Abstract
Objective(S)
To determine the HIV prevalence and extent of engagement with HIV prevention and care among a representative sample of Zimbabwean sex workers working in Victoria Falls, Hwange and Mutare.
Design
Respondent driven sampling (RDS) surveys conducted at each site.
Methods
Sex workers were recruited using respondent driven sampling with each respondent limited to recruiting 2 peers. Participants completed an interviewer-administered questionnaire and provided a finger prick blood sample for HIV antibody testing. Statistical analysis took account of sampling method.
Results
870 women were recruited from the three sites. HIV prevalence was between 50 and 70%. Around half of those confirmed HIV positive were aware of their HIV status and of those 50-70% reported being enrolled in HIV care programmes. Overall only 25-35% of those with laboratory-confirmed HIV were accessing antiretroviral therapy. Among those reporting they were HIV negative, 21-28% reported having an HIV test in the last 6 months. Of those tested HIV negative, most (65-82%) were unaware of their status. Around two-thirds of sex workers reported consistent condom use with their clients. As in other settings, sex workers reported high rates of gender based violence and police harassment.
Conclusions
This survey suggests that prevalence of HIV is high among sex workers in Zimbabwe and that their engagement with prevention, treatment and care is sub-optimal. Intensifying prevention and care interventions for sex workers has the potential to markedly reduce HIV and social risks for sex workers, their clients and the general population in Zimbabwe and elsewhere in the region.
Introduction
Female sex workers (FSWs) are a marginalised group experiencing poor health, high levels of partner abuse and other forms of violence. In some settings their HIV prevalence is very high, often 4-20 times that in the general population[1]. In sub-Saharan Africa, sex work takes many forms ranging from brothel-based activities to more informal solicitation in bars and through mobile phone and other contact networks. Despite acknowledgement of their enhanced risk, FSWs continue to be underserved by HIV programs in Africa[1]. Delivering services is complicated by the fact that sex work is illegal in countries throughout the region including in Zimbabwe[2-4]. Stigma, marginalization, and abuse of human rights have been highlighted as key determinants of reduced access to health care among FSWs in southern Africa[5].
FSWs were recognised in Zimbabwe's National HIV/AIDS Strategic Plan 2006-2010[6] as an important group to reach with HIV prevention and treatment services but currently there are few data to accurately estimate HIV infection rates among this group and the extent to which they are engaged with HIV prevention and care.
In 2009, Zimbabwe's National AIDS Council (NAC) initiated a programme for FSWs[7] which provides HIV testing, syndromic management for sexually transmitted infections (STIs), contraception, primary care, health education and access to legal advice all supported by a network of peer educators. In 2011, three sites were selected for intensified programming in the form of community mobilisation of FSWs, and additional training for health care workers to reduce stigma and discrimination in health care settings. Prior to intensification of services, a survey was conducted among FSWs at these three sites to estimate their HIV prevalence and describe their engagement with HIV prevention, treatment and care services. We discuss our findings in light of potential areas to strengthen a proposed ‘treatment as prevention’ intervention for FSWs in Zimbabwe.
Methods
Study Population and Setting
The study was conducted in three sites in Zimbabwe; Mutare, a small city bordering Mozambique, Victoria Falls, a tourist town bordering Zambia, and Hwange, the site of a large colliery. Women were eligible for inclusion if they were aged >18 years, worked at the study site and reported that they had exchanged sex for money in the past 30 days. The surveys were conducted around 8 months after the introduction of NAC's HIV and STI prevention programme for FSWs.
Sampling
Rapid ethnographic mapping to determine the spatial and social organisation of sex work was conducted at each site to identify key geographic areas where sex work was being conducted, the main typologies of sex work, and inform preliminary estimates of the size of the FSW population.
A bio-behavioural survey was conducted at each site. Respondents were recruited to the survey through respondent-driven sampling (RDS). This variant of chain-sampling can be used to recruit research participants from “hidden” populations. In contrast with other chain sampling approaches, RDS rations the number of recruits per respondent, tends to deploy a larger number of waves of recruitment, and provides financial incentives to recruiters[8]. The aim is to recruit a representative sample of research participants of the hidden population of which the respondents are members. There is ongoing debate in the literature about the extent to which RDS achieves this aim and the conditions under which this may be more or less likely[9-11]. Nevertheless, RDS offers a systematic and theoretically grounded approach to recruiting hidden populations.
The initial recruiters (seeds) were FSWs with close links with other FSWs. The seeds were purposively selected so as to represent a range of ages, geographic areas and sex-work typologies identified during preliminary mapping. Ten seeds were recruited from Mutare and six each from Hwange and Victoria Falls.
Each seed respondent completed a questionnaire, had a finger prick blood sample collected for HIV antibody testing, and was issued with two uniquely identified coupons. The seeds passed these coupons on to individuals meeting the study inclusion criteria, and on presenting a coupon to study staff these individuals were asked to give consent and be recruited to the study. These recruits were themselves given two coupons to refer further peers, and so on. Each respondent was provided with US$5 to cover costs of participation. A further US$2 was provided to the recruiter for each successfully recruited referral. Six waves of coupons were issued. In Mutare, up to 630 individuals could be recruited if all coupons resulted in referral and 378 each in Hwange and Victoria Falls. These “total possible” numbers were roughly double initial estimates of the size of the sex work population at each site. We chose to limit each respondent to two referral coupons to increase the number of waves since we anticipated that estimates would stabilise after 4-5 of our potential 6 waves of recruitment.
Voluntary counselling and testing was made freely available for those participants who wanted to know their HIV results.
Data collection
The questionnaire was developed in English and translated into Shona and Ndebele, the local languages. It was piloted before being implemented to check that all wording was understood and unambiguous. Responses were compiled directly into a computer-assisted survey instrument (QDS™ Nova research Company) by trained female interviewers. The questionnaire data were collected anonymously and included information on socio-demographic and economic characteristics, sexual behaviour, psychological health, physical health, past history of STIs, sexual and social networks, social capital, utilisation of services including HIV testing, provision of antiretroviral therapy (ART), prevention of mother to child transmission (PMTCT) and contraception. Where appropriate we used questions that had been previously used or validated in Zimbabwe or elsewhere[12-15]. The questionnaire took an average of 40 minutes to complete.
