem carbon pool. Our study suggests that these plantations have a greater potential to sequestrate C despite poor soil conditions. The expansion of C. equisetifolia plantations in the South China can play important roles in the regional C budget and coastal protection. Long-term monitoring and research are needed to further explore the ecological and social-economic factors that affect the C sequestration and ecosystem health of these shelterbelt forests.
Supporting Information
Soil physical properties in the four age classes C. equisetifolia plantations.
(DOCX)
The relative growth equations for C. equisetifolia plantations.
(DOCX)
Acknowledgments
We thank Prof. Deborah Neher (University of Vermont, USA), the editor and two anonymous reviewers for their comments on an early version of this paper.
Funding Statement
This work was funded by National Basic Research Program of China (2011CB403200), NSFC-Guangdong Joint Project (U1131001), Natural Science Foundation of China (31300419), the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-J-28), the “Strategic Priority Research Program - Climate Change: Carbon Budget and Related Issues” of the Chinese Academy of Sciences (XDA05070307) and the key projects in the National Science and Technology Pillar program (2009BAD2B0604). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.FAO (2001) Global forest resources assessment 2000. Rome. 479.
- 2. Schlamadinger B, Marland G (1996) The role of forest and bioenergy strategies in the global carbon cycle. Biomass & Bioenergy 10: 275–300. [Google Scholar]
- 3. Dmitry R, Pablo C, Benítezb., Florian K, Ian M, et al. (2007) Geographically explicit global modeling of land-use change, carbon sequestration, and biomass supply. Technological Forecasting and Social Change 74: 1057–1082. [Google Scholar]
- 4.State Forestry Administration of PR. China (SFA) (2009) China forestry statistical year book 2008. Beijing: China Forestry Publisher.
- 5. Hardman CJ, Williams S, Manco BN, Hamilton MA (2012) Predicting the potential threat of Casuarina equisetifolia to three endemic plant species on the Turks and Caicos Islands. Oryx 46: 204–212. [Google Scholar]
- 6. Zoysa MD (2008) Casuarina Coastal Forest Shelterbelts in Hambantota City, Sri Lanka: Assessment of Impacts. Small-scale Forestry 7: 17–27. [Google Scholar]
- 7. Mascarenhas A, Jayakumar S (2008) An environmental perspective of the post-tsunami scenario along the coast of Tamil Nadu, India: Role of sand dunes and forests. Journal of Environmental Management 89: 24–34. [DOI] [PubMed] [Google Scholar]
- 8. Srivastava AK (1995) Biomass and energy production in Casuarina equisetifolia plantation stands in the degraded dry tropics of the Vindhyan plateau, India. Biomass & Bioenergy 9: 465–471. [Google Scholar]
- 9. Resh SC, Binkley D, Parrotta JA (2002) Greater soil carbon sequestration under nitrogen-fixing trees compared with Eucalyptus species. Ecosystems 5: 217–231. [Google Scholar]
- 10. Zhong CL, Zhang Y, Chen Y, Jiang QB, Chen Z, et al. (2010) Casuarina research and applications in China. Symbiosis 50: 107–114. [Google Scholar]
- 11.El-Lankany M, Tumbull JW, Brewbake J (1990) Advances in Casuarina research and utilizatio. CSIRO. Cairo.
- 12. Zhong C, Zhang Y (2003) Introduction, cultivation and management of Casuarina equisetifolia in China. China Forestry Science and Technology 17: 3–5. [Google Scholar]
- 13. Parrotta JA, Baker DD, Fried M (1996) Changes in dinitrogen fixation in maturing stands of Casuarina equisetifolia and Leucaena leucocephala. Canadian Journal of Forest Research 26: 1684–1691. [Google Scholar]
- 14. Gauthier D, Diem HG, Dommergues YR, Ganry F (1985) Assessment of N2 Fixation by Casuarina equisetifolia inoculated with Frankia Ors021001 using N15 methods. Soil Biology & Biochemistry 17: 375–379. [Google Scholar]
- 15. Muthukumar T, Udaiyan K (2010) Growth response and nutrient utilization of Casuarina equisetifolia seedlings inoculated with bioinoculants under tropical nursery conditions. New Forests 40: 101–118. [Google Scholar]
- 16.Clements FE (1916) Plant succession: an analysis of the development of vegetation. Washington: Carnegie Insitution of Washington.