Laboratory procedures
Finger prick blood samples were collected[16] by nurse counselors and transported to the National Microbiology Reference Laboratory in Harare. They were tested for HIV-1 antibody in series using AniLabsytems EIA kit (AniLabsystems Ltd, FIN-01720, Vantaa, Finland) with those specimens testing positive also tested using Vironostika® HIV Microelisa System bioMerieux, Inc., Durham NC 27704. Discrepant results were resolved using western blot.
Key variables
We identified a set of primary outcome variables: laboratory-confirmed HIV status, reported HIV testing within the past 6 months among those HIV negative, and whether currently taking ART among those HIV positive. We explored discrepancies in self-reported and laboratory-confirmed HIV status.
We also investigated consistent (“always”) condom use with commercial clients and with steady/permanent non-commercial partners. The question used to assess condom use was “In the past month, how often did you use condoms with your steady partner?” Participant responses were grouped as “Always” and “Not always”.
The variable used to estimate network degree was "how many of those sex workers whom you know personally would you consider recruiting into this study?”
Statistical analysis
We first used unadjusted statistics to describe the population. All of our analyses were stratified by site. As recommended for RDS, we excluded seeds from the analysis.
Our analysis sought to account for the RDS design of the study, while recognising that there remains debate about which methods best achieve this. It is theorised that successive waves of recruits should become more representative of the source population. Individuals may recruit individuals more like themselves than would be expected on average. To explore these issues we assessed whether the prevalence of key outcome measures stabilised over waves of recruitment and whether socio-demographic characteristics of recruiters were associated with characteristics of their recruits.
To estimate outcome prevalences we first used the Stata RDS package [17] The multiplicity estimate of average network degree was used to correct for over-representation of those with large degree in the sample. Bootstrapping with 1000 repetitions was used to obtain percentages and standard errors except for subgroup analyses, for which Taylor linearization was used. Next, we adapted a method proposed by Szwarcwald to estimate predicted prevalences of the key outcome variables in the source population[18]. We used random effects logistic regression for this purpose. We fitted models including weighting for the inverse of participant degree (using the same question as for RDS analysis). We also specified a random effect term for “seed” such that the variance of responses from individuals who were part of the same recruitment chain was modeled to take some account of potential clustering.
Institutional Review Board approval
All participants gave written consent collected according to the principles of Good Clinical Practice. Institutional Review Board approval (IRB) for the study was given by the Medical Research Council of Zimbabwe (MRCZ) The UCL Ethics Committee. The consent form was approved by all IRBs in English and the translations to Shona and Nedebele were approved by MRCZ.
Results
Recruitment
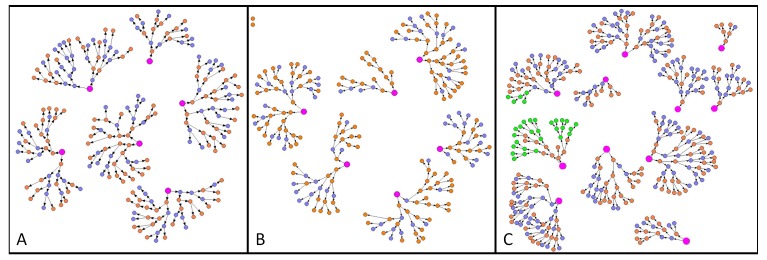
Over six rounds of recruitment 370 FSWs were recruited in Mutare, 237 in Hwange and 229 in Victoria Falls. All 22 seeds recruited women into the study. The recruitment chains are depicted in Figure 1. One batch of dried blood samples from 32 individuals was misplaced by the laboratory and so were not tested for HIV, these individuals were associated with two recruitment chains in Mutare (also shown).
Figure 1. Chains of recruitment in the RDS sites for Hwange (A), Victoria Falls (B) and Mutare (C).
Each circle represents a participant in the survey and the lines represent the passing of recruitment coupons. Orange circles represent women who tested HIV positive and blue circles HIV negative. The green circles in Mutare represent women for whom the HIV status is missing. Finally, the pink circles represent the seeds.
About 90% of seeds in Mutare and Hwange and all the Victoria Falls seeds were HIV positive (Figure 2). HIV prevalence was higher in the first wave of recruitment before falling to a roughly stable prevalence in subsequent waves. Seeds were more likely than recruits in all waves to report consistent use of condoms in all three sites, but there was no discernible pattern in consistent condom use by wave after the first wave of recruits. There was no consistent trend in HIV testing in the previous 6 months by wave in the three sites. Figure 2 suggests that the number of waves required to reach equilibrium was less than the number of waves in the study for all of the outcomes. Later waves also include more women. There was little evidence for strong association between socio-demographic characteristics of recruiters and outcomes in their recruits. The associations for which there was some evidence of autocorrelation were different across sites. There were no associations in any site that showed strong evidence for autocorrelation between recruiters and recruitees (p values of less than p=0.01).
Figure 2. RDS validation: Outcome measure estimates over waves of recruitment.
Characteristics of sampled population
In the three sites FSWs were on average 27-30 years old, and in Mutare and Hwange had generally been long term residents (Table 1). The majority in all sites were widowed, or divorced from a partner (68.3%-75.0%). FSWs in Hwange and Mutare had longer duration in sex work than those in Victoria Falls. Most solicited clients in bars, with significant numbers also soliciting by phone or at home or on the street. In all sites, 17 years was the median age of first sex, and 22-23 years the median age of first commercial sex. Client load was highest in Victoria Falls (median 14 in the last month) and lowest in Hwange (6). FSWs reported a median of 2 commercial partners in the past week, most of whom were new clients. A minority reported anal sex, though among those, 35-58% reported it in the last month. Most participants had at least one close friend who was a sex worker, with 2-4 sex worker close friends most common in all three sites (62.4% of participants in Mutare, 44.5% in Hwange and 58.0% in Victoria Falls).
Table 1. Sociodemographic characteristics of sex workers in three sites in Zimbabwe, RDS weighted.
| Mutare (n=370) |
Hwange (n=237) |
Victoria Falls (n=229) |
|||||
|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95 % CI) | n | % (95 % CI) | ||
| Age | <25 years | 97 | 30.0 (22.8-37.2) | 57 | 28.4 (19.2-37.6) | 80 | 39.5 (30.3-48.7) |
| >25≤30 | 75 | 19.0 (13.5-24.5) | 50 | 21.5 (14.8-28.2) | 62 | 24.9 (16.9-32.9) | |
| >30≤35 | 74 | 20.1 (14.8-25.4) | 44 | 19.2 (12.4-26.0) | 45 | 15.9 (9.5-22.4) | |
| >35≤40 | 46 | 12.3 (7.5-17.1) | 41 | 15 (9.7-20.3) | 19 | 7.3 (3.4-11.2) | |
| >40 | 78 | 18.0 (12.6-23.4) | 45 | 15.9 (9.9-21.9) | 23 | 12.3 (6.2-18.4) | |
| Marital | Single, never married | 64 | 23.4 (16.9-29.8) | 77 | 24.5 (19.0-30.2) | 68 | 29.5 (21.8-37.3) |
| Status | Married or Living together | 9 | 3.4 (0.9-6.6) | 1 | 0.5 (0.1-1.3) | 2 | 2.2 (0.6-5.7) |
| Divorced | 126 | 56.1 (47.9-64.4) | 216 | 57.4 (51.1-64.2) | 118 | 46.0 (38.3-54.4) | |
| Widowed | 38 | 17.0 (10.7-24.2) | 75 | 17.6 (12.1-23.5) | 41 | 22.3 (13.5-31.2) | |
| Don't know | 0 | 0 (0-0) | 1 | * | 0 | 0 (0-0) | |
| Education | None or incomplete primary | 49 | 12.9 (8.4-17.4) | 21 | 12.7 (7.1-18.3) | 34 | 15.5 (9.1-21.9) |
| Incomplete secondary | 186 | 49.7 (42.8-56.6) | 118 | 55.3 (46.9-63.8) | 146 | 67.5 (59.1-75.9) | |
| Complete secondary or higher | 134 | 37.3 (30.4-44.2) | 87 | 32.0 (24.1-39.9) | 49 | 17.0 (10.3-23.7) | |
| Duration of sex work | <2 years | 42 | 9.8 (5.6-14.1) | 38 | 18.3 (12.0-24.6) | 59 | 31.2 (22.8-39.6) |
| >2<4 years | 102 | 33.5 (25.9-41.1) | 70 | 29.3 (21.0-37.6) | 76 | 30.5 (22.1-38.9) | |
| >4<6 years | 59 | 14.3 (9.7-18.9) | 37 | 16.9 (9.6-24.1) | 39 | 13.4 (7.3-19.5) | |
| >6<10 years | 70 | 18.4 (13.4-23.4) | 35 | 13.8 (7.8-19.8) | 32 | 17.1 (10.6-23.6) | |
| >10 years | 97 | 24.1 (18.5-29.7) | 57 | 21.8 (14.8-28.8) | 23 | 7.9 (3.6-12.2) | |
| Duration at that site | <5 years | 60 | 20.7 (14.3-27.1) | 57 | 25.04 (17.5-32.6) | 74 | 40.1 (30.5-49.7) |
| >5<10 | 53 | 14 (9.4-18.6) | 28 | 16.01 (8.7-23.3) | 52 | 21.8 (14.1-29.6) | |
| >10<20 | 66 | 14.7 (9.8-19.6) | 32 | 14.9 (7.7-22.2) | 57 | 20.0 (13.0-27.0) | |
| >20<40 | 153 | 43.2 (35.7-50.8) | 97 | 36.3 (27.8-44.7) | 44 | 18.1 (11.7-24.4) | |
| 40+ | 38 | 7.3 (3.8-10.8) | 23 | 7.7 (3.3-12.2) | 2 | ** | |
| Where do they solicit clients | Bars/nightclubs/entertainment | 298 | 80.7 (75.5-85.9) | 192 | 80.5 (74.2-86.8) | 217 | 94.5 (90.7-98.3) |
| Telephone/at home | 106 | 26.8 (20.7-32.9) | 89 | 38.9 (30.9-46.9) | 42 | 15.8 (9.0-22.6) | |
| Market Stalls | 32 | 8.2 (4.8-11.6) | 6 | 3.5 (0.4-6.6) | 2 | 0.4 (-0.2-1.0) | |
| On the street | 133 | 35.5 (29.4-41.6) | 71 | 26.0 (18.7-33.3) | 30 | 9.8 (5.7-13.9) | |
| Lodges | 30 | 5.9 (3.1-8.7) | 18 | 5.0 (2.4-7.7) | 12 | 2.0 (0.4-3.6) | |
| Hotels | 19 | 6.2 (3.1-9.3) | 11 | 2.4 (0.7-4.2) | 14 | 3.4 (1.0-5.8) | |
| Other | 32 | 7.5 (4.4-10.6) | 20 | 7.3 (3.8-10.9) | 6 | 1.7 (-1.2-4.6) | |
| No. of commercial partners in last week | 0 | 30 | 7.5 (4.5-11.0) | 25 | 12.9 (6.7-17.8) | 19 | 7.7 (3.3-12.8) |
| 1-3 | 146 | 45.3 (38.3-52.0) | 142 | 64.7 (57.2-72.0) | 98 | 41.8 (32.6-50.9) | |
| 4-9 | 140 | 36.4 (30.1-42.9) | 54 | 18.6 (13.0-24.7) | 82 | 39.2 (30.8-48.7) | |
| 10+ | 54 | 10.8 (7.6-14.5) | 16 | 4.6 (2.3-7.7) | 30 | 11.3 (6.1-17.1) | |
| How many times stopped by police in last week | 0 | 154 | 39.3 (32.1-46.7) | 150 | 66.5 (58.9-73.8) | 70 | 33.5 (25.7-41.6) |
| 1-4 | 164 | 49.4 (42.1-56.7) | 28 | 27.0 (20.1-34.5) | 92 | 41.3 (33.4-49.5) | |
| 5+ | 52 | 11.4 (7.6-15.7) | 19 | 6.6 (3.4-9.8) | 67 | 25.2 (18.8-32.7) | |
| No. of FSWs who are close friends | 0 | 8 | 2.5 (1.0-4.2) | 22 | 9.1 (4.8-14.2) | 11 | 4.6 (16.7-7.7) |
| 1 | 56 | 19.6 (13.3-26.4) | 76 | 34.5 (27.1-42.5) | 60 | 28.8 (19.6-37.4) | |
| 2-4 | 214 | 62.4 (55.4-69.3) | 108 | 44.5 (36.6-53.0) | 133 | 58.0 (49.0-68.1) | |
| 5+ | 92 | 15.6 (11.5-20.0) | 31 | 11.9 (6.9-18.1) | 25 | 8.7 (4.7-13.9) | |
Included 'don't know' with married in order to calculate weighted estimates (too few observations to estimate weighting otherwise).