- 17. Goulden ML, McMillan AMS, Winston GC, Rocha AV, Manies KL, et al. (2011) Patterns of NPP, GPP, respiration, and NEP during boreal forest succession. Global Change Biology 17: 855–871. [Google Scholar]
- 18. Hong Y, Xu W, Ye G, Zhang L (2010) Model for Estimating Biomass of Casuarina equisetifolia Plantation in Coastal Region of the Southeastern China. Journal of Zhejiang Forestry Science and Technology 30: 66–69. [Google Scholar]
- 19.Liu G, Jiang N, Zhang L, Liu Z (1996) Standard methods for observation and analysis in Chinese Ecosystem Research Network. Beijing: Chinese Standard Press.
- 20. Hughes RF, Kauffman JB, Jaramillo VJ (1999) Biomass, carbon, and nutrient dynamics of secondary forests in a humid tropical region of Mexico. Ecology 80: 1892–1907. [Google Scholar]
- 21. Usuga JCL, Toro JAR, Alzate MVR, Tapias ADL (2010) Estimation of biomass and carbon stocks in plants, soil and forest floor in different tropical forests. Forest Ecology and Management 260: 1906–1913. [Google Scholar]
- 22. Borja I, De Wit HA, Steffenrem A, Majdi H (2008) Stand age and fine root biomass, distribution and morphology in a Norway spruce chronosequence in southeast Norway. Tree Physiology 28: 773–784. [DOI] [PubMed] [Google Scholar]
- 23. Makkonen K, Helmisaari HS (2001) Fine root biomass and production in Scots pine stands in relation to stand age. Tree Physiology 21: 193–198. [DOI] [PubMed] [Google Scholar]
- 24. Harmand JM, Njiti CF, Bernhard-Reversat F, Puig H (2004) Aboveground and belowground biomass, productivity and nutrient accumulation in tree improved fallows in the dry tropics of Cameroon. Forest Ecology and Management 188: 249–265. [Google Scholar]
- 25. Williams-Linera G (1983) Biomass and nutrient content in two successional stages of tropical wet forest in Uxpanapa, Mexico. Biotropica 15: 275–284. [Google Scholar]
- 26. Miao S, Chen G, Chen Z (1998) Biomasses and distribution patterns of mangrove populations in Zhanjiang Nature Reserve, Guangdong, China. Guihaia 18: 16–19. [Google Scholar]
- 27. Fonseca W, Benayas JMR, Alice FE (2011) Carbon accumulation in the biomass and soil of different aged secondary forests in the humid tropics of Costa Rica. Forest Ecology and Management 262: 1400–1408. [Google Scholar]
- 28. Davis MR, Allen RB, Clinton PW (2003) Carbon storage along a stand development sequence in a New Zealand Nothofagus forest. Forest Ecology and Management 177: 313–321. [Google Scholar]
- 29. Sartori F, Lal R, Ebinger MH, Eaton JA (2007) Changes in soil carbon and nutrient pools along a chronosequence of poplar plantations in the Columbia Plateau, Oregon, USA. Agriculture Ecosystems & Environment 122: 325–339. [Google Scholar]
- 30. Cote L, Brown S, Pare D, Fyles J, Bauhus J (2000) Dynamics of carbon acid nitrogen mineralization in relation to stand type, stand age and soil texture in the boreal mixedwood. Soil Biology & Biochemistry 32: 1079–1090. [Google Scholar]
- 31. Johnston MH, Homann PS, Engstrom JK, Grigal DF (1996) Changes in ecosystem carbon storage over 40 years on an old-field forest landscape in east-central Minnesota. Forest Ecology and Management 83: 17–26. [Google Scholar]
- 32. Paul KI, Polglase PJ, Nyakuengama JG, Khanna PK (2002) Change in soil carbon following afforestation. Forest Ecology and Management 168: 241–257. [Google Scholar]
- 33. Covington WW (1981) Changes in forest floor organic-matter and nutrient content following clear cutting in Northern Hardwoods. Ecology 62: 41–48. [Google Scholar]
- 34. Yang YH, Luo YQ, Finzi AC (2011) Carbon and nitrogen dynamics during forest stand development: a global synthesis. New Phytologist 190: 977–989. [DOI] [PubMed] [Google Scholar]
- 35.Harrison FA, Howard A, Howard PJ, et al.. (1995) Carbon storage in forest soils. In: Greenhouse Gas Balance in Forestry. Forestry Research Coordination Committee Conference. 335–348.