Combined with >20<40 category as too few observations in the response category to calculate weighted estimates.
Most FSWs (72-77%) reported having a “permanent partner” with whom they had had sex 4.5-6 times in the last month (A steady or permanent partner was defined as a husband, or a boyfriend with whom there is an expectation of commitment to a longer term relationship).
Levels of intimate partner violence (IPV) were high with 13-24% reporting physical and 8-23% reporting sexual violence in the last year. IPV was more common within permanent partnerships than with clients. Many FSWs reported they had experienced harassment or abuse from the police. The percentage of FSWs who had been stopped by the police at least once in the previous month was lower in Hwange at 33.6% than in Mutare (60.7%) or Victoria Falls (66.5%). 27-32 percentage points of FSWs reported recent experience of genital sores, ulcers or unusual genital discharge, with about 66% having sought treatment for this.
Outcome variables
The approaches to calculating prevalence estimates for the outcome variables made a small difference to the estimates obtained. For example, in Mutare and Hwange, the weighted HIV prevalence was slightly lower than the unweighted, while in Victoria Falls weighted results were slightly higher. The RDS weighted prevalence and the regression weighted prevalence were similar for each of the outcomes except for the percentage testing for HIV in the previous 6 months (table 2).
Table 2. Main outcome measures.
| Mutare | n / N | Unweighted % | RDS weighted % and 95% confidence intervals | Regression predicted weighted % and 95% confidence intervals |
|---|---|---|---|---|
| HIV Prevalence | 188/338 | 55.6 | 50.6 (43.5-58.6) | 52 (45.1-58.8) |
| Consistent condom use - transactional partners | 242/361 | 67 | 65.7 (58.4-71.2) | 65.2 (58.3-71.5) |
| Consistent condom use - non transactional partners | 96/271 | 35.4 | 33.3 (26.5-40.9) | 33.6 (30-37.4) |
| HIV testing within last 6 months* | 73/262 | 27.9 | 30.3 (23.2-38.6) | 30.2 (22.2-39.6) |
| Victoria Falls | ||||
| HIV Prevalence | 156/229 | 68.1 | 69.6 (61.7-76.7) | 70 (61.0-77.7) |
| Consistent condom use - transactional partners | 160/220 | 72.7 | 71.6 (63.2-78.7) | 72.6 (62.8-80.5) |
| Consistent condom use - non transactional partners | 60/166 | 36.1 | 31.3 (23.1-40.9) | 33.3 (22.4-46.4) |
| HIV testing within last 6 months (HIV negatives only) | 31/146 | 21.2 | 13.4 (8.7-19.9) | 13.2 (10.8-16.1) |
| Hwange | ||||
| HIV Prevalence | 136/237 | 57.4 | 50.6 (41.7-59.0) | 51.8 (46.5-56.9) |
| Consistent condom use - transactional partners | 140/220 | 63.6 | 65.7 (57.3-73.3) | 66.3 (57.6-74.1) |
| Consistent condom use - non transactional partners | 62/185 | 33.9 | 33.4 (24.8-42.6) | 34.2 (24.2-45.8) |
| HIV testing within last 6 months* | 46/167 | 27.5 | 25.1 (17.4-34.7) | 25.6 (17.9-35.2) |
Amongst only those who did not report previously testing HIV positive
The majority of FSWs recruited were HIV positive. HIV prevalence was highest among FSWs recruited in Victoria Falls with RDS-weighted prevalence of 69.6% and a regression weighted prevalence of 70.0%. In Mutare, 50.6% (RDS) - 52% (regression) of FSWs were HIV positive, while in Hwange, this figure was similar at 50.6% (RDS) - 51.8% (regression).
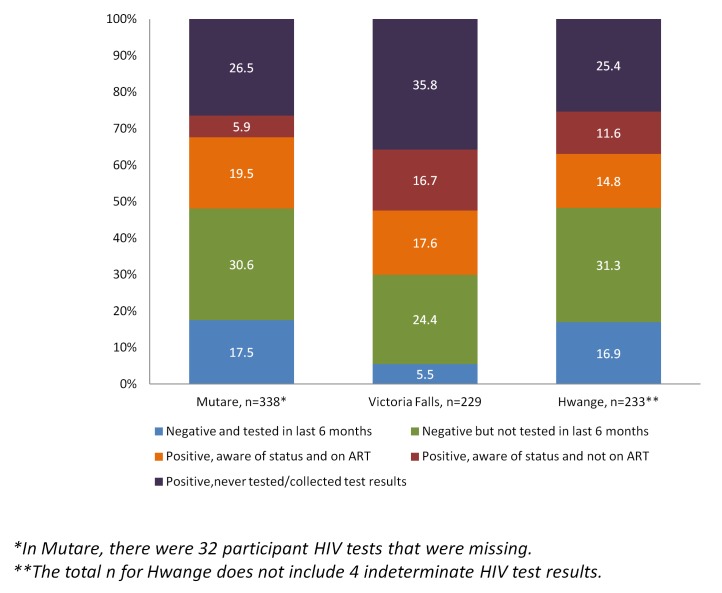
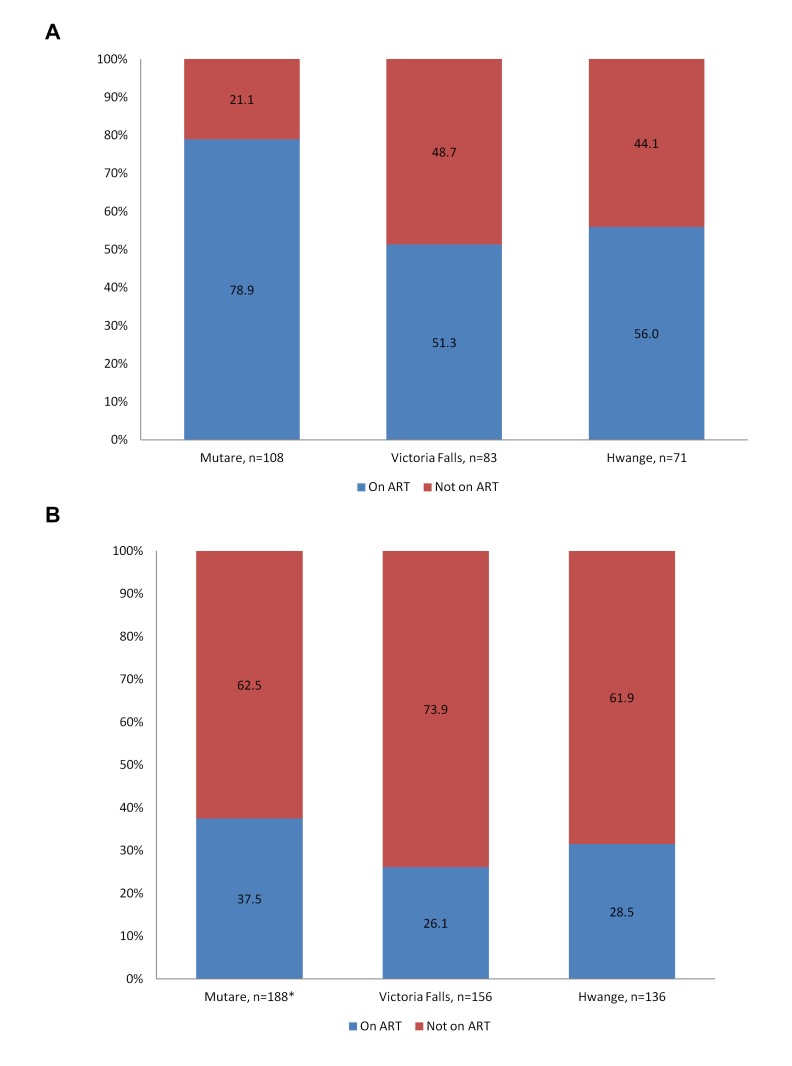
Because we had information on testing, self-reported HIV status amongst FSWs and laboratory confirmed serostatus in the whole sample, we were able to investigate the proportion of FSWs who were aware of their true HIV status and who were aware and on treatment (Figure 3). The category of most concern is FSWs with laboratory-confirmed infection but who are unaware of their status, either because they had never tested, their status had since changed or because they never collected the results of their test. In all three sites, this category was approximately half of all HIV positive participants (percentages are weighted by degree), see Figure 3. Of those who reported in the questionnaire that they were HIV positive, between 51% and 74% reported being on ART (see Figure 4a). This means that of all those with laboratory-confirmed HIV infection, the majority (61.9% to 73.9%) were not on ART (see Figure 4b). Of those who were HIV negative, the majority (65-82%) were also unaware of their status (see Figure 3). This was either because they had not tested in the last 6 months, because they never collected their results or because, in the case of Mutare only, 8 had previously thought they were HIV positive. Only 21-28% of self-reported HIV negative individuals reported having had an HIV test in the last 6 months.
Figure 3. Previous knowledge of HIV status.
Figure 4. Percentage of HIV positive female sex workers who knew their status and were on ART (a).
Percentage of all HIV+ female sex workers who reported that they were on ART (b).
The most common location of last HIV test amongst those who had ever tested was the hospital (38.7% in Mutare, 58.8% in Victoria Falls and 63.2% in Hwange). In Hwange and Victoria Falls, almost all those who reported that they were on ART were receiving treatment at the hospital (42/43 and 41/44 respectively). In Mutare, 55.5% were receiving treatment through the primary care ART clinic while 41.2% were treated at the hospital.
In all sites, FSWs were more likely to report that they always used condoms with commercial partners than with steady/permanent partners. The regression-weighted prevalences of consistent condom use with transactional partners were 65.2% (Mutare), 72.6% (Victoria Falls) and 66.3% (Hwange). For consistent condom use with steady/permanent partners, prevalence was 33.6%, 33.3% and 34.2% respectively.
Discussion
This is one of the first systematic surveys of HIV and associated factors among FSWs to be conducted in Zimbabwe. Of note we explored rates of engagement in HIV prevention, treatment and care, critical if UNAIDS goal of zero new infections and zero deaths are to be achieved[19]. We recruited FSWs from a tourist town, a mining town and Zimbabwe's third city. The FSWs we recruited were at extremely high risk of HIV with a prevalence of HIV between 50-70%, 3-4 times that of the women in the general population. While the majority of those who reported that they were HIV positive on the questionnaire said they were accessing ART services, overall only 26-38% of those with laboratory-confirmed infection (60% of HIV positives in Zimbabwe are estimated to be eligible for ART using current National Guidelines). Few of those who were positive but were unaware of their diagnosis reported having tested recently. Although reported condom use was high the majority of women reported that they had not used condoms at each recent sexual encounter either with client or regular partners.