- 36. Richter DD, Markewitz D, Trumbore SE, Wells CG (1999) Rapid accumulation and turnover of soil carbon in a re-establishing forest. Nature 400: 56–58. [Google Scholar]
- 37. Trouve C, Mariotti A, Schwartz D, Guillet B (1994) Soil organic-carbon dynamics under eucalyptus and pinus planted on savannas in the Congo. Soil Biology & Biochemistry 26: 287–295. [Google Scholar]
- 38. Zak DR, Grigal DF, Gleeson S, Tilman D (1990) Carbon and nitrogen cycling during old-field succession - constraints on plant and microbial biomass. Biogeochemistry 11: 111–129. [Google Scholar]
- 39. Guo LB, Gifford RM (2002) Soil carbon stocks and land use change: a meta analysis. Global Change Biology 8: 345–360. [Google Scholar]
- 40. Schedlbauer JL, Kavanagh KL (2008) Soil carbon dynamics in a chronosequence of secondary forests in northeastern Costa Rica. Forest Ecology and Management 255: 1326–1335. [Google Scholar]
- 41. Wilde SA (1964) Changes in Soil Productivity Induced By Pine Plantations. Soil Science 97: 276–278. [Google Scholar]
- 42. Berthrong ST, Jobbagy EG, Jackson RB (2009) A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation. Ecological Applications 19: 2228–2241. [DOI] [PubMed] [Google Scholar]
- 43. Liu L, Gundersen P, Zhang T, Mo JM (2012) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biology & Biochemistry 44: 31–38. [Google Scholar]
- 44. Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, et al. (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10: 1135–1142. [DOI] [PubMed] [Google Scholar]
- 45. Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, et al. (1995) Changes in soil-phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76: 1407–1424. [Google Scholar]
- 46. Mo JM, Brown S, Peng SL, Kong G (2003) Nitrogen availability in disturbed, rehabilitated and mature forests of tropical China. Forest Ecology and Management 175: 573–583. [Google Scholar]
- 47. Campo J, Maass M, Jaramillo VJ, Martinez-Yrizar A, Sarukhan J (2001) Phosphorus cycling in a Mexican tropical dry forest ecosystem. Biogeochemistry 53: 161–179. [Google Scholar]
- 48. Chen D, Zhang C, Wu J, Zhou L, Lin Y, et al. (2011) Subtropical plantations are large carbon sinks: Evidence from two monoculture plantations in South China. Agricultural and Forest Meteorology 151: 1214–1225. [Google Scholar]
- 49. Kun Yang, Dongsheng Guan (2006) Biomass and its distribution of forest in the Pearl River Delta. Journal of Ecology and Environment 15: 84–88. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Soil physical properties in the four age classes C. equisetifolia plantations.
(DOCX)
The relative growth equations for C. equisetifolia plantations.
(DOCX)