This study confirms that FSWs in Zimbabwe have a much higher risk of HIV than those in the general population[1] in addition they report high rates of symptomatic STIs likely to further increase risk of contracting and transmitting HIV[20]. HIV prevalence rates as high as 60-90% have been reported for sex workers in Kenya[21], Central and East Africa[22], and Rwanda[23]. Importantly, over the past 10 years, Zimbabwe has experienced a well-documented decline in HIV prevalence in the general population with the prevalence in women now around 15%[15,24,25]. By contrast the prevalence among these FSWs remains at the very high rates found much earlier in the epidemic[26] suggesting that they remain relatively untouched by prevention services.
The majority of women recruited to this study were unaware of their HIV status. Historically data on sex worker rates of HIV testing are scant, although sub-optimal when reported, e.g. 4% of FSWs surveyed in Somalia in 2008 had ever tested[27], and 38% in the Democratic Republic of Congo in 2005-6[28]. Previous studies have found that barriers to testing include lack of awareness of services, distance to facilities, transportation costs, opportunity costs, time constraints, and fear of a positive result[29-31]. Barriers unique to sex work include anxiety about contact with authorities and concern about confidentiality, particularly that other FSWs or potential clients may learn their status[32].
Learning one’s HIV status is a prerequisite for entry into HIV care, and there is evidence that people modify their behaviour to engage in less high risk behaviour after testing HIV positive[33-37]. There have been successful interventions to increase HIV counselling and testing among FSWs[38]. Furthermore, strengthened peer support and a more cohesive social environment are associated with sex workers’ willingness to engage in care (testing, treatment initiation and adherence)[39,40]. WHO recommends that retesting ‘should be at least annually’ for those at high risk[41]. There is little evidence on FSWs’ uptake of and retention in HIV treatment programs in Africa; little is known about their progression through the “care cascade” compared to HIV positive clients in the general population.
Of those FSWs who reported that they were HIV positive in the questionnaire, Figure 3 shows that the majority also reported that they were on anti-retroviral therapy (ART). From a public health perspective, it is important to also know what percentage of all HIV positive sex workers, including those unaware of their status, were on ART. Figure 3 shows that for Mutare, this figure was 37.5%, for Victoria Falls it was 26.1% and for Hwange, 28.5%.
Although it is well documented that FSWs face numerous barriers to health seeking, relatively little has been written about their ability to engage with HIV prevention and care services. These data suggest that while reported engagement is relatively high among those who are accurately aware of their status, the majority are not accessing prevention and care services. We have shown previously that Zimbabwean FSWs attending general health services experience high levels of stigma and discrimination [42]. Programmes to increase FSWs engagement with services are critical both for their own wellbeing and likely for the wider public health. There is some evidence that community mobilisation, which seeks to empower women individually and collectively, can increase condom usage and improve uptake of health services and it is now recommended by WHO[43]. What is less clear is whether it is cost effective to include treatment and care for HIV positive FSWs within sex worker specific services (and pre-exposure prophylaxis to HIV negative women). Mathematical modeling suggests that there are potential benefits of intensifying treatment among FSWs in generalized epidemics[44] but empirical data to support this is lacking.
This survey has some limitations. The recruitment of survey participants through RDS poses several challenges to analysis and there remains debate about the methods of analysis. As in all applications of RDS it was not possible for us to empirically verify the extent to which the sample we recruited reflects the characteristics of FSWs working in the three sites. Nevertheless, we deployed several approaches to estimating the prevalence of key outcome variables and while there were some differences in our estimates these were minor. The characteristics of participants appeared to converge over waves of recruitment as would be expected if later waves are more likely to approximate the source population. We found a random effects regression approach to be the most flexible with regard to missing data and providing an approach to dealing with potential homophily, though evidence for homophily was weak. We had high rates of acceptance to participate in the survey.
The survey would have been strengthened if we had been able to collect samples for CD4 count testing among those infected with HIV (to determine what proportion of those eligible for care were accessing it). In addition, it would have been helpful to determine the proportion of women with STI diagnoses as STI symptoms correlate poorly with diagnosis in women[45]. The proportion of FSWs that were recruited early in their sex work careers was relatively low in Mutare and Hwange.
This survey suggests that prevalence of HIV is exceedingly high among FSWs in Zimbabwe and that their engagement with prevention, treatment and care is sub-optimal. In addition, women suffer high rates of intimate partner violence, police harassment and discrimination by the wider community. Intensifying interventions is likely to markedly reduce HIV and social risks for FSWs, their clients and the general population in sub-Saharan Africa [46]. We plan to assess the impact of the increasing scope of Zimbabwe's National Sex Work on HIV and other social outcomes for both FSWs and the population more widely.
Supporting Information
Respondent driven sampling questionnaire – English version.
(XLSX)
Funding Statement
The study was funded by Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH commissioned by the German Federal Ministry for Economic Cooperation and Development (BMZ). Two members of GIZ's team acted as co-investigators. In addition, Stefan Weinmann and Sabine Flessenkämper commented on the manuscript.
References
- 1. WHO (2011) Preventing HIV in Sex Work Settings in sub Saharan Africa. Geneva: World Health Organization. [Google Scholar]
- 2. Wojcicki JM, Malala J (2001) Condom use, power and HIV/AIDS risk: sex workers bargain for survival in Hillbrow/Joubert Park/Berea, Johannesburg. Soc Sci Med 53: 99-121. doi: 10.1016/S0277-9536(00)00315-4. PubMed: 11380165. [DOI] [PubMed] [Google Scholar]
- 3. Pauw I, Brener L (2003) 'You are just whores - you can't be raped': barriers to safer sex practices among women street sex workers in Cape Town. Cult Health Sex 5: 465-481. doi: 10.1080/136910501185198. [DOI] [Google Scholar]
- 4. Scorgie F, Nakato D, Akoth DO, Netshivhambe M, Chakuvinga P et al. (2011) I Expect to be Abused and I Have Fear: Sex workers' Experiences of Human rights Violations and Barrers to Accessing Healthcare in Four African Countries. Cape Town: African Sex Worker Alliance. [Google Scholar]
- 5. Arnott J, Crago A-L (2009) Rights Not Rescue: A Report on Female, Male, Trans Sex Workers' Human RIghts in Botswana, Namibia, and South Africa. New York City: Open Society Institute. [Google Scholar]
- 6. National AIDS Council MoHaCW, UNAIDS (2006) Zimbabwe National HIV and AIDS Strategic Plan (ZNASP). Harare: National AIDS Council; pp. 2006-2010. [Google Scholar]
- 7. Council NA (2006) Zimbabwe National Behavioural Change Strategy 2006-2010. Harare Zimbabwe.
- 8. Heckathorn D (1997) Respondent-driven sampling: a new approach to the study of hidden populations Soc Probl: 174-199. [Google Scholar]
- 9. McCreesh N, Frost SD, Seeley J, Katongole J, Tarsh MN et al. (2012) Evaluation of respondent-driven sampling. Epidemiology 23: 138-147. doi: 10.1097/EDE.0b013e31823ac17c. PubMed: 22157309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salganik MJ (2012) Commentary: Respondent-driven Sampling in the Real World. Epidemiology 23: 148-150. doi: 10.1097/EDE.0b013e31823b6979. PubMed: 22157310. [DOI] [PubMed] [Google Scholar]
- 11. White RG, Lansky A, Goel S, Wilson D, Hladik W et al. (2012) Respondent driven sampling--where we are and where should we be going? Sex Transm Infect 88: 397-399. doi: 10.1136/sextrans-2012-050703. PubMed: 23012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mavhu W, Langhaug LF, Manyonga B, Power R, Cowan FM (2008) What is 'sex' exactly? Using cognitive interviewing to improve validity of sexual behaviour reporting among young people in rural Zimbabwe. Cult Health Sex 10: 563-572. doi: 10.1080/13691050801948102. PubMed: 18649195. [DOI] [PubMed] [Google Scholar]
- 13. Langhaug LF, Buzdugan R, Dirawo J, Benedikt C, Mundida O et al. (2012) Change over time (2007-2009) in HIV preventive behaviours, evidence from a population-based survey in Zimbabwe. TMIH 17: 827-835. [DOI] [PubMed] [Google Scholar]
- 14. Cowan FM, Pascoe SJS, LF L, Mavhu W, Chidiya S, et al (2010) The Regai Dzive Shiri Project: results of a randomised trial of an HIV prevention intervention for Zimbabwean youth. AIDS 24: 2541-2542. doi: 10.1097/QAD.0b013e32833e77c9. PubMed: 20881473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zimbabwe Central Statistical A, Measure; DHS; (2011) Zimbabwe Demographic and Health Survey 2010-11: Preliminary Report. Calverton, Maryland: CSO and Macro International Inc [Google Scholar]
- 16. US National Committee for Clinical Laboratory (1997) Standards Blood Collect Filter Paper for Neonatal Screening Programs (LA4A)
- 17. Schonlau M, Liebau E (2012) Respondent-driven sampling. STATA J 12: 72-93. [Google Scholar]
- 18. Szwarcwald CL, de Souza Júnior PR, Damacena GN, Junior AB, Kendall C (2011) Analysis of data collected by RDS among sex workers in 10 Brazilian cities, 2009: estimation of the prevalence of HIV, variance, and design effect. J Acquir Immune Defic Syndr 57 Suppl 3: S129-S135. doi: 10.1097/QAI.0b013e31821e9a36. PubMed: 21857308. [DOI] [PubMed] [Google Scholar]
- 19. UNAIDS (2012) Report on the Global AIDS Epidemic. Geneva: U.N. AIDS. [Google Scholar]
- 20. Cwikel JG, Lazer T, Press F, Lazer S (2008) Sexually transmissible infections among female sex workers: an international review with an emphasis on hard-to-access populations. Sex Health 5: 9-16. doi: 10.1071/SH07024. PubMed: 18361849. [DOI] [PubMed] [Google Scholar]
- 21. Kreiss JK, Koech D, Plummer FA, Holmes KK, Lightfoote M et al. (1986) AIDS virus infection in Nairobi prostitutes. Spread of the epidemic to East Africa. N Engl J Med 314: 414-418. doi: 10.1056/NEJM198602133140704. PubMed: 3484804. [DOI] [PubMed] [Google Scholar]
- 22. Quinn TC, Mann JM, Curran JW, Piot P (1986) AIDS in Africa: an epidemiologic paradigm. Science 234: 955-963. doi: 10.1126/science.3022379. PubMed: 3022379. [DOI] [PubMed] [Google Scholar]
- 23. Piot P, Laga M (1988) Prostitutes: a high risk group for HIV infection? Soz Praventivmed 33: 336-339. doi: 10.1007/BF02084300. PubMed: 3066058. [DOI] [PubMed] [Google Scholar]
- 24. Gregson S, Gonesse E, Hallett TB, Tabuberekera N, Hargrove JW et al. (2010) HIV decline in Zimbabwe due to reductions in risky sex? Evidence from a comprehensive epidemiological review. Int J Epidemiol. doi: 10.1093/ije/dyq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halperin DT, Mugurungi O, Hallett TB, Muchini B, Campbell B et al. (2011) A surprising prevention success: Why did HIV epidemic decline in Zimbabwe? PLOS Med 8: e1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cowan FM, Hargrove J, Langhaug LF, Jaffar S, Mhuriyengwe L et al. (2005) The appropriateness of core group interventions using presumptive periodic treatment among rural Zimbabwean women who exchange sex for gifts or money. J Acquir Immunodefic Syndr 38: 202-207. doi: 10.1097/00126334-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 27. Kriitmaa K, Testa A, Osman M, Bozicevic I, Riedner G et al. (2010) HIV prevalence and characteristics of sex work among female sex workers in Hargeisa, Somaliland, Somalia. AIDS, 24 Suppl 2: 24: S61-S67 doi: 10.1097/1001.aids.0000386735.0000387177.0000386732a. PubMed: 20610951. [DOI] [PubMed] [Google Scholar]
- 28. Kayembe PK, Mapatano MA, Busangu AF, Nyandwe JK, Musema GM et al. (2008) Determinants of consistent condom use among female commercial sex workers in the Democratic Republic of Congo: implications for interventions. Sex Transm Infect 84: 202-206. doi: 10.1136/sti.2007.028324. PubMed: 18055581. [DOI] [PubMed] [Google Scholar]
- 29. Gage AJ, Ali D (2005) Factors associated with self-reported HIV testing among men in Uganda. AIDS Care 17: 153-165. doi: 10.1080/09540120500119833. PubMed: 15763711. [DOI] [PubMed] [Google Scholar]
- 30. Maman S, Mbwambo J, Hogan NM, Kilonzo GP, Sweat M (2001) Women's barriers to HIV-1 testing and disclosure: challenges for HIV-1 voluntary counselling and testing. AIDS Care 13: 595-603. doi: 10.1080/09540120120063223. PubMed: 11571006. [DOI] [PubMed] [Google Scholar]
- 31. Mshana GH, Wamoyi J, Busza J, Zaba B, Changalucha J et al. (2006) Barriers to accessing antiretroviral therapy in Kisesa, Tanzania: a qualitative study of early rural referrals to the national program. AIDS Patient Care STDs 20: 649-657. doi: 10.1089/apc.2006.20.649. PubMed: 16987051. [DOI] [PubMed] [Google Scholar]
- 32. Munoz J, Adedimeji AA, Alawode O (2010) 'The bring AIDS to us and say we give it to them': Socio-structural context of female sex workers' vulnerabiity to HIV infection in Ibadan Nigeria. J Soc Aspects HIV/AIDS 7: 51-61. doi: 10.1080/17290376.2010.9724969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. The Voluntary HIVC, Testing Efficacy Study G (2000) Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania and Trinidad. (2000) a randomised trial. Lancet 356: 103-112 doi:10.1016/S0140-6736(00)02446-6. PubMed: 10963246. [Google Scholar]
- 34. Kachroo S (2006) Promoting self-testing for HIV in developing countries: potential benefits and pitfalls. Bull World Health Organ 84: 999-1000. doi: 10.2471/BLT.06.032656. PubMed: 17242837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marks G, Crepaz N, Senterfitt JW, Janssen RS (2005) Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. Journal of Acquired Immune Deficiency Syndromes: JAIDS 39: 446-453 PubMed; : 16010168 [DOI] [PubMed] [Google Scholar]
- 36. Valdiserri RO, Ogden LL, McCray E (2003) Accomplishments in HIV prevention science: implications for stemming the epidemic. Nat Med 9: 881-886. doi: 10.1038/nm0703-881. PubMed: 12835709. [DOI] [PubMed] [Google Scholar]
- 37. Weinhardt LS, Carey MP, Johnson BT, Bickham NL (1999) Effects of HIV counselling and testing on sexual risk behaviour: a meta-analytic review of published research, 1985-1997. Am J Public Health 89: 1397-1405. doi: 10.2105/AJPH.89.9.1397. PubMed: 10474559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luchters S, Chersich MF, Rinyiru A, Barasa M-S, King'ola N et al. (2008) Impact of five years of peer-mediated interventions on sexual behavior and sexually transmitted infections among female sex workers in Mombasa, Kenya. BMC Public Health 8: 143. doi: 10.1186/1471-2458-8-143. PubMed: 18445258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hong YANP, Fang XP, Li XP, Liu YM, Li MM (2008) Environmental Support and HIV Prevention Behaviors Among Female Sex Workers in China. Sex Transm Dis 35: 662-667. doi: 10.1097/OLQ.0b013e31816b322c. PubMed: 18418288. [DOI] [PubMed] [Google Scholar]
- 40. Deering KN, Shannon K, Sinclair H, Parsad D, Gilbert E et al. (2009) Piloting a Peer-Driven Intervention Model to Increase Access and Adherence to Antiretroviral Therapy and HIV Care among Street-Entrenched HIV-Positive Women in Vancouver. AIDS Patient Care STDs 23: 603-609. doi: 10.1089/apc.2009.0022. PubMed: 19591602. [DOI] [PubMed] [Google Scholar]
- 41. WHO UNAIDS, nswp (2012) Prevention and Treatment of HIV and other Sexually Transmitted Infections for Sex Workers in Low- and Middle-income Countries: Recommendations for a public health approach. Geneva [Google Scholar]
- 42. Mtetwa SB, Chidiya S, Mungofa S, Cowan FM (2013) "You are wasting our drugs": Health Service Barriers to HIV Treatment for Sex Workers in Zimbabwe.BMC Public Health 13: 698. doi: 10.1186/1471-2458-13-698. PubMed: 23898942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. World Health Organisation. DoMH (2005) Toolkit for targeted HIV/AIDS prevention and care in sex work settings. Geneva: World Health Organisation. [Google Scholar]
- 44. Nagelkerke NJD, Jha P, de Vlas SJ, Korenromp EL, Moses S et al. (2002) Modelling HIV/AIDS epidemics in Botswana and India: impact of interventions to prevent transmission. Bull WHO 80: 89-96. PubMed: 11953786. [PMC free article] [PubMed] [Google Scholar]
- 45. Hawkes S, Morison L, Foster S, Gausia K, Chakraborty J et al. (1999) Reproductive-tract infections in women in low-income, low-prevalence situations: assessment of syndromic management in Matlab, Bangladesh. Lancet 354: 1776-1781. doi: 10.1016/S0140-6736(99)02463-0. PubMed: 10577639. [DOI] [PubMed] [Google Scholar]
- 46. Chersich MF, Luchters SM, Malonza IM, Mwarogo P, King'ola N et al. (2007) Heavy episodic drinking among Kenyan female sex workers is associated with unsafe sex, sexual violence and sexually transmitted infections. Int J STD AIDS 18: 764–9. PubMed: 18005511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Respondent driven sampling questionnaire – English version.
(XLSX)